ABSTRACT
The present study evaluated the antibacterial and antibiofilm activity of carvacrol against Salmonella Typhimurium. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined and the time-kill curve and scanning electron microscopy (SEM) were performed to evaluate antibacterial activity. Antibiofilm activity was evaluated by quantifying total biomass using crystal violet assay, and metabolic activity was determined using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. The action of carvacrol against preformed biofilm on polypropylene and stainless steel was also evaluated by colony counting and SEM. The MIC and MBC was 312 µg mL-1. Carvacrol at MIC and 2 x MIC eliminated cells after 6 and 1 h of treatment, respectively, as exhibited in the time-kill curve. The greatest reduction in biofilm biomass and metabolic activity was 1,719 OD550 and 0,089 OD550 respectively, both at 4 x MIC of carvacrol. In carvacrol treated biofilms of S. Typhimurium on polypropylene, a reduction of 5.12 log was observed with 4 x MIC, while on stainless steel, carvacrol at 4 x MIC reduced bacterial counts by 5 log. The results showed that carvacrol exhibits antibacterial activity and can be used as an alternative for the control of S. Typhimurium biofilms.
Keywords
:
Carvacrol; Biofilm; Antibacterial activity; Salmonella Typhimurium.
INTRODUCTION
Food contamination by pathogenic bacteria is a public health problem and an important cause of mortality worldwide (WHO, 2015). It is estimated that 48 million cases of foodborne diseases, 128,000 hospitalizations, and 3,000 deaths occur in the USA each year (CDC, 2015). Of these 48 million, Salmonella spp. is responsible for over 1 million, causing more than 19,000 hospitalizations and around 380 deaths (Scallan et al., 2011Scallan E, Hoekstra RM, Angul, FJ, Tauxe RV, Widdowson MA, Roy S L, et al. Foodborne Iliness acquired in the United States - major pathogens. Emerg Infect Dis. 2011;17(1):7-15.. According to the Sistema de Informação de Agravos e Notificações (Disease Information and Notification System) (SINAN) (Brasil, 2016Brasil. Sistema de Informação de Agravos de Notificação (SINAN). Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, Coordenação Geral de Vigilância das Doenças Transmissíveis, 2016. [cited 2November 07th, 2017]. Available in: <http://portalarquivos.saude.gov.br/images/pdf/2016/junho/08/Apresenta----o-Surtos-DTA-2016.pdf>), Salmonella spp. was the main agent involved in foodborne outbreaks in Brazil from 2000 to 2015. All Salmonella serotypes can cause disease in humans, and the most common serotypes implicated in the majority of outbreaks are Typhimurium and Enteritidis in most parts of the world (WHO, 2016).
An important characteristic of Salmonella spp. is its ability to form biofilms on different surfaces present in food processing environments, such as glass, stainless steel, wood, and plastic (Steenackers et al., 2012Steenackers H, Hermans K, Vanderleyden J, De Keersmaecker SCJ. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res Int. 2012;45(2):502-531.). Polypropylene and stainless steel are commonly used in the food processing industries, and some studies have shown the formation of Salmonella Typhimurium biofilm on these surfaces (Bayoumi et al., 2012Bayoumi MA, Kamal RM, Abd El Aal SF, Awad EI. Assessment of a regulatory sanitization process in Egyptian dairy plants in regard to the adherence of some food-borne pathogens and their biofilms. Int J Food Microbiol. 2012;158(3):225-231.; Soni et al., 2013Soni KA, Oladunjoye A, Nannapaneni R, Schilling MW, Silva JL, Mikel B, et al. Inhibition and inactivation of Salmonella Typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J Food Protect. 2013;76(2):205-212.; Amaral et al., 2015Amaral VCS, Santos PR, Silva AF, Santos AR, Machinski Jr M, Mikcha JMG. Effect of carvacrol and thymol on Salmonella spp. biofilms on polypropylene. Int J Food Sci Tech. 2015;50(12):2639-2643.).
In the food industry, biofilms can be source of cross-contamination, as food can come into contact with contaminated surfaces (Cappitelli, Polo, Villa, 2014Cappitelli F, Polo A, Villa F. Biofilm formation in food processing environments is still poorly understood and controlled. Food Eng Rev. 2014;6(1-2):29-42.). Another problem is that the biofilms are more resistant than bacteria in planktonic form (Simões, Simões, Vieira, 2010Simões M, Simões LC, Vieira MJ. A review of current and emergent biofilm control strategies. LWT Food Sci Tech. 2010;43(4):573-583.; Nguyen, Yuk, 2013Nguyen HDN, Yuk HG. Changes in resistance of Salmonella Typhimurium biofilms formed under various conditions to industrial sanitizers. Food Control 2013;29(1):236-240.). In this context, developing new strategies for the control of biofilms is important (Shi, Zhu, 2009Shi X, Zhu X. Biofilm formation and food safety in food industries. Trends in Food Sci Tech. 2009;20(9):407-413.).
