Abstracts
Arctiinae are a species-rich subfamily of moth, with approximately 1,400 species in Brazil and 723 recorded in the Cerrado biome. A list of species of these moths was compiled during three years of sampling in four vegetation types within the Emas National Park. A total of 5,644 individuals belonging to 149 species were collected. About 67% of these species are new records for the Emas National Park, 31% for the State of Goiás and 9% for the Cerrado biome. Cerrado sensu stricto and semideciduous forests have higher species richness, followed by campo cerrado and campo sujo. The vegetation type with the highest number of exclusive species was the semideciduous forest, followed by cerrado sensu stricto, campo cerrado and campo sujo. The high species richness and the high proportion of new species records for Goiás and Cerrado reinforce the importance of the Emas National Park region as a center of diversity for this group of moths. The conservation of areas not yet cleared around the Park, including the creation of new protected areas, and the establishment of ecological corridors between these areas and the Park would be strategies to preserve the fauna of these moths.
light trap; Lithosiini; Arctiini
Arctiinae é uma das subfamílias de mariposas mais ricas em espécies. Já foram registradas cerca de 1400 espécies no Brasil e 723 no bioma Cerrado. Uma lista de espécies destas mariposas foi compilada de três anos de amostragens realizadas em quatro fitofisionomias do Parque Nacional das Emas. Um total de 5.644 indivíduos, pertencentes a 149 espécies foram coletados. Cerca de 67% das espécies representam novos registros para o Parque Nacional das Emas, 31% para o Estado de Goiás e 9% para o bioma Cerrado. Cerrado sensu stricto e mata estacional semidecídua apresentaram maior riqueza de espécies, seguidas por campo cerrado e campo sujo. A fitofisionomia que apresentou maior número de espécies exclusivas foi a mata estacional semidecídua, seguida por cerrado sensu stricto, campo cerrado e campo sujo. A grande riqueza de espécies e a alta proporção de novos registros de espécies para Goiás e para o Cerrado reforçam a importância da região do Parque Nacional das Emas como um centro de diversidade para esse grupo de mariposas. A conservação das áreas ainda não desmatadas no entorno do Parque, incluindo a criação de novas unidades de conservação, e o estabelecimento de corredores ecológicos entre essas áreas e o Parque seriam estratégias para preservar a fauna dessas mariposas.
armadilha luminosa; Lithosiini; Arctiini
Introduction
The biodiversity knowledge is still insuficient due the Linnean and Wallacean shortfalls (Bini et al., 2006BINI, L.M., DINIZ-FILHO, J.A.F., RANGEL, T.F.L.V.B., BASTOS, R.P. & PINTO, M.P. 2006. Chalenging Wallacean and Linnean shortfalls: knowledge gradients and conservation planning in a biodiversity hotspot. Diversity Distrib. 12:475-482, 10.1111/j.1366-9516.2006.00286.x
https://doi.org/10.1111/j.1366-9516.2006...
). The first is related to the lack of taxonomists, since a significant proportion of species have not been described for many taxa, mainly the rich tropical invertebrate groups. The second is related to the limited knowledge of species occurrences, since, for the majority of taxa, geographical distributions are poorly understood and contain many gaps. The best way to reduce the Wallacean shortfall is to invest in biodiversity inventories and publish lists of species. The results of these inventories, i.e. the species lists, provide support for actions on conservation and management, which are especially important in areas undergoing rapid environmental degradation (Lewinsohn et al. 2005LEWINSOHN, T.M., FREITAS, A.V.L. & PRADO, P.I. 2005. Conservation of terrestrial invertebrates and their habitats in Brazil. Conserv. Biol. 19:640-645, 10.1111/j.1523-1739.2005.00682.x
https://doi.org/10.1111/j.1523-1739.2005...
). They are also important in macroecological and evolutionary studies.
