Abstracts
A simple, rapid, and sensitive fluorimetric method was developed and validated to quantify curcumin in lipid and polymeric nanocapsule suspensions, using acetonitrile as a solvent. The excitation and emission wavelengths were set at 397 nm and 508 nm, respectively. The calibration graph was linear from 0.1 to 0.6 µg/mL with a correlation coefficient of 0.9982. The detection and quantitation limits were 0.03 and 0.10 µg/mL, respectively. The validation results confirmed that the developed method is specific, linear, accurate, and precise for its intended use. The current method was successfully applied to the evaluation of curcumin content in lipid and polymeric nanocapsule suspensions during the early stage of formulation development.
Curcumin; Fluorimetric method; Polymeric nanocapsules; Lipid nanocapsules
Um método fluorimétrico simples, rápido e sensível foi desenvolvido e validado para quantificação da curcumina em suspensões de nanocápsulas lipídicas e poliméricas, usando acetonitrila como solvente. Os comprimentos de onda de excitação e emissão foram 397 nm e 508 nm, respectivamente. Nas condições testadas, a curva de calibração demonstrou-se linear na faixa de 0,1 a 0,6 µg/mL, exibindo coeficiente de correlação de 0,9982. Os limites de detecção e quantificação foram 0,03 e 0,10 µg/mL, respectivamente. Os resultados da validação confirmaram que o método desenvolvido é específico, linear, exato e preciso para o uso proposto. O presente método foi aplicado com sucesso para a avaliação do teor de curcumina nas suspensões de nanocápsulas lipídicas e poliméricas durante o estágio inicial do desenvolvimento da formulação.
Curcumina; Método fluorimétrico; Nanocápsulas poliméricas; Nanocápsulas lipídicas
ARTICLE
Development and validation of a fluorimetric method to determine curcumin in lipid and polymeric nanocapsule suspensions
Letícia MazzarinoI; Ismael Casagrande BellettiniII; Edson MinattiII; Elenara Lemos-SennaI,* * Correspondence: E. L. Senna. Laboratório de Farmacotécnica, Departamento de Ciências Farmacêuticas, Universidade Federal de Santa Catarina, Campus Universitário Trindade - 88040-900 - Florianópolis - SC, Brazil. E-mail: lemos@ccs.ufsc.br
ILaboratory of Pharmaceutical Technology, Department of Pharmaceutical Sciences, Federal University of Santa Catarina
IILaboratory of Polymer and Surfactant Solutions, Department of Chemistry, Federal University of Santa Catarina
ABSTRACT
A simple, rapid, and sensitive fluorimetric method was developed and validated to quantify curcumin in lipid and polymeric nanocapsule suspensions, using acetonitrile as a solvent. The excitation and emission wavelengths were set at 397 nm and 508 nm, respectively. The calibration graph was linear from 0.1 to 0.6 µg/mL with a correlation coefficient of 0.9982. The detection and quantitation limits were 0.03 and 0.10 µg/mL, respectively. The validation results confirmed that the developed method is specific, linear, accurate, and precise for its intended use. The current method was successfully applied to the evaluation of curcumin content in lipid and polymeric nanocapsule suspensions during the early stage of formulation development.
Uniterms: Curcumin/determination. Fluorimetric method/quantitative analysis. Polymeric nanocapsules/evaluation. Lipid nanocapsules/evaluation.
RESUMO
Um método fluorimétrico simples, rápido e sensível foi desenvolvido e validado para quantificação da curcumina em suspensões de nanocápsulas lipídicas e poliméricas, usando acetonitrila como solvente. Os comprimentos de onda de excitação e emissão foram 397 nm e 508 nm, respectivamente. Nas condições testadas, a curva de calibração demonstrou-se linear na faixa de 0,1 a 0,6 µg/mL, exibindo coeficiente de correlação de 0,9982. Os limites de detecção e quantificação foram 0,03 e 0,10 µg/mL, respectivamente. Os resultados da validação confirmaram que o método desenvolvido é específico, linear, exato e preciso para o uso proposto. O presente método foi aplicado com sucesso para a avaliação do teor de curcumina nas suspensões de nanocápsulas lipídicas e poliméricas durante o estágio inicial do desenvolvimento da formulação.
Unitermos: Curcumina/determinação. Método fluorimétrico/análise quantitativa. Nanocápsulas poliméricas/avaliação. Nanocápsulas lipídicas/avaliação.
