Abstract:
In this study we described the diet of Hylaeamys megacephalus (G. Fisher, 1814) and investigated the degree of individual variation in the diet of this species among the Amazon Forest and the oil palm plantation. We analyzed the stomach contents of 36 individuals, of whom 11 were collected in the forest and 25 captured in the palm oil palm plantation. The H. megacephalus diet consisted of 18 food items, of which 12 were animal composition and eight were vegetable composition. The niche amplitude of the species was narrower in the forest area (Baforest = 0.013) compared to the palm tree plantation area (Bapalm = 0.478). This shows that individuals have greater niche overlap in forest areas, while in the plantation areas the animals expand their food niche. In addition, the values of the mean of the individual diet in relation to the diet of the entire population were lower in the palm oil palm plantation environment (ISpalm = 0.164) than in the Forest environment (ISforest = 0.357), indicating a high specialization in the palm oil plantation. These results indicate a population mechanism to reduce intraspecific competition in response to scarce resources.
Keywords:
Diet; food importance; individual specialization; niche amplitude; Rodentia
Resumo:
Neste estudo nós descrevemos a dieta de Hylaeamys megacephalus (G. Fisher, 1814) e investigamos o grau de variação individual na dieta desta espécie entre os hábitats de Floresta Amazônica e Plantação de Palmeira de Dendê. Analisamos o conteúdo estomacal de 36 indivíduos, dos quais 11 foram coletados na floresta e 25 capturados na plantação de palmeira de dendê. A dieta de H. megacephalus consistiu em 18 itens alimentares, dos quais 12 de composição animal e oito de composição vegetal. A amplitude de nicho da espécie foi mais estreita na área de floresta (Baforest = 0,013) comparada com a área de plantação de palmeira de dendê (Bapalm = 0,478). Isto sugere que os indivíduos têm maior sobreposição de nicho nas áreas de floresta, enquanto que nas áreas de plantação os animais expandem seu nicho alimentar. Além disto, os valores da média de similaridade da dieta individual em relação à dieta de toda a população foi menor no ambiente de plantação de palmeira de dendê (ISpalm = 0,164) do que no ambiente de Floresta (ISforest = 0,357), indicando uma alta especialização individual na plantação de dendê. Estes resultados indicam um mecanismo populacional para reduzir a competição intraespecífica em resposta à escassez de recursos.
Palavras-chave:
Dieta; importância alimentar; especialização individual; amplitude de nicho; Rodentia
Introduction
The individual specialization has been considered as an endogenous mechanism of population in response to several factors, such as environmental variations (eg. habitat changes, seasonality or daily periods), ecological pressures (eg. predation, competition, reproductive period) and resource polymorphisms (Skulason & Smith 1995SKULASON, S. & SMITH, T.B. 1995. Resource polymorphisms in vertebrates. Trends in Ecology & Evolution 10:366-370., Bolnick et al. 2003BOLNICK, D.I., SVANBÄCK R., FORDYCE, J.A., YANG, L.H., DAVIS, J.M., HULSEY, C.D. & FORISTER, M.L. 2003. The Ecology of Individuals: Incidence and Implications of Individual Specialization. The American Naturalist vol. 161, nº 1, pp.1-28.). The resource polymorphism can be explained by the discrete morphological discrepancies related to intraspecific differences in resource use, which not only interferes with the size of a population's niche but may also influence the isolation reproductive behaviour of individuals, representing an intermediate stage of speciation (Smith & Skúlasson 1996SMITH, T.B. & SKULASON, S. 1996. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annual Review of Ecology and Systematics 27:111-133.). The individual specialization would be a mechanism to reduce intraspecific competition (Roughgarden 1972ROUGHGARDEN, J. 1972. Evolution of niche width. American Naturalist 106:683-718., Svanbäck & Persson 2004SVANBÄCK, R. & PERSSON, L. 2004. Individual specialization, niche width and population dynamics: implications for trophic polymorphisms. Journal of Animal Ecology 73: 973-982.) in populations with high rates of individual specialization, where each individual typically uses a resource set significantly smaller than the set of resources used by the population as a whole and with less overlap of individual niches (Bolnick et al. 2003BOLNICK, D.I., SVANBÄCK R., FORDYCE, J.A., YANG, L.H., DAVIS, J.M., HULSEY, C.D. & FORISTER, M.L. 2003. The Ecology of Individuals: Incidence and Implications of Individual Specialization. The American Naturalist vol. 161, nº 1, pp.1-28.).
This scenario usually occurs when resources are meager, for example, where individuals vary their diet in order to decrease intraspecific competition (Svanbäck & Bolnick 2005SVANBÄCK, R. & BOLNICK, D.I. 2005. Intraspecific competition affects the strength of individual specialization: an optimal diet theory model. Evolutionary Ecology Research 7, 993-1012.). The opposite would occur when resources are abundant, where the overlap of individual niches is high and individuals would use a pool of resources close to the niche size of the population as a whole (Werner & Hall 1974WERNER, E.E. & HALL, D.J. 1974. Optimal foraging and the size selection of prey by the bluegill sunfish (Lepomis macrochirus). Ecology 55:1042-1052., Bolnick et al. 2003BOLNICK, D.I., SVANBÄCK R., FORDYCE, J.A., YANG, L.H., DAVIS, J.M., HULSEY, C.D. & FORISTER, M.L. 2003. The Ecology of Individuals: Incidence and Implications of Individual Specialization. The American Naturalist vol. 161, nº 1, pp.1-28.). Most of these theories have been commonly studied in communities of fish, insects and amphibians (Araújo & Gonzaga 2007ARAÚJO, M.S. & GONZAGA, M.O. 2007. Individual specialization in the hunting wasp Trypoxylon (Trypargilum) albonigrum (Hymenoptera, Crabronidae). Behav Ecol Sociobiol 61:1855-1863., Araújo et al. 2009ARAÚJO, M.S., BOLNICK, D.I., MARTINELLI, L.A., GIARETTA, A.A. & DOS REIS, S.F. 2009. Individual-level diet variation in four species of Brazilian frogs. Journal of Animal Ecology, 78, 848-856., Hannah et al. 2013HANNAH, B., ZANDEN, V., BJORNDAL, K.A. & BOLTEN, A.B. 2013. Temporal consistency and individual specialization in resource use by green turtles in successive life stages. Oecologia, 173(3):767-77., Bolnick et al. 2014BOLNICK, D. I., SNOWBERG, L.K., HIRSCH, P.E., LAUBER, C.L., KNIGHT, R., CAPORASO, J.G. & SVANBÄCK, R. 2014. Individuals' diet diversity influences gut microbial diversity in two freshwater fish (threespine stickleback and Eurasian perch). Ecology Letters, 17: 979-987., Costa et al. 2015COSTA, A., SALVIDIO, S., POSILLICO, M., MATTEUCCI, G., DE CINTI, B. & ROMANO, A. 2015. Generalisation within specialization: inter-individual diet variation in the only specialized salamander in the world. Sci. Rep, 5, 13260.), but in mammalian species the knowledge about individual specialization and polymorphism of resources is scarce (Olsson et al. 2007OLSSON, J., QUEVEDO, M., COLSON, C. & SVANBÄCK, R. 2007. Gut length plasticity in perch: into the bowels of resource polymorphisms. Biological Journal of the Linnean Society, 90, 517-523., Martins et al. 2008MARTINS, E.G., ARAÚJO, M.S., BONATO, V. & DOS REIS, S.F. 2008. Sex and Season Affect Individual Level Diet Variation in the Neotropical Marsupial Gracilinanus microtarsus (Didelphidae). Biotropica 40(1): 132-135., Camargo et al. 2013CAMARGO, N.F., RIBEIRO, J.F., DE CAMARGO, A.J.A. & VIEIRA, E.M. 2013. Intra- and inter-individual variation show distinct trends as drivers of seasonal changes in the resource use of a neotropical marsupial. Biological Journal of the Linnean Society, 111, 737-747., Pires et al. 2013PIRES, M.M., MARTINS, E.G., ARAÚJO, M.S. & DOS REIS, S.F. 2013. Between-individual variation drives the seasonal dynamics in the trophic niche of a Neotropical marsupial. Austral Ecology, 38: 664-671., Svanbäck et al. 2015SVANBÄCK, R., QUEVEDO, M., OLSSON, J. & EKLÖV, P. 2015. Individuals in food webs: the relationships between trophic position, omnivory and amongindividual diet variation. Oecologia, 178:103-114.).