Arctiinae moths are a species-rich subfamily of Lepidoptera (Heppner 1991HEPPNER, J.B. 1991. Faunal regions and the diversity of Lepidoptera. Trop. Lepid. 2(1):1-85.). There are approximately 11,000 species worldwide, with 6,000 in the Neotropics (Watson & Goodger 1986WATSON, A. & GOODGER, D.T. 1986. Catalogue of the Neotropical tiger-moths. Occas. Pap. Syst. Entomol. 1(1):1-70.) and 1,400 in Brazil (Ferro & Diniz 2010FERRO, V.G. & DINIZ, I.R. 2010. Riqueza e composição de mariposas Arctiidae (Lepidoptera) no Cerrado. In Cerrado, Conhecimento científico quantitativo como subsídio para ações de conservação (I.R. Diniz, J. Marinho-Filho, R.B. Machado & R.B. Cavalcanti, eds). Thesaurus, Brasília, p. 255-313.). According to Ferro et al. (2010)FERRO, V.G., MELO, A.S. & DINIZ, I.R. 2010. Richness of tiger moths (Lepidoptera: Arctiidae) in the Brazilian Cerrado: how much do we know? Zoologia. 27(5):725-731, 10.1590/S1984-46702010000500009
https://doi.org/10.1590/S1984-4670201000...
, 723 Arctiinae species have been recorded in the Brazilian Cerrado. However, the number of species occurring in this biome should be much higher, because less than 60% of the one degree latitude/longitude cells that cover the area of the Cerrado have sampling records of Arctiinae, and only one of these cells was adequately sampled (Ferro et al. 2010FERRO, V.G., MELO, A.S. & DINIZ, I.R. 2010. Richness of tiger moths (Lepidoptera: Arctiidae) in the Brazilian Cerrado: how much do we know? Zoologia. 27(5):725-731, 10.1590/S1984-46702010000500009
https://doi.org/10.1590/S1984-4670201000...
).
According to the new classification of Zahiri et al. (2012)ZAHIRI, R., HOLLOWAY, J.D., KITCHING, I.J., LAFONTAINE, J.D., MUTANEN, M. & WAHLBERG, N. 2012. Molecular phylogenetics of Erebidae (Lepidoptera, Noctuoidea). Syst. Entomol. 37(1):102-124, 10.1111/j.1365-3113.2011.00607.x
https://doi.org/10.1111/j.1365-3113.2011...
, Arctiinae moths consist of the tribes Arctiini, Lithosiini, Amerilini and Syntomini, with the two latter not occurring in the Neotropics (Heppner 1991HEPPNER, J.B. 1991. Faunal regions and the diversity of Lepidoptera. Trop. Lepid. 2(1):1-85., Hauser & Boppré 1997HAUSER, C.L. & BOPPRÉ, M. 1997. A revision of the Afrotropical taxa of the genus Amerila Walker (Lepidoptera: Arctiidae). Syst. Entomol. 22(1):1-44, 10.1046/j.1365-3113.1997.d01-21.x
https://doi.org/10.1046/j.1365-3113.1997...
). The vast majority of Lithosiini species have a small body size (Weller et al. 2009WELLER, S., DACOSTA, M., SIMMONS, R., DITTMAR, K. & WHITING, M. 2009. Evolution and taxonomic confusion in Arctiidae. In Tiger Moths and Wolly Bears, Behavior, Ecology and Evolution of the Arctiidae (W.E. Conner, ed.). Oxford University Press, New York, p. 11-30.). The moths of this tribe mainly feed on mosses, lichens and algae (Weller et al. 2009WELLER, S., DACOSTA, M., SIMMONS, R., DITTMAR, K. & WHITING, M. 2009. Evolution and taxonomic confusion in Arctiidae. In Tiger Moths and Wolly Bears, Behavior, Ecology and Evolution of the Arctiidae (W.E. Conner, ed.). Oxford University Press, New York, p. 11-30.) and are generally more associated with vegetation types in early stages of succession (Hilt & Fiedler 2006HILT, N. & FIEDLER, K. 2006. Arctiid moth ensembles along a successional gradient in the Ecuadorian montane rain forest zone: how different are subfamilies and tribes? J. Biogeogr. 33(1):108-120, 10.1111/j.1365-2699.2005.01360.x
https://doi.org/10.1111/j.1365-2699.2005...