INTRODUCTION
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] (Figure 1) is a yellow polyphenol extracted from the rhizome of the plant Curcuma longa, commonly known as turmeric. It is widely used in food as a spice flavoring and coloring agent. The medicinal use of this plant has been recognized in Ayurveda for over 6000 years (Aggarwal et al., 2003; Heath et al., 2003). Curcumin has been shown to possess several pharmacological actions including anti-inflammatory, anticancer, antioxidant and antimicrobial effects (Maheshwari et al., 2006). Curcumin has demonstrated chemopreventive properties, suppressing the tumorigenic activity of a wide variety of carcinogens in several kinds of cancer. In culture cell and animal studies, curcumin has been shown to exhibit antiproliferative, anti-invasive, and antiangiogenic properties. Curcumin has also demonstrated its usefulness for the treatment of other diseases such as diabetes, Alzheimer's disease, Parkinson's disease, and arthritis (Syng-ai et al., 2004).
In spite of the great potential of the use of curcumin in therapeutics, its clinical application has been limited due to several drawbacks. This drug displays a poor aqueous solubility, preventing its administration by the intravenous route. When administrated orally, the majority of curcumin is secreted in the feces and only negligible amounts in the urine, indicating that this drug is poorly absorbed from the gut. Pharmacokinetic studies have indicated that curcumin is rapidly metabolized in the liver, undergoing extensive reduction via alcohol dehydrogenase (Syng-ai et al., 2004). Besides the low systemic bioavailability expected from its pharmacokinetic characteristics, curcumin is quickly hydrolyzed at neutral and basic pH and is susceptible to photochemical degradation (Wang et al., 1997; Sharma et al., 2001; Tonnesen et al., 2002; Tomren et al., 2007). In order to overcome these problems, curcumin has been associated with colloidal carriers. Among them, polymeric nanoparticles have been considered as promising drug delivery systems due to their potential to increase the therapeutic efficacy and to reduce the undesirable side effects of drugs. Other important advantages of nanoparticles include their ability to carry water-insoluble drugs by intravenous route, their protection of active molecules against in vivo degradation, their ability to control drug release, ease of preparation and high stability in biological fluids and during storage (Gref et al., 1995; Fonseca et al., 2002; Gupte, Ciftci, 2004). On the other hand, some drawbacks of polymeric nanoparticles stem from the residues of organic solvents required in their preparation, polymer cytotoxicity, and difficulties scaling up the production processes.
In recent years, the main objective of researchers concerning the preparation of colloidal drug carriers has been the development of drug delivery systems using only excipients acceptable for human use (Heurtault et al., 2002; Mehnert, Mäder, 2002). Recently, lipid nanocapsules have been proposed as carriers for hydrophobic drugs. Lipid nanocapsules are submicronic particles composed of an oily liquid core surrounded by a solid or semi-solid shell of surfactant. These systems are prepared with lipids and a combination of hydrophilic/lipophilic surfactants approved by the FDA (Heurtault et al., 2002; Dulieu, Bazile, 2005). Lipid nanocapsules have been prepared using the phase inversion process, which exploits the ability of polyethoxylated surfactants to change their affinities by the water and oil phase, as a function of the emulsion temperature, resulting in more stable and smaller nanoparticles. This method also has the advantages of being relatively simple and low-energy consuming, allowing easy industrial scale-up and in particular, avoiding the use of organic solvents, the residues of which represent a potential risk to human health (Witschi, Doelker, 1997; Heurtault et al., 2003; Anton et al., 2008).
The preparation of lipid and polymeric nanocapsules has been explored in our research group with the aim of improving the stability and biological performance of curcumin. Firstly, we sought to evaluate the effect of the type of the materials (lipid or polymer) and the procedures employed in the preparation of the nanocapsule suspensions on curcumin encapsulation. As support for formulation development as well as for future preclinical studies, a specific and sensitive analytical method must be developed and validated. The most widely used method for quantification of curcumin is a direct ultraviolet spectrophotometry method, but a lack of reproducibility has been observed. High performance liquid chromatography methods have also been developed to quantify curcumin, but they have the disadvantages of being more time-consuming and expensive (Bisht et al., 2007; Nam et al., 2007; Tiyaboonchai et al., 2007; Sou et al., 2008). Given that curcumin exhibits strong fluorescence in organic solvents (Díaz, Peinado, 1992), and the fact that no validated method has been described to quantify curcumin in colloidal suspensions, the development and validation of a spectrofluorimetric method was undertaken to determine curcumin loading in polymeric and lipid nanocarrier suspensions.