In an attempt to find effective alternatives to control bacteria, studies have revealed the antibacterial (Burt, 2004Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol. 2004;94(3):223-253.; Luz et al., 2012Luz IS, Gomes Neto NJ, Tavares AG, Nunes PC, Magnani M, Souza EL. Evidence for lack of acquisition of tolerance in Salmonella enterica serovar Typhimurium ATCC 14028 after exposure to subinhibitory amounts of Origanum vulgare L. essential oil and carvacrol. Appl Environ Microbiol. 2012;78(14):5021-5024.; Chauhan, Kang, 2014Chauhan AK, Kang S. Thymol disrupts the membrane integrity of Salmonella ser. Typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res Microbiol. 2014;165(7):559-565.; Nair et al., 2014Nair DVT, Nannapaneni R, Kiess A, Schilling W, Sharma CS. Reduction of Salmonella on Turkey breast cutlets by plant-derived compounds. Foodborne Pathog Dis. 2014;11(12):981-987.) and antibiofilm activity of essential oils and their components (Bersan et al., 2014Bersan SMF, Galvão LCC, Goes VFF, Sartoratto A, Figueira GM, Rehder VLG, et al. Action of essential oils from Brazilian native and exotic medicinal species on oral biofilms. BMC Complement Altern Med. 2014;14:451.; Piovezan et al., 2014Piovezan M, Uchida NS, Silva AF, Grespan R, Santos PS, Silva EL, et al. Effect of cinnamon essential oil and cinnamaldehyde on Salmonella Saintpaul biofilm on a stainless steel surface. J Gen Appl Microbiol. 2014;60(3):119-121.). Carvacrol is an important component of the essential oil of Origanum vulgari, and is present in the essential oils such as Thymus vulgaris (Lambert et al., 2001Lambert RJW, Skandamis PN, Coote PJ, Nychas GJE. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol. 2001;91(3):453-462.; Burt, 2004) and Satureja bachtiarica (Falsafi et al., 2015Falsafi T, Moradi P, Mahboubi M, Rahimi E, Momtaz H, Hamedi B. Chemical composition and anti-Helicobacter pylori effect of Satureja bachtiarica Bunge essential oil. Phytomedicine 2015;22(1):173-177.). It can be used as an antifungal (Ahmad et al., 2011Ahmad A, Khan A, Akhtar F, Yousuf S, Xess I, Khan LA, et al. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur J Clin Microbiol Infect Dis. 2011;30(1):41-50.), anti-inflammatory (Lima et al., 2013Lima MS, Quintans-Junior LJ, De Santana WA, Kaneto CM, Soares MBP, Villarreal CF. Anti-inflammatory effects of carvacrol: evidence for a key role of interleukin-10. Eur J Pharmacol. 2013;699(1-3):112-117.) agent, among other uses, and has a broad antimicrobial spectrum (Burt, 2004). In addition, studies have evaluated its antibiofilm activity on various surfaces common to the food industry (Soni et al., 2013Soni KA, Oladunjoye A, Nannapaneni R, Schilling MW, Silva JL, Mikel B, et al. Inhibition and inactivation of Salmonella Typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J Food Protect. 2013;76(2):205-212.; Neyret et al., 2014Neyret C, Herry JM, Meylheuc T, Dubois-Brissonnet F. Plant derived compounds as natural antimicrobials to control paper mill biofilms. J Ind Microbiol Biotechnol. 2014;41(1):87-96.; Amaral et al., 2015Amaral VCS, Santos PR, Silva AF, Santos AR, Machinski Jr M, Mikcha JMG. Effect of carvacrol and thymol on Salmonella spp. biofilms on polypropylene. Int J Food Sci Tech. 2015;50(12):2639-2643.). The aim of this study was therefore to evaluate the antibacterial and antibiofilm action of carvacrol against the Salmonella enterica serotype Typhimurium.
MATERIAL AND METHODS
Bacterial strain and culture conditions
The bacterial strain used was Salmonella enterica serotype Typhimurium (ATCC 14028). The culture was maintained in Trypic Soy Broth (TSB, Difco, Le Pont de Claix, France) supplemented with 20% glycerol at -20 °C. Before use, an aliquot was transferred to Brain Heart Infusion broth (BHI, Difco, Le Pont de Claix, France) and incubated for 24 h at 35 °C. The culture was transferred to Hektoen Agar (Difco, Le Pont de Claix, France) and incubated under the same conditions.