In the Amazon, the conversion of natural ecosystems into oil palm monocultures (Elaeis guineensis) has expanded over the last 40 years (Fitzherbert et al. 2008FITZHERBERT, E.B., STRUEBIG, M.J., MOREL, A., DANIELSEN, F., BRÜHL, C.A., DONALD, P.F. & PHALAN, B. 2008. How will oil palm expansion affect biodiversity? Trends Ecol. Evol., 23, 538-545., Butler & Laurence 2009BUTLET, R.A. & LAURANCE, W.F. 2009. Is oil palm the next emerging threat to the Amazon? Tropical Conservation Science, 2:1-10., Wilcove & Koh 2010WILCOVE, D.S. & KOH, L.P. 2010. Addressing the threats to biodiversity from oil palm agriculture. Biodiversity and Conservation, 19:999-1007.). The conversion of forest habitats to oil palm plantations causes loss of environmental complexity and reduction of ecosystem resources. Different from the original native forests, the oil palm plantations present a uniform spatial distribution and tree age structure (Fitzherbert et al. 2008FITZHERBERT, E.B., STRUEBIG, M.J., MOREL, A., DANIELSEN, F., BRÜHL, C.A., DONALD, P.F. & PHALAN, B. 2008. How will oil palm expansion affect biodiversity? Trends Ecol. Evol., 23, 538-545.) with exposed soils, reduced leaf litter (Yeboua, 2000YEBOUA, K. & BALLO, K. 2000. Caracteristiques chimiques du sol sous palmeraie. Cahiers d'études et de recherches francofones/Agricultures, 9:73-76., Nelson 2002NELSON, B.P.N., RHEBERGEN, T., BERTHELSEN, S., WEBB, M.J., BANABAS, M., OBERTHÜR, T., DONOUGH, C.R., RAHMADSYAH, INDRASUARA, K. & LUBIS, A. 2002. Soil Acidification under Oil Palm: Rates and Effects on Yield. Slides 22-25.), low-density understory, highly volatile microclimate (Chung et al. 2000CHUNG, A.Y.C., EGGLETON, P., SPEIGTH, M.R., HAMMOND, P.M. & CHEY, V.K. 2000. The diversity of beetle assemblages in different habitat types in Sabah, Malaysia. Bulletin of Entomological Research, 90:475-496.), and a lower discontinuous canopy (Turner 2009TURNER, E.C. & FOSTER, W.A. 2009. The impact of forest conversion to oil palm on arthropod abundance and biomass in Sabah, Malaysia. J. of Trop. Ecol., 25:23-30.). All these drastic structural changes induced by the conversion to oil palm plantation, have been affecting the fauna in different ways in the tropical forests all over the world (Danielsen & Heegaard 1995DANIELSEN, F. & HEEGAARD, M. 1995. Impact of logging and plantation development on species diversity: a case study from Sumatra. In: Sandbukt, O. (Ed) Management of tropical forest: towards an integrated perspective. Oslo: University of Oslo, Centre for Development and the Environment., Chung et al. 2000CHUNG, A.Y.C., EGGLETON, P., SPEIGTH, M.R., HAMMOND, P.M. & CHEY, V.K. 2000. The diversity of beetle assemblages in different habitat types in Sabah, Malaysia. Bulletin of Entomological Research, 90:475-496., Peh et al. 2006PEH, K.S.H., SODHI, N.S., DE JONG, J., SEKERCIOGLU, C.H., YAP, C.A.M. & LIM, S.L.H. 2006. Conservation value of degraded habitats for forest birds in southern peninsular malaysia. divers. distrib. 12:572-581., Barlow et al. 2007BARLOW, J., GARDNER, T.A., ARAUJO, I.S., ÁVILA-PIRES, T.C., BONALDO, A.B., COSTA, J.E., ESPOSITO, M.C., FERREIRA, L.V., HAWES, J., HERNANDEZ, M.I.M., HOOGMED, M.S., LEITE, R.N., LO-MAN-HUNG, N.F., MALCOLM, J.R., MARTINS, M.B., MESTRE, L.A.M., MIRANDA-SANTOS, R., NUNES-GUTJAHR, A.L., OVERAL, W.L., PARRY, L., PETERS, S.L., RIBEIRO-JUNIOR, M.A., DA SILVA, M.N.F., SILVA-MOTA, C. & PERES, C.A. 2007. Quantifying the biodiversity value of tropical primary, secondary and plantation forest. PNAS, 20 (1040):18555-18560., Butler & Laurence 2009BUTLET, R.A. & LAURANCE, W.F. 2009. Is oil palm the next emerging threat to the Amazon? Tropical Conservation Science, 2:1-10., Yaap et al. 2010YAAP, B., STRUEBIG, J., PAOLI, G. & KOH, L.P. 2010. Mitigating the biodiversity impacts of oil palm development. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 5(19):1-11., Correa et al. 2015CORREA, F.S., JUEN, L., RODRIGUES, L.C., SILVA-FILHO, H.F. & SANTOS-COSTA, M.C. 2015. Effects of oil palm plantations on anuran diversity in the eastern Amazon. Animal Biology 65: 321-335., Cunha et al. 2015CUNHA, E.J., MONTAG, L.F.A. & JUEN, L. 2015. Oil palm crops effects on environmental integrity of Amazonian streams and heteropteran (Hemiptera) species diversity. Ecol. Indic., 52, 422-429., Ferreira et al. 2018FERREIRA, M.C., BEGOT, T.O., PRUDENTE, B.S., JUEN, L. & MONTAG, L.F.A. 2018. Effects of oil palm plantation on habitats structure and fish assemblages in Amazon streams. Environmental Biology of Fishes 101.4: 547-562.).
Van Valen (1965)VAN VALEN, L. 1965. Morphological variation and width of ecological niche. American Naturalist 99:377-389. was one of the first ecologists to propose the individual-level niche variation as a consequence of different genotypes in the same population. Age classes and sex are factors that could influence the individual variability of niche use in a population (Shine 1991SHINE, R. 1991. Intersexual dietary divergence and the evolution of sexual dimorphism in snakes. American Naturalist 138:103-122., Bolnick et al. 2003BOLNICK, D.I., SVANBÄCK R., FORDYCE, J.A., YANG, L.H., DAVIS, J.M., HULSEY, C.D. & FORISTER, M.L. 2003. The Ecology of Individuals: Incidence and Implications of Individual Specialization. The American Naturalist vol. 161, nº 1, pp.1-28., Martins et al. 2008MARTINS, E.G., ARAÚJO, M.S., BONATO, V. & DOS REIS, S.F. 2008. Sex and Season Affect Individual Level Diet Variation in the Neotropical Marsupial Gracilinanus microtarsus (Didelphidae). Biotropica 40(1): 132-135.). However, differences in habitat and resource availability would also influence individual variations (Lomnicki 1980LOMNICKI, A. 1980. Regulation of population density due to individual differences and patchy environment. Oikos 35:185-193., Bourke et al. 1997BOURKE, P., MAGNAN P. & RODRIQUEZ M.A. 1997. Individual variations in habitat use and morphology in brook charr. Journal of Fish Biology 51:783-794.). Individual-level niche variations have relevant effects, in the medium and long term, on diversification of species, since they affect the stability of population (Dieckmann & Doebeli 1999DIECKMANN, U. & DOEBELI, M. 1999. On the origin of species by sympatric speciation. Nature 400:354-357.). Here we investigate the diet of the small rodent Hylaeamys megacephalus (G. Fisher 1814) and evaluated the degree of individual variation in response to the difference of habitats, including native Amazon Forest and oil palm plantation. We hypothesize that in the oil palm plantation the individual variation of the Hylaeamys megacephalus diet would be greater than in the forest, because of the lower supply of food resources in the first habitat.