MATERIAL AND METHODS
Chemicals and reagents
Curcumin and poly (D,L-lactide) (PLA, MW 90,000 - 120,000) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Soybean hydrogenated lecithin (LIPOID S 75-3N) was provided by Lipoid GmbH (Ludwigshafen, Germany) and castor oil was obtained from Via Farma Importadora Ltda. (São Paulo, Brazil). Hydroxystearic acid-polyethylene glycol copolymer (Solutol HS 15) and Poloxamer (Pluronic F 68) were kindly donated by the BASF Chemical Company (Ludwigshafen, Germany). Sodium chloride was obtained from Vetec (Rio de Janeiro, Brazil). Except for the HPLC grade acetonitrile used in the analysis (Carlo Erba, Milan, Italy), all other reagents and solvents were analytical grade.
Preparation of the colloidal suspensions
Lipid nanocapsule suspensions
Lipid nanocapsule suspensions were prepared by the phase inversion method as described by Heurtault et al. (2002). An aqueous phase containing 8.40 g distilled water, 0.75 g NaCl and 4.20 g Solutol HS 15 was added to the oil phase containing 1.40 g castor oil, 0.14 g lecithin, and 5 or 10 mg curcumin, both previously heated to 90 ºC, under magnetic stirring. Three temperature cycles alternating from 60 to 95 ºC were applied to achieve the inversion process. During the last cooling period, the suspensions were rapidly diluted with 25.0 mL cold water (approximately 0 ºC) and continuously stirred for 30 min.
Polymeric nanocapsule suspensions
Polymeric nanocapsule suspensions were prepared using the interfacial deposition process after solvent displacement as described by Fessi et al. (1989). Briefly, 60.0 mg PLA, 0.250 mL castor oil, and 2.5 or 5.0 mg curcumin were dissolved in 2 mL of acetone. The resulting solution was mixed with a 10 mL acetone:ethanol (60:40, v/v) mixture containing 25.0 mg lecithin, and the final volume was adjusted to 12.0 mL. This organic phase was poured into an aqueous phase (26.0 mL) containing 0.375% (w/v) of Pluronic F 68 or Solutol HS 15, maintained under magnetic stirring. The organic solvents were then eliminated by evaporation under reduced pressure, and the final volume of the colloidal suspension was adjusted to 10.0 mL.
Lipid and polymeric nanocapsule suspensions were filtered through filter paper with a pore size of 8 mm (J-Prolab, São José dos Pinhais, Brazil). Unloaded lipid and polymeric nanocapsule suspensions were prepared and treated in the same manner as the samples. The nanocapsule suspensions were analyzed in the fluorimetric system after dissolution in acetonitrile.
Fluorimetric method
Steady-state fluorescence
The steady-state fluorescence spectra of curcumin were recorded on a Hitachi F4500 Spectrofluorimeter equipped with a thermostatted cell holder set at 25.0 ºC. The sample was continuously stirred in a standard 1 cm quartz cell. Both slits of excitation and emission monochromators were adjusted to 5.0 nm. The samples were excited at 397 nm and the emission spectra were recorded from 440 to 600 nm. The relative fluorescence intensities were measured at λemi = 508 nm.
Preparation of stock and working solutions
A stock solution was prepared by accurately dissolving 100.0 mg of curcumin in 100.0 mL of acetonitrile. From this solution, a working standard solution of 10.0 µg/mL was prepared in acetonitrile. This solution was then diluted as needed to prepare different standard solutions: 0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 µg/mL in acetonitrile.
Method validation
The validation parameters evaluated were specificity, linearity and range, accuracy, precision, limit of quantification (LOQ) and limit of detection (LOD). The performance characteristics were based on the pharmaceutical regulatory guidelines of the International Conference on Harmonization (2005) and United States Pharmacopeia (2007).
(a) Specificity. The specificity of the fluorimetric method was evaluated by analyzing unloaded nanocapsule suspensions in order to verify whether the excipients used in the formulations of the nanocapsule suspensions interfered in the curcumin quantification. The samples were analyzed in the fluorimetric system in triplicate.
(b) Linearity and range. Six standard solutions ranging from 0.1 to 0.6 µg/mL, in concentration, 3 replicates each, were analyzed in the fluorescence system. The calibration graph was obtained by plotting the fluorescence intensity of the standard solutions against the theoretical standard concentrations. The linearity was evaluated by linear least-square regression analysis.