Minimum inhibitory concentration and minimum bactericidal concentration
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of carvacrol (purity ≥ 98%; Sigma-Aldrich) were determined according to the Clinical and Laboratory Standards Institute (CLSI, 2012), using the broth microdilution method in 96-well microtiter plates (TPP, Trasadingen, Switzerland). Carvacrol was diluted in Mueller-Hinton Broth (MHB; Difco, Le Pont de Claix, France) and 0.5 % ethanol (v/v) and 100 µL was added to each well at concentrations ranging from 19 to 5000 µg mL-1. MIC was visually determined as the lowest concentration at which bacterial growth was not observed. Bacterial growth control consisting of MHB and 0.5 % ethanol (v/v) and a control that consisted of carvacrol in MHB were included. After MIC determination, 10 µL was removed from wells in which bacterial growth was not observed and inoculated into agar Hektoen plates which was incubated for 24 h at 35 °C. The MBC was determined as the lowest concentration, which no bacterial growth was observed on agar plates. The experiment was performed in duplicate and the results were obtained from three experiments.
Time-Kill curve assay
The test was performed according to Isenberg (2004Isenberg HD. Clinical Microbiology Procedures Handbook Washington: ASM Press; 2004; p. 5.10.2.) with some modifications. Overnight culture of S. Typhimurium ATCC 14028, was standardized and transferred to MHB, supplemented with carvacrol at concentrations of 0.5 x MIC, MIC and 2 x MIC, obtaining a final inoculum of 6 x 105 CFU mL-1. A quantity of 100 µL was removed at intervals of 0, 1, 2, 3, 4, 5, 6, 12 and 24 h, serially diluted and plated on Mueller Hinton Agar (MHA, Difco Pont-de-Claix, France). Plates were incubated at 35 °C for 24 h and the CFU counted. The tests were performed in duplicate and repeated three times.
Effect of carvacrol in biofilm total biomass
The assay was performed in a 96-well microplate, according to Djordjevic, Wiedmann and McLandsborough (2002Djordjevic D, Wiedmann M, McLandsborough LA. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol. 2002;68(6):2950-2958.), with modifications. The bacterial strain was grown in TSB at 35 °C overnight, the culture was diluted to obtain 107 CFU mL-1, and 100 µL of bacterial suspension was added to the microplates, which were incubated at 35 °C for 48 h. After incubation, the biofilm was treated with 100 µL of carvacrol at MIC, 2 x MIC and 4 x MIC, or 100 µL of TSB (control), for one hour. Microplates were washed with 0.85% sterile saline, fixed with methanol PA for 15 min. and stained with 1% crystal violet for 20 min. The microplates were washed with sterile distilled water, dried, and bound dye adhered to the bacterial cells. They were then solubilized with 200 µL 95% ethanol and a reading was performed with a microplate reader (ASYS, EXPERT PLUS Model UV) at 550 nm. The tests were performed in duplicate and repeated three times.
Effect of carvacrol in biofilm metabolic activity.
Determination of biofilm metabolic activity was performed using a MTT [3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] staining assay, according to Jia et al. (2011Jia P, Xue YJ, Duan XJ, Shao SH. Effect of cinnamaldehyde on biofilm formation and sarA expression by methicillin-resistant Staphylococcus aureus. Lett Appl Microbiol. 2011;53(4):409-416.), with some modifications. The overnight culture of S. Typhimurium was diluted in TSB to obtain 107 CFU mL-1 and added to the microplates. After 48 h incubation at 35 ºC, the wells were rinsed, carvacrol at MIC, 2 x MIC and 4 x MIC was added, and the plates were incubated for 1 h at 35 ºC. A quantity of 100 μL of 0.05% MTT (Sigma - Aldrich Corp., St. Louis, Missouri, USA) was then added to the wells. After incubation for 1 h at 35 ºC, the MTT solution was replaced by isopropanol acid and mixed for 15 min, and the absorbance was measured at 550 nm. The tests were performed in duplicate and repeated three times.
Effect antibiofilm of carvacrol on polypropylene and stainless steel
Biofilm formation was initially performed according to Uchida et al. (2014Uchida NS, Grespan R, Piovezan M, Ferreira EC, Machinski Jr M, Cuman RKN, et al. Effect of carvacrol on Salmonella Saintpaul biofilms on stainless steel surface. Trop J Pharm Res. 2014;13(12):2021-2025.). Polypropylene and stainless steel coupons (1 x 8 x 8 mm) were sanitized according to Bayoumi et al. (2012Bayoumi MA, Kamal RM, Abd El Aal SF, Awad EI. Assessment of a regulatory sanitization process in Egyptian dairy plants in regard to the adherence of some food-borne pathogens and their biofilms. Int J Food Microbiol. 2012;158(3):225-231.) and Uchida et al. (2014), respectively. The overnight culture of S. Typhimurium ATCC 14028 was diluted in TSB to yield 107 CFU mL-1 and placed in microtubes containing polypropylene or stainless-steel coupons, which were incubated for 48 h at 35 ºC. After incubation, coupons were washed with 0.85% sterile saline solution and subjected to ultrasound at 25 Hz for 5 min. (Ultra Cleaner 750 A Unique). Serial dilutions were performed in 0.85% sterile saline solution, plated on MHA, and incubated at 35 °C for 24 h. The results were expressed as log10 CFU cm-2. The experiment was performed in triplicate and repeated three times.