Material and Methods
1. Hylaeamys megacephalus
The species H. megacephalus (G. Fisher 1814) is a small rodent from the Cricetidae Family, Subfamily Sigmodontinae (Figure 1). According to this study, adults weight an average of 58g ± 2g. They are considered as frugivorous/gramnivorous (Paglia et al. 2012PAGLIA, A.P., FONSECA, G.A.B., RYLANDS, A.B., HERRMANN, G., AGUIAR, L.M.S., CHIARELLO, A.G., LEITE, Y.L.R., COSTA, L.P., SICILIANO, S., KIERULFF, M.C.M., MENDES, S.L., TAVARES, V.C., MITTERMEIER, R.A. & PATTON, J.L. 2012. Lista Anotada dos Mamíferos do Brasil. 2ª Ed. Occasional Papers in Conservation Biology, Number 6, Conservation International, Arlington, VA.) and are primarily terrestrial (Nitikman & Mares 1987NITIKMAN, L.Z. & MARES, M.A. 1987. Ecology small mammal in a gallery forest of central Brazil. Annals of Carnegie Museum 56 (1-2):75-99.) but also scansorial (Voss et al. 2001VOSS, R.S., LUNDE, D.P. & SIMMONS, N.B. 2001. The mammals of Paracou, French Guiana: A neotropical lowland rainforest fauna - part 2. Nonvolant species. Bulletin of the American Museum of Natural History, v. 263, p. 3-236.). Despite the low sampling of this species in Amazon Forest, it is probably widely distributed in this biome (Musser et al. 1998MUSSER, G.G.; CARLETON, M.D.; BROTHERS, E. & GARDNER, A.L. 1998. Systematic studies of Oryzomyine rodents (Muridae, Sigmodontinae): diagnoses and distributions of species formerly assigned to Oryzomys "capito." Bull. Am. Mus. Nat. Hist. 236:1-376., Patton et al. 2000PATTON, J.L., DA SILVA, M.N.F. & MALCOLM, J.R. 2000. Mammals of the Rio Juruá and the evolutionary and ecological diversifi cation of Amazonia. Bull. Am. Mus. Nat. Hist. 244:1-306.). In addition, its distribution is known for the Cerrado and the Atlantic Forest (Paglia et al. 2012PAGLIA, A.P., FONSECA, G.A.B., RYLANDS, A.B., HERRMANN, G., AGUIAR, L.M.S., CHIARELLO, A.G., LEITE, Y.L.R., COSTA, L.P., SICILIANO, S., KIERULFF, M.C.M., MENDES, S.L., TAVARES, V.C., MITTERMEIER, R.A. & PATTON, J.L. 2012. Lista Anotada dos Mamíferos do Brasil. 2ª Ed. Occasional Papers in Conservation Biology, Number 6, Conservation International, Arlington, VA., Percequillo et al. 2015PERCEQUILLO, A.R. 2015. Genus Hylaeamys Weksler, Percequillo, and Voss, 2006, In: Patton, J.L., PARDIÑAS, U.F.G. & D'Elía, G. (Eds). Mammals of South America - Rodents. Volume 2. The University of Chicago Press, Ltd., London. p. 335-346.), always associated with forest habitats (Mares et al. 1986MARES, M.A.; ERNEST, K.A. & GETTINGER, D.A. 1986. Small mammal community structure and composition in the Cerrado province of central Brazil. J. Trop. Ecol. 2:289- 300., Ochoa et al. 1993OCHOA, G.J.C., MOLINA, C. & GINER, S. 1993. Inventario y estudio comunitario de los mamíferos del Parque Nacional Canaima, con una lista de las espécies registradas para la Guayana Venezolana. Acta Cient. Venez. 44:245-62., Percequillo et al. 2015PERCEQUILLO, A.R. 2015. Genus Hylaeamys Weksler, Percequillo, and Voss, 2006, In: Patton, J.L., PARDIÑAS, U.F.G. & D'Elía, G. (Eds). Mammals of South America - Rodents. Volume 2. The University of Chicago Press, Ltd., London. p. 335-346.).
Location of the study area in the State of Pará, Brazil, delimiting the forest fragments (in gray) and areas of oil palm plantation (in white), and location of the sampling points (in black).
2. Study Site
We collected the H. megacephalus (G. Fisher 1814) specimens within the 103,000 ha Agropalma private landholding (01˚55'57" S/02º24'4" S, 48˚45'49" W/48º48'2" W). The study area contains 39,000 ha of oil palm plantations and 64,000 ha of terra firme primary forest (Figure 1). The remaining forest patches interspersed with oil palm plantations ranged from 1,500 to 15,000 ha. The region of the study is currently a mosaic of open habitat areas and natural forest remnants under several other natural phytophysionomic types. The height of the forest canopy range from 25 to 35 m, dominated by some plant species, such as the Attalea maripa (inajá), Cecropia distachya, Cordia scabrifolia, Tapiriri guianensis (tapiriri), Rollinia exsuca, Inga thibaudiana (ingá) and Vismia guianensis (sealing). The climate in the region is tropical humid, according to Köppen's classification adapted by Peel et al. (2007)PEEL, M.C., FINLAYSON, B.L. & MCMAHON, T.A. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 11(5):1633-1644., with a rainy season from December to May and a dry season from June to November. Mean temperature in the region is 26 °C and mean air humidity reaches up to 85% (Oliveira et al. 2002OLIVEIRA, L.L., FONTINHAS, R.L., LIMA, A.M.M. & LIMA, R.J.S. 2002. Mapas dos parâmetros climatológicos do Estado do Pará: umidade, temperatura e insolação, médias anuais. Anais do XIII Congresso Brasileiro de Meteorologia. Sociedade Brasileira de Meteorologia, Fortaleza.). The Elaeis guineensis has an economic life of 20 to30 years, reaching about 20 m in height. After that, the trees are cut for new planting, and the remains of the previous planting are stacked in lines interspersed with the new planting. These clumps of organic matter provide a good environment for invertebrate and small vertebrate fauna. The oil palm tree produces fibrous fruits most of the year, that are rich in beta-carotene which gives them an orange color (Luskin & Potts 2011LUSKIN, M.S. & POTTS, M.D. 2011. Microclimate and habitat heterogeneity through the oil palm lifecycle. Basic and Applied Ecology 12:540-551.).