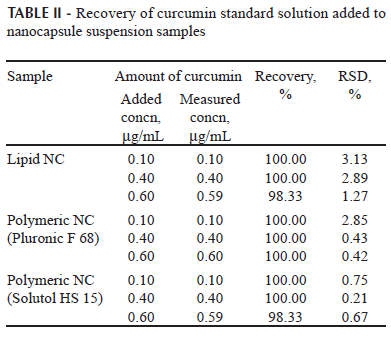
(c) Accuracy. The accuracy was investigated by spiking unloaded nanocapsule suspensions with known concentrations of curcumin standard solution at 3 different levels (lower, medium and upper concentration) corresponding to curcumin final concentrations of 0.1, 0.4 and 0.6 µg/mL. The analysis was performed in triplicate. The recovery (R) was estimated after fluorescence analysis using the following equation:
where R is recovery, Csnc is the curcumin concentration in the spiked nanocapsule suspension, Cnc is the curcumin concentration in unloaded nanocapsule suspension, and Css is the curcumin concentration in standard solution.
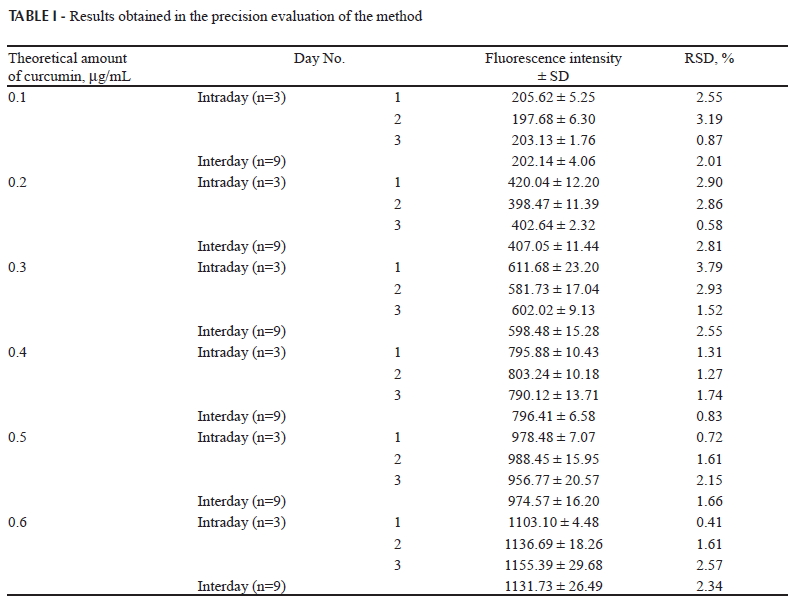
(d) Precision. The precision of the method was determined by measuring the repeatability (intraday precision) and the intermediate precision (interday precision), both expressed as relative standard deviation (RSD). The precision was evaluated by assaying standard solutions with different concentrations of curcumin (0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 µg/mL). The repeatability was evaluated by measuring 3 samples of each concentration of curcumin on the same day under the same experimental conditions. The intermediate precision was evaluated by assaying each standard solution on 3 different days. The RSD was calculated.
(e) Limit of Quantification (LOQ). The LOQ value was calculated directly from the calibration graph. It is defined as the lowest concentration in the standard curve that can be measured with suitable precision and accuracy. LOQ may be expressed as:
where δ is the standard deviation (SD) of y-intercepts of regression lines and S is the slope of the calibration graph.
(f) Limit of Detection (LOD). The LOD value was calculated directly from the calibration graph. It is defined as the lowest concentration in the standard curve that can be detected, but not necessarily quantified as an exact value. LOD may be expressed as:
where δ is the standard deviation (SD) of y-intercepts of regression lines and S is the slope of the calibration graph.
Determination of curcumin loading in nanocapsules
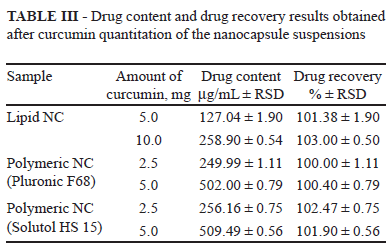
Entrapment efficiency and drug content were estimated after determination of the curcumin concentration in the nanocapsule suspensions by fluorescence spectrophotometry. The entrapment efficiency (%) was estimated as being the difference between the total concentration of curcumin found in the nanocapsule suspensions after their complete dissolution in acetonitrile and the concentration of drug in the supernatant obtained by the suspension ultrafiltration/centrifugation procedure using Microcon Centrifugal Filter Devices with Ultracel YM-100 membrane (100 000 nominal molecular weight limit, Millipore Corp., USA). The drug content was expressed in mg of curcumin/mL of suspension.