Results obtained from the MIC tests were used to evaluate the effects of carvacrol in biofilm. The following concentrations were used: 0.5 x MIC, MIC, 2 x MIC and 4 x MIC respectively. After biofilm formation on polypropylene and stainless steel for 48 h, the coupons were washed with 0.85% sterile saline solution and exposed to different concentrations of carvacrol. After 1h, the coupons were washed again with 0.85% sterile saline solution, subjected to ultrasound, and the bacterial cells were quantified on MHA. The results were expressed as log10 CFU cm-2. The experiment was performed in triplicate and repeated three times.
Scanning Electron Microscopy (SEM)
The treated planktonic and biofilm cells and untreated control cells were analyzed by scanning electron microscopy (SEM) according to Wong et al. (2010Wong HS, Townsend KM, Fenwick SG, Trengove RD, O'Handley RM. Comparative susceptibility of planktonic and 3-day-old Salmonella Typhimurium biofilms to disinfectants. J Appl Microbiol. 2010;108(6):2222-2228.), with some modifications.
The preparation of the planktonic cells was carried out according to Liu et al. (2015aLiu H, Pei H, Han Z, Feng G, Li D. The antimicrobial effects and synergistic antibacterial mechanism of the combination of e-Polylysine and nisin against Bacillus subtilis. Food Control. 2015a;47:444-450.), with some modifications. The bacterial culture in logarithmic phase in TSB was divided in two portions of 30 mL, one of which was used as control (untreated) and the other of which was treated with carvacrol at 2 x MIC for 3 h. Samples were centrifuged at 4500 x g for 5 min and washed with 0.85% sterile saline solution. They were subsequently fixed in glutaraldehyde 2.5% (Sigma-Aldrich, St. Louis, MO, USA) in 0.1 M cacodylate buffer (SEM, Hatfield, PA, USA) for 2 h at 4 °C. The samples were then centrifuged, washed three times in cacodylate buffer and fixed on glass coverslips containing poly-L-lysine for 1 hour. After fixation, the samples were washed three times in cacodylate buffer and dehydrated in a graded ethanol series (50%, 70%, 80%, 90% and 100%). They were critical point dried in CO2 and covered with gold and the images were obtained using a scanning electron microscope (Shimadzu SS 550).
The pre-formed biofilm on polypropylene and stainless-steel coupons untreated and treated at 2 x MIC of carvacrol for 1 h were fixed in glutaraldehyde 2.5% in 0.1 M cacodylate and maintained for 48 h at 4 ºC. The coupons were then washed three times with cacodylate buffer, dehydrated in a series of graded ethanol, critical point dried in CO2, covered with gold and examined by scanning electron microscope (Shimadzu SS 550).
Statistical analysis
The results were analyzed using the GraphPad Prism 5.0 Software. The groups that showed significant differences were analyzed using the Kruskal-Wallis test to compare three or more groups, followed by the Dunn tests to determine differences between groups. The test results with crystal violet and metabolic activity were assessed by ANOVA. Values of p < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Antimicrobial activity of carvacrol against Salmonella Typhimurium
The antimicrobial activity of carvacrol against S. Typhimurium ATCC 14028 was evaluated in vitro and MIC and MBC values were 312 µg mL-1. Similar results were obtained by other studies using carvacrol against S. Typhimurium and other serotypes (Soni et al., 2013Soni KA, Oladunjoye A, Nannapaneni R, Schilling MW, Silva JL, Mikel B, et al. Inhibition and inactivation of Salmonella Typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J Food Protect. 2013;76(2):205-212.; Nair et al., 2014Nair DVT, Nannapaneni R, Kiess A, Schilling W, Sharma CS. Reduction of Salmonella on Turkey breast cutlets by plant-derived compounds. Foodborne Pathog Dis. 2014;11(12):981-987.). MIC and MBC values ranging from 64 to 512 µg mL-1 were obtained by Miladi et al. (2016Miladi H, Zmantar T, Chaabouni Y, Fedhila K, Bakhrouf A, Mahdouani K, et al. Antibacterial and efflux pump inhibitors of thymol and carvacrol against food-borne pathogens. Microb Pathog. 2016;99:95-100.) in S. Typhimurium and S. Enteritidis. Du et al. (2015Du E, Gan L, Li Z, Wang W, Liu D, Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J Anim Sci Biotechnol. 2015;6:58.) also evaluated the effect of carvacrol against Salmonella spp. and identified MICs of 187.5 and 375 µg mL-1 and an MBC value of 750 µg mL-1.