). The Arctiini tribe are composed of small to medium sized moths whose larvae feed on a wide variety of plants, including grasses, herbs, shrubs and trees (Weller et al. 2009WELLER, S., DACOSTA, M., SIMMONS, R., DITTMAR, K. & WHITING, M. 2009. Evolution and taxonomic confusion in Arctiidae. In Tiger Moths and Wolly Bears, Behavior, Ecology and Evolution of the Arctiidae (W.E. Conner, ed.). Oxford University Press, New York, p. 11-30.). Furthermore, Arctiini moths can explore different vegetation types because they are polyphagous (Singer & Bernays 2009SINGER, M.S. & BERNAYS, E.A. 2009. Specialized generalists: Behavioral and evolutionary ecology of polyphagous wooly bear caterpillars. In Tiger Moths and Wolly Bears, Behavior, Ecology and Evolution of the Arctiidae (W.E. Conner, ed.). Oxford University Press, New York, p. 103-114.) and can therefore inhabit a wide variety of terrestrial habitats.
The Cerrado is a biodiversity hotspot for conservation priorities (Myers et al. 2000MYERS, N., MITTERMEIER, R.A., MITTERMEIER, C.G., FONSECA, G.A.B. & KENT, J. 2000. Biodiversity hotspots for conservation priorities. Nature. 403(6772):853-858, 10.1038/35002501
https://doi.org/10.1038/35002501...
). Only 2% of its area is legally protected (Klink & Machado 2005KLINK, C.A. & MACHADO, R.B. 2005. Conservation of the Brazilian Cerrado. Conserv. Biol. 19(3):707-713, 10.1111/j.1523-1739.2005.00702.x
https://doi.org/10.1111/j.1523-1739.2005...
) and the Arctiinae fauna is not known properly, even in this area. The Cerrado biome is composed by a wide variety of vegetation types ranging from open areas with no shrub element to areas with a high density of tall trees (Oliveira-Filho & Ratter 2002OLIVEIRA-FILHO, A.T. & RATTER, J.A. 2002. Vegetation physiognomies and woody flora of the Cerrado biome. In The Cerrados of Brazil. Ecology and natural history of a Neotropical savanna (P.S. Oliveira & R.J. Marquis, eds). Columbia University Press, New York, p. 91-120.). The vegetation types that comprise the cerrado sensu lato are campo limpo, campo sujo, campo cerrado, cerrado sensu stricto and cerradão. These five vegetation types represent a gradient of vegetation increasing in woody plant density and decreasing in herbaceous density (Oliveira-Filho & Ratter 2002OLIVEIRA-FILHO, A.T. & RATTER, J.A. 2002. Vegetation physiognomies and woody flora of the Cerrado biome. In The Cerrados of Brazil. Ecology and natural history of a Neotropical savanna (P.S. Oliveira & R.J. Marquis, eds). Columbia University Press, New York, p. 91-120.). The Cerrado also contains forest formations, which are not considered to belong to the cerrado sensu lato (Oliveira-Filho & Ratter 2002MYERS, N., MITTERMEIER, R.A., MITTERMEIER, C.G., FONSECA, G.A.B. & KENT, J. 2000. Biodiversity hotspots for conservation priorities. Nature. 403(6772):853-858, 10.1038/35002501
https://doi.org/10.1038/35002501...
). The Arctiinae moths respond to these differences in vegetation, and the fauna of the forest formations differs from the savanna formations (Ferro & Diniz 2007FERRO, V.G. & DINIZ, I.R. 2007. Composition of the Arctiidae species (Insecta, Lepidoptera) in Cerrado areas. Rev. Bras. Zool. 24(3):635-646, 10.1590/S0101-81752007000300015
https://doi.org/10.1590/S0101-8175200700...