RESULTS AND DISCUSSION
The validation of analytical procedures is proof of the suitability of the method for the intended purpose. The current guidelines define the validation characteristics needed for various types of test procedures, but it is the responsibility of the analyst to identify the critical performance parameters and to design the validation study adequately (Ermer, 2001). Fluorimetric methods have shown to be sensitive, simple, rapid and reproducible for the quantitative determination of many compounds (Aktas et al., 2003; Abdellatef et al., 2006). Considering the biological interest of curcumin, several studies have previously reported the determination of this drug by fluorimetric methods (Wang et al., 2006; Wang, Huang, 2007; Wang et al., 2008). However, the steady-state absorption and fluorescence characteristics of curcumin have been found to be sensitive to the solvent's characteristics. Diaz and Peinado (1992) demonstrated that the relative fluorescence intensity of curcumin increases with increasing solvent dielectric constant. The highest value of relative fluorescence intensity was obtained using acetonitrile. Degradation of curcumin in acetonitrile solution was also reported after irradiation with light (400-700 nm), but the live-times were long enough to perform fluorimetric analysis of curcumin; fluorescence readings were constant for at least 1 h (Khopde et al., 2000). Besides the use of acetonitrile to provide higher values of fluorescence intensity, this solvent is able to dissolve curcumin and the lipid and polymeric constituents of nanocapsule suspensions, and for these reasons this solvent was chosen to carry out the analysis (Tonnesen, 2002).
A representative emission spectrum of curcumin obtained under the specified fluorescence conditions is shown in Figure 2A. This spectrum shows an intense emission band in the 450 - 600 nm wavelength region, exhibiting a fluorescence maximum at 508 nm. In order to prove the specificity of the method, unloaded lipid and polymeric nanocapsule suspensions containing the same concentration of the excipients were analyzed. The fluorescence spectra obtained from unloaded lipid and polymeric nanocapsule suspensions are shown in Figures 2B, 2C and 2D.The spectra showed that the method was specific because no interference from the excipients was detected at 508 nm under the fluorimetric conditions employed.
Fluorescence intensity
The calibration graph for curcumin in lipid and polymeric nanocapsules was linear over the range of 0.1 to 0.6 µg/mL with a correlation coefficient of 0.9982. The regression equation of the media calibration graph (n=3) was:
y = 1871x + 30.22
The slope and the intercept SDs were 50.98 and 18.53, respectively. The validity of the assay was verified by analysis of variance (ANOVA), which confirmed that the regression equation was linear (Fcalculated = 1557.48 > Fcritical = 3.58x10-5, P = 5%). The LOQ and LOD of the method calculated were 0.10 and 0.03 µg/mL, respectively, indicating that the method was sufficiently sensitive to be used for the drug entrapment evaluation.
The precision of the developed method is displayed in Table I. The results showed that the intraday and interday RSD values were lower than 5.0 % (Brasil, 2003). The fluorescence intensities indicated satisfactory intraday and interday variability.
The accuracy of the method was evaluated using three developed nanocapsule suspensions: lipid prepared using Solutol HS15, and polymeric prepared using Pluronic F 68 or Solutol HS 15 as hydrophilic surfactants. According to the ICH, several quantitative approaches can be used to demonstrate the accuracy of the method. Spiking experiments for recovery investigations should be performed mimicking authentic conditions as closely as possible so that putative interferences between analyte and matrix can be observed. This ranges from the direct preparation of a drug product with various contents of active ingredient to which the whole analytical procedure is applied, to the addition of a drug substance stock solution to a placebo formulation (Ermer, 2001). In our case, the latter procedure was used in this study. The recovery was determined as a percentage of the difference between the experiments at 3 different levels. Each level was tested 3 times. The results given in Table II demonstrate that the method exhibits acceptable accuracy.