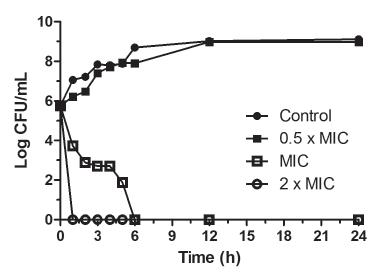
In Time-kill curve assay (Figure 1) the control reached a bacterial population of approximately 9 log10 CFU mL-1 after 24 h of incubation at 35 ºC. It was also observed that the bacterial reduction promoted by the treatment was dependent on the carvacrol concentration and the exposure time. However, treatment with 0.5 x MIC of carvacrol did not reduce the bacterial population at any of the different times, when compared to the control. A gradual reduction of the bacterial counts at times between 0 and 6 h was observed with treatment at MIC and no viable cells were detected after 6 h. No viable cells were observed in the treatment with 2 x MIC after 1 h of treatment.
Time-kill curve assay of carvacrol at 0.5 x MIC (156 µg mL-1), MIC (312 µg mL-1) and 2 x MIC (624 µg mL-1), against planktonic cells of Salmonella Typhimurium ATCC 14028.
It is important to note that the bactericidal action of carvacrol was verified at 312 and 624 µg mL-1, eliminating bacterial cells after only 6 h and 1 h of treatment, respectively. Oladunjoye et al. (2013Oladunjoye A, Soni KA, Nannapaneni R, Schilling MW, Silva JL, Mikel B, et al. Synergistic activity between lauric arginate and carvacrol in reducing Salmonella in ground turkey. Poultry Sci. 2013;92(5):1357-1365.) also evaluated different concentrations of carvacrol against mixed S. Typhimurium and eradicated cells with 0.05% (approximately 500 µg mL-1) of carvacrol at 22 °C after 30 min of exposure.
Planktonic untreated cells of S. Typhimurium and those treated with carvacrol were observed by SEM (Figure 2 A and B). The treated cells (Figure 2 B) had a shriveled and retracted appearance, compared with the untreated cells, consistent with Di Pasqua et al. (2007Di Pasqua R, Betts G, Hoskins N, Edwards M, Ercolini D, Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J Agric Food Chem. 2007:55(12):4863-4870.) that found alterations in structure and membrane of S. Typhimurium cells after treatment with carvacrol. These authors also showed a decrease in the percentage of unsaturated fatty acids for the treated cells, and suggested that this results would support a mechanism of action proposed to carvacrol against the outer cell envelope, probably interacting with the membrane lipids and causing structural alterations visible by SEM. Other studies suggest that essential oils with antimicrobial activity interact with the plasma membrane of the bacteria, which can result in damage to the integrity causing a leakage of intracellular material (Ultee, Kets, Smid, 1999Ultee A, Kets EPW, Smid EJ. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 1999;65(10):4606-4610.; Burt, 2004Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol. 2004;94(3):223-253.).
Scanning electron microscopy of Salmonella Typhimurium ATCC 14028. A) Planktonic cells without treatment (6000x). B) Planktonic cells treated with 2 x MIC (624 µg mL-1) of carvacrol for 3 h (6000x). C) Biofilm on polypropylene without treatment (3000x). D) Biofilm on polypropylene treated with 2 x MIC of carvacrol for 1 h (3000x). E) Biofilm on stainless steel without treatment (3000x). F) Biofilm on stainless steel treated with 2 x MIC of carvacrol for 1 h (3000x).
Effect of carvacrol in biofilm
Crystal violet assay is widely used to quantify total biofilm biomass and also to evaluate the effect of different substances such as antibiofilm (Zhang et al., 2014Zhang J, Rui X, Wang L, Guan Y, Sun X, Dong M. Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food Control 2014;42:125-131.). This study evaluated different concentrations of carvacrol, namely MIC, 2 x MIC, 4 x MIC and control (without carvacrol), and found a total biomass reduction of the treated biofilm cells compared to the control (Figure 3). Biofilm biomass was reduced to 1.221 OD550, 0.738 OD550 and 0.562 OD550 after treatment with carvacrol at MIC, 2 x MIC, and 4 x MIC, respectively, as compared with 2.281 OD550 for the control (p < 0,05).
Effects of carvacrol at MIC (312 µg mL-1), 2 x MIC (624 µg mL-1), 4 x MIC (1250 µg mL-1) on total biofilm biomass of Salmonella Typhimurium ATCC 14028. * p < 0,05, compared with control.
Soni et al. (2013Soni KA, Oladunjoye A, Nannapaneni R, Schilling MW, Silva JL, Mikel B, et al. Inhibition and inactivation of Salmonella Typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J Food Protect. 2013;76(2):205-212.) showed that the treatment of preformed biofilm of S. Typhimurium with carvacrol at 0.025%, 0.05% and 0.1 % produced a significant reduction in biofilm mass. In contrast, Burt et al. (2014Burt SA, Ojo-Fakunle VTA, Woertman J, Veldhuizen EJA. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. Plos One 2014;9(4):e93414.) observed a small, but not significant reduction in the biofilm biomass of S. Typhimurium with a treatment of carvacrol at 0.8 mM (approximately 120 µg mL-1) for 24 h.