3. Specimens Collection and Procedures
We sampled the small non-flying mammals in the study area in two field campaigns conducted in 2012, one in the rainy season and the other in the dry season. Each campaign lasted about 25 days. We installed 16 sampling points with a minimum of 1 km distance between them, with eight points located in the Forest and eight points located in the Oil Palm Plantation. At each sampling point we established a collection station, with a minimum distance of 500m from the edge of each habitat (forest or oil palm plantation). At each collection station we used pitfalls (Bury and Corn 1987BURY, R.B. & CORN, P.S. 1987. Evaluation of pitfall trapping in northwestern forests: trap arrays with drift fences. J. Wildl. Manage 51:112-119.; Ribeiro-Júnior et al. 2011) and live traps, like Sherman and Tomahawk (Voss and Emmons 1996). However, we used only H. megacephalus specimens collected in pitfalls, since the Sherman and Tomahawk traps were baited, and this would influence the results. At each collection station we implemented a pitfall line with a set of four buckets of 60 litters, buried with a distance of 15m between them. The sampling effort was 2,240 traps/nights, divided between the two types of habitats. All the points were sampled at the same time. We collected the specimens of H. megacephalus just in 11 points from the 16 points sampled (Figure 1). The collection of samples was authorized by ICMBio/SISBIO, through the permanent license number 4628-1 assigned to Ana Cristina Mendes-Oliveira.
We withdraw the gastrointestinal tract from subjects using the stomach and fecal contents for diet analysis. We measured the total weight of the stomach and intestine, and the dry weight of the stomach and intestinal contents using a high precision scale (20 g x 0.001 g). The content was displayed on a petri dish using a microscope. We calculate the frequency of occurrence (Hyslop 1980HYSLOP, E.J. 1980. Stomach contents analysis-a review of methods and their application. The Fisheries Society of the British Isles. 17, 41 1-429.), and each food item was weighted to calculate the mass frequency (Hahn et al., 1997HAHN, N.S., ANDRIAN, I.F.; FUGI, R. & ALMEIDA, V.L.L. 1997. Ecologia trófica. Pp. 209-228. In: Vazzoler, A. E. A. M.; Agostinho, A. A. & Hahn, N. S. (Eds.). A planície de inundação do Alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. Maringá, Eduem, 460p.) . We sorted each item into categories within the groups of plants and animals. The only plant item identified was palm oil. Plant items included the following categories: plant fragments (bark, pulp and fiber), fragments of palm oil (bark, pulp, fiber and almond) and whole seeds. Items of animals were identified by order or by family.
4. Data analyses
We calculated the Frequency of Occurrence (FOi%) for each item. We also calculated the mass frequency of each food item on the total weight of all items of diet (Hynes 1950HYNES, H.B.N. 1950. The food of freshwater sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius) with a review of methods used in studies of the food of fishes. J. Anim. Ecol. 19, 36-58., Hyslop 1980HYSLOP, E.J. 1980. Stomach contents analysis-a review of methods and their application. The Fisheries Society of the British Isles. 17, 41 1-429.). To evaluate the relative importance of each food item in the diet, we calculated the Food Index (IAi%) (Kawakami & Vazzoler 1980KAWAKAMI, E. & VAZZOLER, G. 1980. Método gráfico e estimativa de índice alimentar aplicado no estudo de alimentação de peixes. Boletim do Instituto Oceanográfico, 29: 205-207., Oliveira et al. 2004OLIVEIRA, A.K., ALVIM, M.C.C., PERET, A.C. & ALVES, C.B.M. 2004. Diet shifts related to body size of the pirambeba Serrasalmus brandtii Lütken, 1875 (Osteichthyes, Serrasalminae) in the Cajuru Reservoir, São Francisco River basin, Brazil. Braz. J. Biol. 64:117-124.) using the formula: IAi% = (FOi% * Pi%/∑FOi% * Pi%) * 100; Where the IAi% means food importance of item i, FOi% means frequency of occurrence of item i, and Pi% means the weight of item i. We calculated the IAi for each food item recorded in each habitat separately, which allowed us to analyze the food items consumed in each of the oil palm and forest habitats.
We evaluated the effect of the environment (oil palm and forest) on the niche width of the H. megacephalus species, using the Niche Amplitude calculation (Hurlbert 1978), which includes the value of the Levins index (Hurlbert 1978) ranging from 0 to 1. This index is equal to 0 when each animal consumes a single type of food category and equals 1 when the population consumes all available food categories similarly (Hulbert 1978HULBERT, S. H. 1978. The measurement of niche overlap and some of its relatives. Ecology, 59: 67-77., Fugi et al. 2008FUGI, R., LUZ-AGOSTINHO, K.D.G. & AGOSTINHO, A.A. 2008. Trophic interaction between an introduced (peacock bass) and a native (dogfish) piscivorous fish in a Neotropical impounded river. Hydrobiologia, 607: 143-150.). Important to mention that the Levins´s measure does not allow for the possibility that the resource vary in abundance (Krebs, 1999KREBS, C.J. 1999. Ecological Methodology. University of British Columbia, 619 pp.), but it´s still very usefully to standardize the niche breath. To evaluate the variation in the diversity of items consumed by the population of H. megacephalus, the Shannon Diversity Index (H') (Zar 1984ZAR, J.H. 1984. Biostatistical analysis, 2nd ed. Prentice-Hall Inc., Englewood Cliffs, New Jersey.) was performed. The higher the H' value the greater the diversity of food items in the diet. We performed a t-test to analyze differences between each sampled environment. The t-test was performed in R (R Development Core Team 2016R DEVELOPMENT CORE TEAM 2016 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org.
http://www.R-project.org...
), using the diversity function of the Vegan package (Oksanen et al. 2013OKSANEN, J., BLANCHET, F.G., FRIENDLY, M., KINDT, R., LEGENDRE, P., MCGLINN, D., MINCHIN, P.R., O'HARA, R.B., SIMPSON, G.L., SOLYMOS, P., STEVENS, M.H.H., SZOECS, E. & WAGNER, H. 2013. Package 'vegan'. Community ecology package, version, 2(9).).
We calculated the individual dietary variation through the Proportional Similarity Index (PSI), which measures the overlap between the diet of individual i and the menu of the population as a whole (Bolnick et al. 2002BOLNICK, D.I., YANG, L.H., FORDYCE, J.A., DAVIS, J.M. & SVANBÄCK, R. 2002. Measuring individual-level resource specialization. Ecology 83:2936-2941., 2003BOLNICK, D.I., SVANBÄCK R., FORDYCE, J.A., YANG, L.H., DAVIS, J.M., HULSEY, C.D. & FORISTER, M.L. 2003. The Ecology of Individuals: Incidence and Implications of Individual Specialization. The American Naturalist vol. 161, nº 1, pp.1-28.). This index allowed us to compare the degree of individual specialization among the sexes and the habitats through the t-test. The measure of the degree of proportional similarity among individuals of a population (IS) is calculated by averaging the individual PSi values (Bolnick et al. 2002). The SI ranges from 0 (individual maximum specialization) to 1 (no individual specialization), and we consider values below 0.6, suggested by Bolnick et al. (2002), to represent high levels of individual specialization of the population. These values were calculated using the RInSp (R Development Core Team 2016R DEVELOPMENT CORE TEAM 2016 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org.
http://www.R-project.org...
) package.
Results
We collected 36 individuals (six in the dry season and 30 in the rainy season), of which 11 were collected in the forest and 25 specimens captured in palm oil planting. The H. megacephalus diet is composed of 18 food item of which 10 are of animal origin (Orthoptera, Coleoptera, Dermaptera, Heteroptera, Lepdoptera, Blattaria, Formicidae, Curculionidae, Cerambycidae and Araneae) and eight are of vegetal origin, as plants fragments (peel, pulp, vegetable fiber and whole seeds) and oil palm fragments (peel, pulp, fiber and almond) (Table 1).
Diet composition of the individuals of the species Hylaeamys megacephalus. Legend: frequency of occurrence (FOi%) and food importance index (IAi%) of each item in the two different habitats (oil palm plantation and forest).