The results obtained in the evaluation of curcumin loading for the two kinds of nanocapsules are summarized in Table III. The concentration of drug in the supernatant obtained by the suspension ultrafiltration/centrifugation procedure was lower than the LOQ of the method, indicating that only a very low concentration (< 0.10 µg/mL) was not encapsulated. Since the total concentration of curcumin ranged from 127.04 to 509.49 µg/mL, it is possible to state that for all formulations, the entrapment efficiency values were higher than 99%. These high values of entrapment efficiency obtained for curcumin could be related to the higher affinity of this hydrophobic drug for the oil core of nanocapsules. Moreover, the pH of the nanocapsule suspension was maintained below 6 to obtain the optimum stability of the preparations, preventing degradation of the drug. The curcumin was completely recovered in the colloidal suspensions, independent of the drug amount initially added to the formulations. The differences of drug content values in the nanocapsule suspensions are related only to the differences in the final volume of the preparations. The results demonstrated that the fluorimetric method can be successfully applied to determine curcumin in lipid and polymeric nanocapsule suspensions.
CONCLUSIONS
The fluorimetric method developed and validated proved to be specific, linear, accurate, precise and sensitive. It was successfully used to quantify curcumin in lipid and polymeric nanocapsule suspensions, and can be applied for the determination of entrapment efficiency and drug content, not only for formulation studies, but also as a pre-requisite to undertaking further chemical stability evaluation and preclinical studies of the nanocapsule suspensions.
Received for publication on 25th February 2009
Accepted for publication on 23rd August 2009
- ABDELLATEF, H. E.; EL-HENAWEE, M. M.; EL-SAYED, H. M.; AYAD, M. M. Spectrophotometric and spectrofluorimetric methods for analysis of acyclovir and acebutolol hydrochloride. Spectrochim. Acta A Mol. Biomol. Spectrosc., v.65, p.997-999, 2006.
- AGGARWAL, B.; KUMAR, A.; BHARTI, A. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res., v.23, p.363-398, 2003.
- AKTAS, E.S.; ERSOY, L.; SAGIRH, O. A new spectrofluorimetric method for the determination of lisinopril in tablets. Farmaco, v.58, p.165-168, 2003.
- ANTON, N.; BENOIT, J.; SAULNIER, P. Design and production of nanoparticles formulated from nano-emulsion templates - a review. J. Control. Release, v.128, p.185-199, 2008.
- BISHT, S.; FELDMANN, G.; SONI, S.; RAVI, R.; KARIKARI, C.; MAITRA, A.; MAITRA, A. Polymeric nanoparticle-encapsulated curcumin (nanocurcumin): a novel strategy for human cancer therapy. J. Nanobiotechnology, v. 5, n. 3, 2007. Available at: <http://www.jnanobiotechnology.com/content/5/1/3>. Accessed on: 27 feb. 2009.
- BRASIL. Agência Nacional de Vigilância Sanitária. Resolução RE nº 899, de 29 de maio de 2003 - Guia para validação de métodos analíticos e bioanalíticos. Available at: <http://www.anvisa.gov.br>. Accessed on: 15 fev. 2009.
- DÍAZ, A. N.; PEINADO, R. Fluorometric determination of curcumin in yogurt and mustard. J. Agric. Food Chem., v.40, p.56-59, 1992.
- DULIEU, C.; BAZILE, D. Influence of lipid nanocapsules composition on their aptness to freeze-drying. Pharm. Res., v.22, p.285-292, 2005.
- ERMER, J. Validation in pharmaceutical analysis. Part I: an integrated approach. J. Pharm. Biomed. Anal., v.24, p.755-767, 2001.
- FESSI, H.; PUISIEUX, F.; DEVISSAGUET, J. P.; AMMOURY, N.; BENITA, S. Nanocapsule formation by interfacial deposition following solvent displacement. Int. J. Pharm., v.55, R1-R4, 1989.
- FONSECA, C.; SIMÕES, S.; GASPAR, R. Paclitaxel-loaded PLGA nanoparticles: preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release, v.83, p.273-286, 2002.
- GREF, R.; DOMB, A.; QUELLEC, P.; BLUNK, T.; MÜLLER, R. H.; VERBAVATZ, J. M.; LANGER, R. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv., v.16, p.215-233, 1995.
- GUPTE, A.; CIFTCI, K. Formulation and characterization of paclitaxel, 5-FU and paclitaxel + 5-FU microspheres. Int. J. Pharm., v.276, p.93-106, 2004.
- HEATH, D.; PRUITT, M.; BRENNER, D.; ROCK, C. Curcumin in plasma and urine: quantitation by high-performance liquid chromatography. J. Chromatogr. B., v.783, p.287-295, 2003.