MTT assay showed that carvacrol at MIC, 2 x MIC and 4 x MIC decreased S. Typhimurium biofilm activity to 0.089 OD550, 0.076 OD550 and 0.053 OD550 respectively, as compared with 0.142 OD550 for the control (p < 0,05) (Figure 4). Previous studies have demonstrated the effect of different essential oils and their components on the reduction metabolic activity of Gram-positive and Gram-negative bacterial biofilms (Bai, Vittal, 2014Bai AJ, Vittal RR. Quorum sensing inhibitory and antibiofilm activity of essential oils and their in vivo efficacy in food systems. Food Biotechnol. 2014;28(3):269-292.; Piovezan et al., 2014Piovezan M, Uchida NS, Silva AF, Grespan R, Santos PS, Silva EL, et al. Effect of cinnamon essential oil and cinnamaldehyde on Salmonella Saintpaul biofilm on a stainless steel surface. J Gen Appl Microbiol. 2014;60(3):119-121.; Liu et al., 2015bLiu Q, Niu H, Zhang W, Mu H, Sun C, Duan J. Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett Appl Microbiol. 2015b;60(5):421-430.) but to our knowledge, no studies have evaluated the effect of carvacrol on the metabolic activity on Gram-negative bacteria.
Effects of carvacrol at MIC (312 µg mL-1), 2 x MIC 624 (µg mL-1), 4 x MIC (1250 µg mL-1) on the metabolic activity of biofilm of Salmonella Typhimurium ATCC 14028. * p < 0,05, compared with control.
Thus, the results of the present study revealed that the addition of carvacrol at different concentrations to the preformed biofilm not only reduced biomass (as indicated by crystal violet assay) but also was able to reduce metabolic activity.
Effects of carvacrol on Salmonella Typhimurium biofilm on polypropylene and stainless steel
After 48 h of contact with the polypropylene surface, the number of viable cells of S. Typhimurium recovered from coupons was 8.27 log CFU cm-2. The reduction obtained after treatment for 1h with 2 x MIC and 4 x MIC of carvacrol, was 4.25 (51.39%) and 5.12 log CFU cm-2 (61.91%) (p <0.05), respectively (Table I). On stainless steel the number of viable cells of S. Typhimurium was 8.25 log CFU cm-2. The reduction obtained with treatment at 2 x MIC and 4 x MIC of carvacrol was 3.82 (46.3%), and 5 log CFU cm-2 (60.6%) (p <0.05) respectively (Table I).
Previous studies by our group evaluated the effect of carvacrol on Salmonella spp. biofilms during and after its formation on stainless steel and polypropylene. In S. Saintpaul established biofilm on stainless steel, the greatest reduction (2.85 log CFU cm-2) was observed with 117 µg mL-1 of carvacrol (Uchida et al., 2014Uchida NS, Grespan R, Piovezan M, Ferreira EC, Machinski Jr M, Cuman RKN, et al. Effect of carvacrol on Salmonella Saintpaul biofilms on stainless steel surface. Trop J Pharm Res. 2014;13(12):2021-2025.). Salmonella spp. biofilms on polypropylene exhibited reductions ranging from 0.87 to 4.72 log CFU cm-2 (Amaral et al., 2015Amaral VCS, Santos PR, Silva AF, Santos AR, Machinski Jr M, Mikcha JMG. Effect of carvacrol and thymol on Salmonella spp. biofilms on polypropylene. Int J Food Sci Tech. 2015;50(12):2639-2643.).
Soni et al. (2013Soni KA, Oladunjoye A, Nannapaneni R, Schilling MW, Silva JL, Mikel B, et al. Inhibition and inactivation of Salmonella Typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J Food Protect. 2013;76(2):205-212.), when evaluating the effect of carvacrol on S. Typhimurium biofilm on stainless steel, verified a reduction of 7 log CFU after treatment with 500 and 1000 µg mL-1 of carvacrol for 1 h. In the present study, treatment with carvacrol at 4 x MIC (1250 µg mL-1) for 1 h produced a reduction of 5 log CFU cm-2 on biofilm on stainless steel. When lower concentrations (250 and 120 µg mL-1) were used for 1 h in the study by Soni et al. (2013) a reduction in biofilm cell counts was not observed, while our results showed a decrease of approximately 3 log CFU cm-2 after treatment with carvacrol at MIC (312 µg mL-1) for 1 h (Table I).
The effect of carvacrol on the biofilm of S. Typhimurium on polypropylene and stainless steel was also evaluated by scanning electron microscopy (Figure 2 C and E). In both surfaces, biofilm without treatment showed the presence of microcolonies, which according to Oliveira, Brugnera, Piccoli (2010Oliveira MMM, Brugnera DF, Piccoli RH. Biofilmes microbianos na indústria de alimentos: uma revisão. Rev Inst Adolfo Lutz. 2010;69(3):277-84.) are bacterial biofilm characteristics. When biofilms were treated (Figure 2 D and F) the microcolonies were not observed, although diffuse cells could be seen. Bridier et al. (2015Bridier A, Sanchesz-Vizuete P, Guilbaud M, Piard C, Naitali M, Briandet R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015;45(Pt B):167-178.) suggests that as the biofilm was disorganized, the cells are more exposed, facilitating the action of other sanitizers and contributing to the eradication of bacteria.