In the whole study, we recorded 17 food items consumed in the oil palm plantation areas and 13 in the forest areas (Table 1). Of the total number of items consumed in the oil palm habitat, nine were of animal origin, Coleoptera being the most frequent and also the most important (Table 1). Among the eight ingested vegetable components, the palm pulp had the highest importance in the diet of the species in the oil palm habitat (Table 1). In the Forest environment, the most consumed animal item was also Coleoptera, among the six registered animal components. But unlike the oil palm plantation, the fibers and bark of native plant species were more important in the diet of H. megacephalus in the forest habitat. Whole seeds also had great importance in the diet, especially in the forest environment (Table 1).
The niche amplitude calculated for H. megacephalus in the forest habitat (Baforest = 0,013) was narrower than the oil palm plantation habitat (Bapalm = 0,478). These results show that forest individuals tend to limit their niche and overlap their diet. The opposite occurs in the areas of oil palm plantation.
The diversity of dietary items of the forest individuals (H'forest = 1.039) did not differ significantly from the diet of the oil palm plantation (H'palm = 0.907) (t = 0.686, df = 34, p = 0.497). However, the analysis of ANOSIN showed differences between the diet composition and abundance of oil palm and forest samples (p = 0.038, r = 0.206).
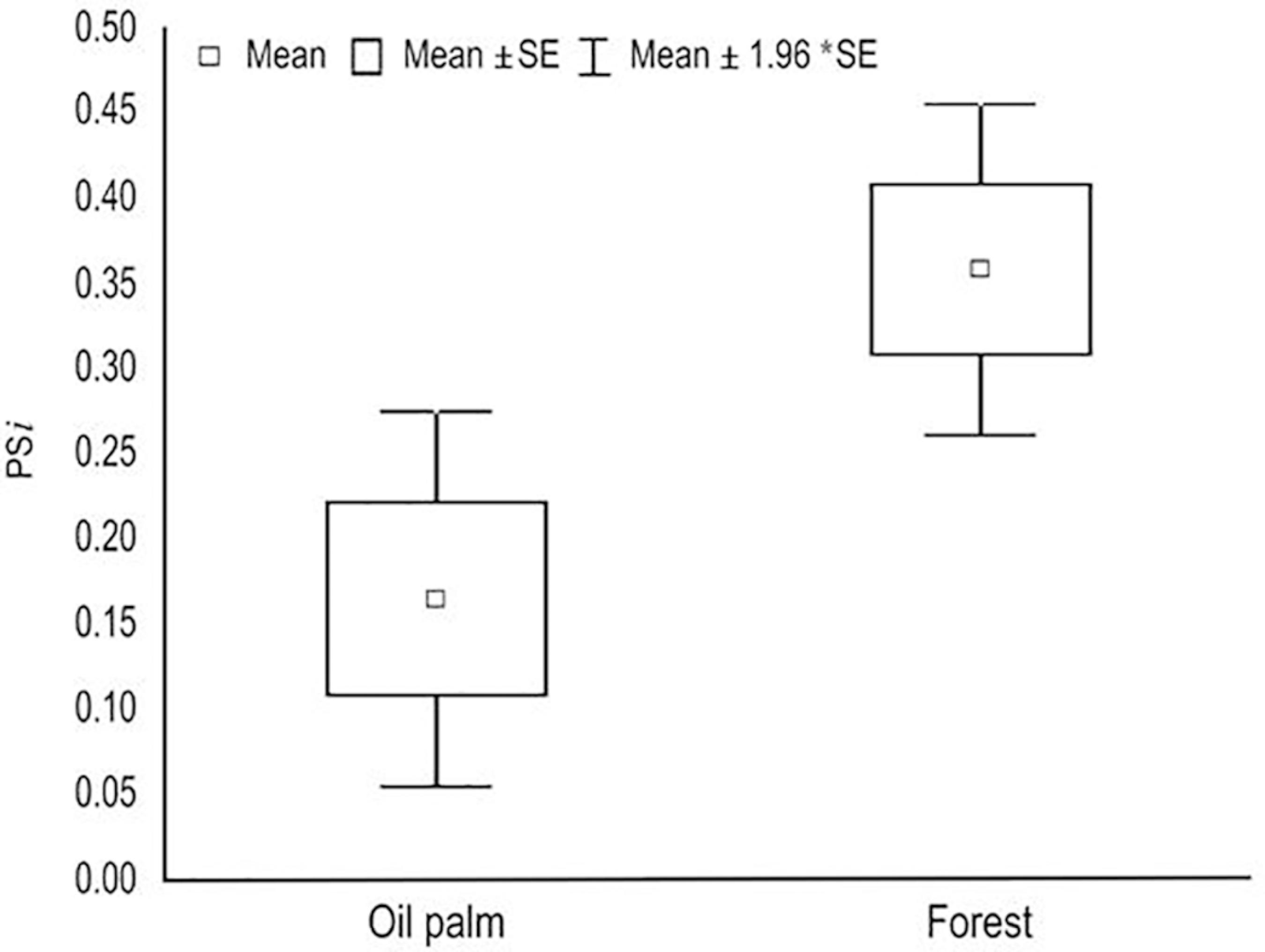
When we evaluated the individuals separately, we observed that in the oil palm plantation the values of PSI are lower than in the forest habitat, which means less overlapping of diet in relation to the diet of the whole population, than the individuals in the oil palm plantation. The t-test (t = -1.964; df = 32; p = 0,048) present an effect of the environment on individual diet specialization of the population (Figure 2). The average values of the degree of proportional similarity between the diet of the individuals, considering the entire population (IS), were lower in the oil palm (ISpalm = 0,164) than in the forest (ISforest = 0,357), indicating greater individual specialization of the diet in oil palm plantation.
Analysis of T-test comparing the variability in the diet of between different habitats (oil palm plantation and forest).
Discussion
We concluded that the changes in the environment has an effect on the H. megacephalus diet, wherein the oil palm plantation has an increase in the individual variation of the diet. This would be a mechanism for reducing intrapopulation competition in response to scarce resources (Roughgarden 1972ROUGHGARDEN, J. 1972. Evolution of niche width. American Naturalist 106:683-718., Svanback & Persson 2004SVANBÄCK, R. & PERSSON, L. 2004. Individual specialization, niche width and population dynamics: implications for trophic polymorphisms. Journal of Animal Ecology 73: 973-982.). The increase in individual variability reflected the increase in the niche amplitude of the population as a whole, in the area of oil palm plantation. In the Forest, theoretically, individuals tend to overlap their diet and decrease their niche breadth. The broader food niche observed in the plantation environment can also be determined by strong intraspecific competition as a result of a higher population density, leading to a behavioral change as a result of depletion of the preferred resources. The description of these patterns for mammals, especially for rodents, may be useful for a better understanding of the mechanisms that promote evolutionary diversification through individual specialization (Svanbäck & Bolnick 2007SVANBÄCK, R. & BOLNICK, D.I. 2007. Intraspecific competition drives increased resource use diversity within a natural population. Proceedings of the Royal Society, Biological Sciences, 274: 839-844.). The studied population is seen as a generalist population composed of individual specialists. Thus, this work reinforces the need to evaluate diet individually in studies that consider evaluating the impacts on the use of these resources (Bolnick et al., 2003BOLNICK, D.I., SVANBÄCK R., FORDYCE, J.A., YANG, L.H., DAVIS, J.M., HULSEY, C.D. & FORISTER, M.L. 2003. The Ecology of Individuals: Incidence and Implications of Individual Specialization. The American Naturalist vol. 161, nº 1, pp.1-28.). It is noteworthy that the increase in the amplitude of niche population was disproportionally greater than the increase in individual niche amplitudes, indicating that individual niches remain relatively limited when the niche of the population expands on the oil palm habitat.