- HEURTAULT, B.; SAULNIER, P.; PECH, B.; PROUST, J.; BENOIT, J. A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharm. Res., v.19, p.875-880, 2002.
- HEURTAULT, B.; SAULNIER, P.; PECH, B.; VENIER-JULIENNE, M.; PROUST, J.; PHAN-TAN-LUU, R.; BENOIT, J. The influence of lipid nanocapsule composition on their size distribution. Eur. J. Pharm. Sci., v.18, p.55-61, 2003.
- INTERNATIONAL CONFERENCE ON HARMONIZATION. ICH. Validation of analytical procedures: text and methodology, Q2(R1), 2005. Available at: <http://www.ich.org>. Accessed: 27 feb. 2009.
- KHOPDE, S. M.; PRIYADARSINI, K. I.; PALIT, D. K.; MUKHERJEE, T. Effect of solvent on the excited-state photophysical properties of curcumin. Photochem. Photobiol., v.72, p.625-631, 2000.
- MAHESHWARI, R.; SINGH, A.; GADDIPATI, J.; SRIMAL, R. Multiple biological activities of curcumin: a short review. Life Sci., v.78, p.2081-2087, 2006.
- MEHNERT, W.; MÄDER, K. Solid lipid nanoparticles. Production, characterization and applications. Adv. Drug Deliv., v.47, p.165-196, 2001.
- NAM, S.; NAM, H.; JOO, J.; BAEK, I.; PARK, J. Curcumin-loaded PLGA nanoparticles coating onto metal stent by electrophoretic deposition techniques. Bull. Korean Chem. Soc., v.28, p.397-402, 2007.
- SHARMA, R.; MCLELLAND, H.; HILL, K.; IRESON, C.; EUDEN, S.; MANSON, M.; PIRMOHAMED, M.; MARNETT, L.; GESCHER, A.; STEWARD, W. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin. Cancer Res., v.7, p.1894-1900, 2001.
- SOU, K.; INENAGA, S.; TAKEOKA, S.; TSUCHIDA, E. Loading of curcumin into macrophages using lipid-based nanoparticles. Int. J. Pharm., v.352, p.287-293, 2008.
- SYNG-AI, C.; KUMARI, A.; KHAR, A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol. Cancer Ther., v.9, p.1101-1108, 2004.
- TIYABOONCHAI, W.; TUNGPRADIT, W.; PLIANBANGCHANG, P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm., v.337, p.299-306, 2007.
- TOMREN, M.; MÁSSON, M.; LOFTSSON, T.; TONNESEN, H. Studies on curcumin and curcuminoids XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrin. Int. J. Pharm., v.338, p.27-34, 2007.
- TONNESEN, H. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Pharmazie, v.57, p.820-824, 2002.
- TONNESEN, H.; MÁSSON, M.; LOFTSSON, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int. J. Pharm., v.244, p.127-135, 2002.
- UNITED STATES PHARMACOPEIA. 30.ed. Validation of compendial procedures. Rockville: United States Pharmacopeial Convention, 2007. chap.1225, p.680-683.
- WANG, F.; HUANG, W. Determination of curcumin by its quenching effect on the fluorescence of Eu3+-tryptophan complex. J. Pharm. Biomed. Anal., v.43, p.393-398, 2007.
- WANG, F.; HUANG, W.; WANG, Y. Fluorescence enhancement effect for the determination of curcumin with yttrium (III)-curcumin-sodium dodecyl benzene sulfonate system. Luminescence, v.128, p.110-116, 2008.
- WANG, F.; WU, X.; WANG, F.; LIU, S.; JIA, Z.; YANG, J. The sensitive fluorimetric method for the determination of curcumin using the enhancement of mixed micelle. J. Fluoresc., v.16, p.53-59, 2006.
- WANG, Y.; PAN, M.; CHENG, A.; LIN, L.; HO, Y., HSIEH, C.; LIN, J. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal., v.15, p.1867-1876, 1997.
- WITSCHI, C.; DOELKER, E. Residual solvents in pharmaceutical products: acceptable limits, influences on physicochemical properties, analytical methods and documented values. Eur. J. Pharm. Biopharm., v.43, p.215-242, 1997.
Publication Dates
-
Publication in this collection
17 Aug 2010 -
Date of issue
June 2010
History
-
Accepted
23 Aug 2009 -
Received
25 Feb 2009