The present study revealed that carvacrol exhibits effective antibacterial activity against S. Typhimurium, as well antibiofilm activity, observed by the reduction in biomass and bacterial counts and by SEM. The compound was not, however, able to eradicate S. Typhimurium in biofilm cells.
CONCLUSION
Carvacrol exhibited antibacterial and antibiofilm action against S. Typhimurium. Thus, we can suggest that carvacrol may be an alternative to conventional sanitizing for the control of bacteria in food processing environments. However, additional tests are needed to evaluate the mechanisms by which the compound acts on biofilm.
ACKNOWLEDGMENTS
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Coordination for the Improvement of Higher Education Personnel) (CAPES) and the Complexo de Central de Apoio a Pesquisa (COMCAP) of the State University of Maringa.
REFERENCES
- Ahmad A, Khan A, Akhtar F, Yousuf S, Xess I, Khan LA, et al. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur J Clin Microbiol Infect Dis. 2011;30(1):41-50.
- Amaral VCS, Santos PR, Silva AF, Santos AR, Machinski Jr M, Mikcha JMG. Effect of carvacrol and thymol on Salmonella spp. biofilms on polypropylene. Int J Food Sci Tech. 2015;50(12):2639-2643.
- Bai AJ, Vittal RR. Quorum sensing inhibitory and antibiofilm activity of essential oils and their in vivo efficacy in food systems. Food Biotechnol. 2014;28(3):269-292.
- Bayoumi MA, Kamal RM, Abd El Aal SF, Awad EI. Assessment of a regulatory sanitization process in Egyptian dairy plants in regard to the adherence of some food-borne pathogens and their biofilms. Int J Food Microbiol. 2012;158(3):225-231.
- Bersan SMF, Galvão LCC, Goes VFF, Sartoratto A, Figueira GM, Rehder VLG, et al. Action of essential oils from Brazilian native and exotic medicinal species on oral biofilms. BMC Complement Altern Med. 2014;14:451.
- Brasil. Sistema de Informação de Agravos de Notificação (SINAN). Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, Coordenação Geral de Vigilância das Doenças Transmissíveis, 2016. [cited 2November 07th, 2017]. Available in: <http://portalarquivos.saude.gov.br/images/pdf/2016/junho/08/Apresenta----o-Surtos-DTA-2016.pdf>
- Bridier A, Sanchesz-Vizuete P, Guilbaud M, Piard C, Naitali M, Briandet R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015;45(Pt B):167-178.
- Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol. 2004;94(3):223-253.
- Burt SA, Ojo-Fakunle VTA, Woertman J, Veldhuizen EJA. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. Plos One 2014;9(4):e93414.
- Cappitelli F, Polo A, Villa F. Biofilm formation in food processing environments is still poorly understood and controlled. Food Eng Rev. 2014;6(1-2):29-42.
- Center for Disease Control and Prevention - CDC. Trends in Foodborne Illness in the United States [internet] 2015. [cited November 07th, 2017]. Available in: <http://www.cdc.gov/foodborneburden/trends-in- foodborne-illness.html>.
» http://www.cdc.gov/foodborneburden/trends-in- foodborne-illness.html - Chauhan AK, Kang S. Thymol disrupts the membrane integrity of Salmonella ser. Typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res Microbiol. 2014;165(7):559-565.
- Clinical Laboratory Standards Institute. CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, 9th ed. NCCLS document M7-A9. Wayne, PA: Clinical and Laboratory Standards Institute; 2012.
- Di Pasqua R, Betts G, Hoskins N, Edwards M, Ercolini D, Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J Agric Food Chem. 2007:55(12):4863-4870.
- Djordjevic D, Wiedmann M, McLandsborough LA. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol. 2002;68(6):2950-2958.
- Du E, Gan L, Li Z, Wang W, Liu D, Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J Anim Sci Biotechnol. 2015;6:58.
- Falsafi T, Moradi P, Mahboubi M, Rahimi E, Momtaz H, Hamedi B. Chemical composition and anti-Helicobacter pylori effect of Satureja bachtiarica Bunge essential oil. Phytomedicine 2015;22(1):173-177.
- Isenberg HD. Clinical Microbiology Procedures Handbook Washington: ASM Press; 2004; p. 5.10.2.
- Jia P, Xue YJ, Duan XJ, Shao SH. Effect of cinnamaldehyde on biofilm formation and sarA expression by methicillin-resistant Staphylococcus aureus. Lett Appl Microbiol. 2011;53(4):409-416.