The reduction in the complexity of the oil palm plantation habitat compared to the forest reflects on the increase of the individual variability of H. megacephalus. This individual specialization is considered an endogenous mechanism of the population, in response to several factors, such as environmental variations (Bolnick et al. 2003BOLNICK, D.I., SVANBÄCK R., FORDYCE, J.A., YANG, L.H., DAVIS, J.M., HULSEY, C.D. & FORISTER, M.L. 2003. The Ecology of Individuals: Incidence and Implications of Individual Specialization. The American Naturalist vol. 161, nº 1, pp.1-28., Skulason & Smith 1995SKULASON, S. & SMITH, T.B. 1995. Resource polymorphisms in vertebrates. Trends in Ecology & Evolution 10:366-370.). Differences in habitat and resource availability can influence individual variations (Lomnicki 1980LOMNICKI, A. 1980. Regulation of population density due to individual differences and patchy environment. Oikos 35:185-193., Bourke et al. 1997BOURKE, P., MAGNAN P. & RODRIQUEZ M.A. 1997. Individual variations in habitat use and morphology in brook charr. Journal of Fish Biology 51:783-794.). In this case, the individual specialization could reduce intraspecific competition (Roughgarden 1972ROUGHGARDEN, J. 1972. Evolution of niche width. American Naturalist 106:683-718., Svanbäck & Persson 2004SVANBÄCK, R. & PERSSON, L. 2004. Individual specialization, niche width and population dynamics: implications for trophic polymorphisms. Journal of Animal Ecology 73: 973-982.). However, in the medium and long-term, the high individual-level niche variations can have as consequence the diversification of the species, as they affect the stability of the population (Dieckmann & Doebeli 1999DIECKMANN, U. & DOEBELI, M. 1999. On the origin of species by sympatric speciation. Nature 400:354-357.).
In this study, we suggest the change of the frugivorous/gramnivorous diet defined to H. megacephalus by Paglia et al (2006)PEH, K.S.H., SODHI, N.S., DE JONG, J., SEKERCIOGLU, C.H., YAP, C.A.M. & LIM, S.L.H. 2006. Conservation value of degraded habitats for forest birds in southern peninsular malaysia. divers. distrib. 12:572-581. to frugivorous/insectivorous. We observe that these animals feed on large quantities of plant items such as bark, pulp, plant fiber and seeds, but also ingest a wide variety of animal food items including insects and arachnids. Other studies using close phylogenetically species, found arthropods in 100% of samples and some seeds and other parts of plants (Palma 1996PALMA, A.R.T. 1996. Separação de nichos entre pequenos mamíferos de Mata Atlântica. M.S. thesis, Universidade Estadual de Campinas, Campinas, Brazil (in Portuguese).). Our results suggest that when these items were not available, H. megacephalus increased the consumption of other items. This was evidenced by the frequency of occurrence of the palm kernel consumed in the forest area. Due to the difficult investigation of the small rodent diet, as these animals fragment food into very small parts (Hansson 1970HANSSON, L. 1970. Methods of morphological diet microanalysis in rodents. Oikos, 21: 255-266.), information about their eating habits is still limited. Few studies, often superficial, elucidate little about food ecology and impacts on communities. For the vast majority of rodent species, it is not known what the true importance of food resources is and how these items vary in their diet.
Although we have not done feasibility tests of the seeds ingested, we suggest a dispersion potential of this species, as the IAi% values for the whole seed item was the third highest among all food items consumed. The high consumption of seeds by terrestrial rodents in the neotropics makes this group of fauna essential in the maintenance and recovery of forest habitats (Forget 1992FORGET, P.M., 1992. Seed removal and seed fate in Gustavia superba (Lecythidaceae). Biotropica, 24: 408-414.), not only as dispersers, but controlling some dominant plant species, allowing greater biodiversity. Several small mammals are being considered as potential dispersers in the neotropics (Pimentel & Tabarelli 2004PIMENTEL, D.S. & TABARELLI, M. 2004. Seed dispersal of the palm Attalea oleifera in a remmant of the Brazilian Atlantic Forest. Biotropica 36: 74-84.). Consumption of fruits and seeds, albeit in variable proportions, indicates a strong dispersal potential for small mammals, although information on the dietary memories of some genera may be scarce and, in some cases, associated with phylogenetically close species (Lessa & Geise 2010LESSA, L.G. & GEISE L. 2010. Hábitos alimentares de marsupiais didelfídeos brasileiros: análise do estado de conhecimento atual. Oecologia Australis, 14(4): 901-910., Casella & Cáceres 2006CASELLA, J. & CÁCERES, N.C. 2006. Diet of four small mammal species from Atlantic forest patches in south Brazilian. Neotropical Biology Conservation 1: 5-11., Lessa & Costa 2009LESSA, L.G. & COSTA, F.N. 2009. Diet and seed dispersal by five marsupials (Didelphimorphia: Didelphidae) in a Brazilian cerrado reserve. Mammal Biology 75: 10-16.).
In general, we demonstrated that the niche used by the H. megacephalus species is narrower in the forest since the individual dietary variation is smaller. While in the oil palm plantation, where the PSI values are lower, indicating a smaller individual niche overlap, the whole niche of the population expanded. However, the richness and composition of the items consumed by the individuals of both habitats are not different. It means that populations of H. megacephalus in oil palm plantation as well as in forest habitats are feeding on almost the same food items. Individuals residing in the oil palm plantation further diversify the use of these food items among themselves. In this way, we can say that there is an impact of the oil palm plantation on the individual variability of the H. megacephalus species diet. We reinforce the importance of considering individual variability in the diet and niche amplitude studies, since if we considered all specimens as ecologically equivalents (DeAngelis & Gross 1992DE ANGELIS, D.L. & GROSS, L.J. 1992. Individual-Based Models and approaches in Ecology: Populations, Communities and Ecosystems. Chapman and Hall, NY.) in the H. megacephalus population, we could erroneously conclude on the comparison of intake between habitats. The description of these patterns for mammals, especially for rodents, may be useful for a better understanding of the mechanisms that promote evolutionary diversification through individual specialization (Svanbäck & Bolnick 2007SVANBÄCK, R. & BOLNICK, D.I. 2007. Intraspecific competition drives increased resource use diversity within a natural population. Proceedings of the Royal Society, Biological Sciences, 274: 839-844.).
Acknowledgments
We kindly acknowledg to the Conservation International (CI) and the AGROPALMA Company for the logistical support to carry out the research. To Dr. Eli Gurgel of the Laboratory of Botany of the Museu Paraense Emílio Goeldi, for the help in the identification of the vegetal items of the diet. To Marcely Valois and Mariano Brandão of the Laboratory of Invertebrates of the Federal University of Pará for the help in the identification of the animal items of the diet. Also to Jesse Carlton for the final English revision.
References
- ARAÚJO, M.S., BOLNICK, D.I., MARTINELLI, L.A., GIARETTA, A.A. & DOS REIS, S.F. 2009. Individual-level diet variation in four species of Brazilian frogs. Journal of Animal Ecology, 78, 848-856.
- ARAÚJO, M.S. & GONZAGA, M.O. 2007. Individual specialization in the hunting wasp Trypoxylon (Trypargilum) albonigrum (Hymenoptera, Crabronidae). Behav Ecol Sociobiol 61:1855-1863.
- BARLOW, J., GARDNER, T.A., ARAUJO, I.S., ÁVILA-PIRES, T.C., BONALDO, A.B., COSTA, J.E., ESPOSITO, M.C., FERREIRA, L.V., HAWES, J., HERNANDEZ, M.I.M., HOOGMED, M.S., LEITE, R.N., LO-MAN-HUNG, N.F., MALCOLM, J.R., MARTINS, M.B., MESTRE, L.A.M., MIRANDA-SANTOS, R., NUNES-GUTJAHR, A.L., OVERAL, W.L., PARRY, L., PETERS, S.L., RIBEIRO-JUNIOR, M.A., DA SILVA, M.N.F., SILVA-MOTA, C. & PERES, C.A. 2007. Quantifying the biodiversity value of tropical primary, secondary and plantation forest. PNAS, 20 (1040):18555-18560.
- BOLNICK, D.I., YANG, L.H., FORDYCE, J.A., DAVIS, J.M. & SVANBÄCK, R. 2002. Measuring individual-level resource specialization. Ecology 83:2936-2941.
- BOLNICK, D.I., SVANBÄCK R., FORDYCE, J.A., YANG, L.H., DAVIS, J.M., HULSEY, C.D. & FORISTER, M.L. 2003. The Ecology of Individuals: Incidence and Implications of Individual Specialization. The American Naturalist vol. 161, nº 1, pp.1-28.
- BOLNICK, D. I., SNOWBERG, L.K., HIRSCH, P.E., LAUBER, C.L., KNIGHT, R., CAPORASO, J.G. & SVANBÄCK, R. 2014. Individuals' diet diversity influences gut microbial diversity in two freshwater fish (threespine stickleback and Eurasian perch). Ecology Letters, 17: 979-987.
- BOURKE, P., MAGNAN P. & RODRIQUEZ M.A. 1997. Individual variations in habitat use and morphology in brook charr. Journal of Fish Biology 51:783-794.
- BURY, R.B. & CORN, P.S. 1987. Evaluation of pitfall trapping in northwestern forests: trap arrays with drift fences. J. Wildl. Manage 51:112-119.
- BUTLET, R.A. & LAURANCE, W.F. 2009. Is oil palm the next emerging threat to the Amazon? Tropical Conservation Science, 2:1-10.
- CASELLA, J. & CÁCERES, N.C. 2006. Diet of four small mammal species from Atlantic forest patches in south Brazilian. Neotropical Biology Conservation 1: 5-11.
- CAMARGO, N.F., RIBEIRO, J.F., DE CAMARGO, A.J.A. & VIEIRA, E.M. 2013. Intra- and inter-individual variation show distinct trends as drivers of seasonal changes in the resource use of a neotropical marsupial. Biological Journal of the Linnean Society, 111, 737-747.
- CORREA, F.S., JUEN, L., RODRIGUES, L.C., SILVA-FILHO, H.F. & SANTOS-COSTA, M.C. 2015. Effects of oil palm plantations on anuran diversity in the eastern Amazon. Animal Biology 65: 321-335.
- COSTA, A., SALVIDIO, S., POSILLICO, M., MATTEUCCI, G., DE CINTI, B. & ROMANO, A. 2015. Generalisation within specialization: inter-individual diet variation in the only specialized salamander in the world. Sci. Rep, 5, 13260.
- CUNHA, E.J., MONTAG, L.F.A. & JUEN, L. 2015. Oil palm crops effects on environmental integrity of Amazonian streams and heteropteran (Hemiptera) species diversity. Ecol. Indic., 52, 422-429.
- CHUNG, A.Y.C., EGGLETON, P., SPEIGTH, M.R., HAMMOND, P.M. & CHEY, V.K. 2000. The diversity of beetle assemblages in different habitat types in Sabah, Malaysia. Bulletin of Entomological Research, 90:475-496.
- DANIELSEN, F. & HEEGAARD, M. 1995. Impact of logging and plantation development on species diversity: a case study from Sumatra. In: Sandbukt, O. (Ed) Management of tropical forest: towards an integrated perspective. Oslo: University of Oslo, Centre for Development and the Environment.
- DE ANGELIS, D.L. & GROSS, L.J. 1992. Individual-Based Models and approaches in Ecology: Populations, Communities and Ecosystems. Chapman and Hall, NY.
- DIECKMANN, U. & DOEBELI, M. 1999. On the origin of species by sympatric speciation. Nature 400:354-357.
- FERREIRA, M.C., BEGOT, T.O., PRUDENTE, B.S., JUEN, L. & MONTAG, L.F.A. 2018. Effects of oil palm plantation on habitats structure and fish assemblages in Amazon streams. Environmental Biology of Fishes 101.4: 547-562.
- FITZHERBERT, E.B., STRUEBIG, M.J., MOREL, A., DANIELSEN, F., BRÜHL, C.A., DONALD, P.F. & PHALAN, B. 2008. How will oil palm expansion affect biodiversity? Trends Ecol. Evol., 23, 538-545.
- FORGET, P.M., 1992. Seed removal and seed fate in Gustavia superba (Lecythidaceae). Biotropica, 24: 408-414.
- FUGI, R., LUZ-AGOSTINHO, K.D.G. & AGOSTINHO, A.A. 2008. Trophic interaction between an introduced (peacock bass) and a native (dogfish) piscivorous fish in a Neotropical impounded river. Hydrobiologia, 607: 143-150.
- HAHN, N.S., ANDRIAN, I.F.; FUGI, R. & ALMEIDA, V.L.L. 1997. Ecologia trófica. Pp. 209-228. In: Vazzoler, A. E. A. M.; Agostinho, A. A. & Hahn, N. S. (Eds.). A planície de inundação do Alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. Maringá, Eduem, 460p.
- HANNAH, B., ZANDEN, V., BJORNDAL, K.A. & BOLTEN, A.B. 2013. Temporal consistency and individual specialization in resource use by green turtles in successive life stages. Oecologia, 173(3):767-77.
- HANSSON, L. 1970. Methods of morphological diet microanalysis in rodents. Oikos, 21: 255-266.
- HULBERT, S. H. 1978. The measurement of niche overlap and some of its relatives. Ecology, 59: 67-77.
- HYNES, H.B.N. 1950. The food of freshwater sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius) with a review of methods used in studies of the food of fishes. J. Anim. Ecol. 19, 36-58.
- HYSLOP, E.J. 1980. Stomach contents analysis-a review of methods and their application. The Fisheries Society of the British Isles. 17, 41 1-429.
- KAWAKAMI, E. & VAZZOLER, G. 1980. Método gráfico e estimativa de índice alimentar aplicado no estudo de alimentação de peixes. Boletim do Instituto Oceanográfico, 29: 205-207.
- KREBS, C.J. 1999. Ecological Methodology. University of British Columbia, 619 pp.
- LESSA, L.G. & COSTA, F.N. 2009. Diet and seed dispersal by five marsupials (Didelphimorphia: Didelphidae) in a Brazilian cerrado reserve. Mammal Biology 75: 10-16.
- LESSA, L.G. & GEISE L. 2010. Hábitos alimentares de marsupiais didelfídeos brasileiros: análise do estado de conhecimento atual. Oecologia Australis, 14(4): 901-910.
- LOMNICKI, A. 1980. Regulation of population density due to individual differences and patchy environment. Oikos 35:185-193.
- LUSKIN, M.S. & POTTS, M.D. 2011. Microclimate and habitat heterogeneity through the oil palm lifecycle. Basic and Applied Ecology 12:540-551.
- MARES, M.A.; ERNEST, K.A. & GETTINGER, D.A. 1986. Small mammal community structure and composition in the Cerrado province of central Brazil. J. Trop. Ecol. 2:289- 300.
- MARTINS, E.G., ARAÚJO, M.S., BONATO, V. & DOS REIS, S.F. 2008. Sex and Season Affect Individual Level Diet Variation in the Neotropical Marsupial Gracilinanus microtarsus (Didelphidae). Biotropica 40(1): 132-135.