- Lambert RJW, Skandamis PN, Coote PJ, Nychas GJE. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol. 2001;91(3):453-462.
- Lima MS, Quintans-Junior LJ, De Santana WA, Kaneto CM, Soares MBP, Villarreal CF. Anti-inflammatory effects of carvacrol: evidence for a key role of interleukin-10. Eur J Pharmacol. 2013;699(1-3):112-117.
- Liu H, Pei H, Han Z, Feng G, Li D. The antimicrobial effects and synergistic antibacterial mechanism of the combination of e-Polylysine and nisin against Bacillus subtilis. Food Control. 2015a;47:444-450.
- Liu Q, Niu H, Zhang W, Mu H, Sun C, Duan J. Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett Appl Microbiol. 2015b;60(5):421-430.
- Luz IS, Gomes Neto NJ, Tavares AG, Nunes PC, Magnani M, Souza EL. Evidence for lack of acquisition of tolerance in Salmonella enterica serovar Typhimurium ATCC 14028 after exposure to subinhibitory amounts of Origanum vulgare L. essential oil and carvacrol. Appl Environ Microbiol. 2012;78(14):5021-5024.
- Miladi H, Zmantar T, Chaabouni Y, Fedhila K, Bakhrouf A, Mahdouani K, et al. Antibacterial and efflux pump inhibitors of thymol and carvacrol against food-borne pathogens. Microb Pathog. 2016;99:95-100.
- Nair DVT, Nannapaneni R, Kiess A, Schilling W, Sharma CS. Reduction of Salmonella on Turkey breast cutlets by plant-derived compounds. Foodborne Pathog Dis. 2014;11(12):981-987.
- Neyret C, Herry JM, Meylheuc T, Dubois-Brissonnet F. Plant derived compounds as natural antimicrobials to control paper mill biofilms. J Ind Microbiol Biotechnol. 2014;41(1):87-96.
- Nguyen HDN, Yuk HG. Changes in resistance of Salmonella Typhimurium biofilms formed under various conditions to industrial sanitizers. Food Control 2013;29(1):236-240.
- Oladunjoye A, Soni KA, Nannapaneni R, Schilling MW, Silva JL, Mikel B, et al. Synergistic activity between lauric arginate and carvacrol in reducing Salmonella in ground turkey. Poultry Sci. 2013;92(5):1357-1365.
- Oliveira MMM, Brugnera DF, Piccoli RH. Biofilmes microbianos na indústria de alimentos: uma revisão. Rev Inst Adolfo Lutz. 2010;69(3):277-84.
- Piovezan M, Uchida NS, Silva AF, Grespan R, Santos PS, Silva EL, et al. Effect of cinnamon essential oil and cinnamaldehyde on Salmonella Saintpaul biofilm on a stainless steel surface. J Gen Appl Microbiol. 2014;60(3):119-121.
- Scallan E, Hoekstra RM, Angul, FJ, Tauxe RV, Widdowson MA, Roy S L, et al. Foodborne Iliness acquired in the United States - major pathogens. Emerg Infect Dis. 2011;17(1):7-15.
- Shi X, Zhu X. Biofilm formation and food safety in food industries. Trends in Food Sci Tech. 2009;20(9):407-413.
- Simões M, Simões LC, Vieira MJ. A review of current and emergent biofilm control strategies. LWT Food Sci Tech. 2010;43(4):573-583.
- Soni KA, Oladunjoye A, Nannapaneni R, Schilling MW, Silva JL, Mikel B, et al. Inhibition and inactivation of Salmonella Typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J Food Protect. 2013;76(2):205-212.
- Steenackers H, Hermans K, Vanderleyden J, De Keersmaecker SCJ. Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res Int. 2012;45(2):502-531.
- Uchida NS, Grespan R, Piovezan M, Ferreira EC, Machinski Jr M, Cuman RKN, et al. Effect of carvacrol on Salmonella Saintpaul biofilms on stainless steel surface. Trop J Pharm Res. 2014;13(12):2021-2025.
- Ultee A, Kets EPW, Smid EJ. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 1999;65(10):4606-4610.
- World Health Organization. WHO. Estimates of the global burden of foodborne diseases: foodborne disease burden epidemiology reference group 2007-2015. Geneva: WHO; 2015.
- World Health Organization. WHO. Salmonella (non-typhoidal). Fact Sheet 139. Geneva: WHO; 2016.
- Wong HS, Townsend KM, Fenwick SG, Trengove RD, O'Handley RM. Comparative susceptibility of planktonic and 3-day-old Salmonella Typhimurium biofilms to disinfectants. J Appl Microbiol. 2010;108(6):2222-2228.
- Zhang J, Rui X, Wang L, Guan Y, Sun X, Dong M. Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food Control 2014;42:125-131.
Publication Dates
-
Publication in this collection
2018
History
-
Received
11 Apr 2017 -
Accepted
20 Sept 2017