- MUSSER, G.G.; CARLETON, M.D.; BROTHERS, E. & GARDNER, A.L. 1998. Systematic studies of Oryzomyine rodents (Muridae, Sigmodontinae): diagnoses and distributions of species formerly assigned to Oryzomys "capito." Bull. Am. Mus. Nat. Hist. 236:1-376.
- NELSON, B.P.N., RHEBERGEN, T., BERTHELSEN, S., WEBB, M.J., BANABAS, M., OBERTHÜR, T., DONOUGH, C.R., RAHMADSYAH, INDRASUARA, K. & LUBIS, A. 2002. Soil Acidification under Oil Palm: Rates and Effects on Yield. Slides 22-25.
- NITIKMAN, L.Z. & MARES, M.A. 1987. Ecology small mammal in a gallery forest of central Brazil. Annals of Carnegie Museum 56 (1-2):75-99.
- OCHOA, G.J.C., MOLINA, C. & GINER, S. 1993. Inventario y estudio comunitario de los mamíferos del Parque Nacional Canaima, con una lista de las espécies registradas para la Guayana Venezolana. Acta Cient. Venez. 44:245-62.
- OKSANEN, J., BLANCHET, F.G., FRIENDLY, M., KINDT, R., LEGENDRE, P., MCGLINN, D., MINCHIN, P.R., O'HARA, R.B., SIMPSON, G.L., SOLYMOS, P., STEVENS, M.H.H., SZOECS, E. & WAGNER, H. 2013. Package 'vegan'. Community ecology package, version, 2(9).
- OLIVEIRA, L.L., FONTINHAS, R.L., LIMA, A.M.M. & LIMA, R.J.S. 2002. Mapas dos parâmetros climatológicos do Estado do Pará: umidade, temperatura e insolação, médias anuais. Anais do XIII Congresso Brasileiro de Meteorologia. Sociedade Brasileira de Meteorologia, Fortaleza.
- OLIVEIRA, A.K., ALVIM, M.C.C., PERET, A.C. & ALVES, C.B.M. 2004. Diet shifts related to body size of the pirambeba Serrasalmus brandtii Lütken, 1875 (Osteichthyes, Serrasalminae) in the Cajuru Reservoir, São Francisco River basin, Brazil. Braz. J. Biol. 64:117-124.
- OLSSON, J., QUEVEDO, M., COLSON, C. & SVANBÄCK, R. 2007. Gut length plasticity in perch: into the bowels of resource polymorphisms. Biological Journal of the Linnean Society, 90, 517-523.
- PAGLIA, A.P., FONSECA, G.A.B., RYLANDS, A.B., HERRMANN, G., AGUIAR, L.M.S., CHIARELLO, A.G., LEITE, Y.L.R., COSTA, L.P., SICILIANO, S., KIERULFF, M.C.M., MENDES, S.L., TAVARES, V.C., MITTERMEIER, R.A. & PATTON, J.L. 2012. Lista Anotada dos Mamíferos do Brasil. 2ª Ed. Occasional Papers in Conservation Biology, Number 6, Conservation International, Arlington, VA.
- PALMA, A.R.T. 1996. Separação de nichos entre pequenos mamíferos de Mata Atlântica. M.S. thesis, Universidade Estadual de Campinas, Campinas, Brazil (in Portuguese).
- PATTON, J.L., DA SILVA, M.N.F. & MALCOLM, J.R. 2000. Mammals of the Rio Juruá and the evolutionary and ecological diversifi cation of Amazonia. Bull. Am. Mus. Nat. Hist. 244:1-306.
- PEH, K.S.H., SODHI, N.S., DE JONG, J., SEKERCIOGLU, C.H., YAP, C.A.M. & LIM, S.L.H. 2006. Conservation value of degraded habitats for forest birds in southern peninsular malaysia. divers. distrib. 12:572-581.
- PEEL, M.C., FINLAYSON, B.L. & MCMAHON, T.A. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 11(5):1633-1644.
- PERCEQUILLO, A.R. 2015. Genus Hylaeamys Weksler, Percequillo, and Voss, 2006, In: Patton, J.L., PARDIÑAS, U.F.G. & D'Elía, G. (Eds). Mammals of South America - Rodents. Volume 2. The University of Chicago Press, Ltd., London. p. 335-346.
- PIMENTEL, D.S. & TABARELLI, M. 2004. Seed dispersal of the palm Attalea oleifera in a remmant of the Brazilian Atlantic Forest. Biotropica 36: 74-84.
- PIRES, M.M., MARTINS, E.G., ARAÚJO, M.S. & DOS REIS, S.F. 2013. Between-individual variation drives the seasonal dynamics in the trophic niche of a Neotropical marsupial. Austral Ecology, 38: 664-671.
- R DEVELOPMENT CORE TEAM 2016 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org
» http://www.R-project.org - ROUGHGARDEN, J. 1972. Evolution of niche width. American Naturalist 106:683-718.
- SHINE, R. 1991. Intersexual dietary divergence and the evolution of sexual dimorphism in snakes. American Naturalist 138:103-122.
- SKULASON, S. & SMITH, T.B. 1995. Resource polymorphisms in vertebrates. Trends in Ecology & Evolution 10:366-370.
- SMITH, T.B. & SKULASON, S. 1996. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annual Review of Ecology and Systematics 27:111-133.
- SVANBÄCK, R. & PERSSON, L. 2004. Individual specialization, niche width and population dynamics: implications for trophic polymorphisms. Journal of Animal Ecology 73: 973-982.
- SVANBÄCK, R. & BOLNICK, D.I. 2005. Intraspecific competition affects the strength of individual specialization: an optimal diet theory model. Evolutionary Ecology Research 7, 993-1012.
- SVANBÄCK, R. & BOLNICK, D.I. 2007. Intraspecific competition drives increased resource use diversity within a natural population. Proceedings of the Royal Society, Biological Sciences, 274: 839-844.
- SVANBÄCK, R., QUEVEDO, M., OLSSON, J. & EKLÖV, P. 2015. Individuals in food webs: the relationships between trophic position, omnivory and amongindividual diet variation. Oecologia, 178:103-114.
- TURNER, E.C. & FOSTER, W.A. 2009. The impact of forest conversion to oil palm on arthropod abundance and biomass in Sabah, Malaysia. J. of Trop. Ecol., 25:23-30.
- VAN VALEN, L. 1965. Morphological variation and width of ecological niche. American Naturalist 99:377-389.
- VOSS, R.S., LUNDE, D.P. & SIMMONS, N.B. 2001. The mammals of Paracou, French Guiana: A neotropical lowland rainforest fauna - part 2. Nonvolant species. Bulletin of the American Museum of Natural History, v. 263, p. 3-236.
- WERNER, E.E. & HALL, D.J. 1974. Optimal foraging and the size selection of prey by the bluegill sunfish (Lepomis macrochirus). Ecology 55:1042-1052.
- WILCOVE, D.S. & KOH, L.P. 2010. Addressing the threats to biodiversity from oil palm agriculture. Biodiversity and Conservation, 19:999-1007.
- YAAP, B., STRUEBIG, J., PAOLI, G. & KOH, L.P. 2010. Mitigating the biodiversity impacts of oil palm development. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 5(19):1-11.
- YEBOUA, K. & BALLO, K. 2000. Caracteristiques chimiques du sol sous palmeraie. Cahiers d'études et de recherches francofones/Agricultures, 9:73-76.
- ZAR, J.H. 1984. Biostatistical analysis, 2nd ed. Prentice-Hall Inc., Englewood Cliffs, New Jersey.
Publication Dates
-
Publication in this collection
28 Mar 2019 -
Date of issue
2019
History
-
Received
13 June 2018 -
Reviewed
11 Feb 2019 -
Accepted
21 Feb 2019