, Moreno et al. 2014MORENO, C., CIANCIARUSO, M.V., SGARBI, L.F. & FERRO, V.G. 2014. Richness and composition of tiger moths (Erebidae: Arctiinae) in a Neotropical savanna: are heterogeneous habitats richer in species? Nat. Conserv. 12(2):138-143, 10.1016/j.ncon.2014.09.006
https://doi.org/10.1016/j.ncon.2014.09.0...
).
We present a list of Arctiinae moth species of the Emas National Park, one of the largest conservation units of the Cerrado biome. The park includes the Brazilian Long Term Ecological Research Network. We have performed sampling in different vegetation types of the park and measured the observed and extrapolated richness of species across the study area and in each vegetation type. We have also measured the exclusive species of each vegetation type and the number of species that are new records for the Park, the State of Goiás and for the Cerrado biome.
Material and Methods
Specimens of Arctiinae were sampled in the Emas National Park (ENP), located between the cities of Mineiros and Chapadão do Céu, in the State of Goiás, Central West of Brazil (17°49′-18°28′S and 52°39′-53°10′W) (Figure 1). The ENP covers 132,941 ha and approximately 80% of this area consists of grasslands (campos limpos and campos sujos). Approximately 15% of the ENP area is composed of campo cerrado and cerrado sensu stricto and 5% of the remaining area consists of campos úmidos, veredas and forests (França et al. 2007FRANÇA, H., RAMOS-NETO, M.B. & SETZER, A. 2007. O fogo no Parque Nacional das Emas. Ministério do Meio Ambiente, Brasília.). The climate is Aw in Koppen classification, i.e. the climate is tropical humid with three to six months of dry winter and rainy summer, which imposes a strong seasonality in the vegetation (Ramos-Neto & Pivello 2000RAMOS-NETO, M.B. & PIVELLO, V.R. 2000. Lightning fires in a Brazilian savanna National Park: rethinking management strategies. Environ. Manage. 26(6):675-684, 10.1007/s002670010124
https://doi.org/10.1007/s002670010124...
). The annual precipitation ranges from 1,200 to 2,000 mm, concentrated between September and March, with an average annual temperature of 24.6°C (Ramos-Neto & Pivello 2000RAMOS-NETO, M.B. & PIVELLO, V.R. 2000. Lightning fires in a Brazilian savanna National Park: rethinking management strategies. Environ. Manage. 26(6):675-684, 10.1007/s002670010124
https://doi.org/10.1007/s002670010124...
).
Map of Brazil showing Goiás State in black (a); map of Goiás State showing the Emas National Park (ENP) in black (b); area of the ENP showing the sample plots (black points, c); smaller scale of c (d); smaller scale of the squares indicated in d (e, f, g). The symbols indicate the vegetation type: semideciduous forest (▪), cerrado sensu stricto (•), campo cerrado (▴) and campo sujo (+). The numbers represent the code of sample plots.
Sampling units consisted of 40 plots of 10x10 m, distributed in four vegetation types: semideciduous forest (n = 10 plots); cerrado sensu stricto (n = 10 plots); campo cerrado (n = 14 plots); campo sujo (n = six plots) (Figure 1). In each plot, the moths were collected from dusk until dawn through a Luiz de Queiroz light trap (Silveira-Neto & Silveira 1969SILVEIRA NETO, S. & SILVEIRA, A. C. 1969. Armadilha luminosa modelo "Luiz de Queiroz". O Solo 61:19-21.) equipped with a 15 W black lamp. The traps were suspended 1.5 m above the ground in the center of each plot. The minimum distance between plots was 100 m to minimize the capture of species from surrounding vegetation types. This distance corresponds to the radius of attraction of a 125 W lamp (Muirhead-Thompson 1991MUIRHEAD-THOMPSON, R.C. 1991. Trap responses of flying insects. Academic Press, London.), a power eight times greater than that we used in this study. Sampling was restricted to periods of new and waning lunar phases due to the radius of attraction of the traps being greater (Yela & Holyoak 1997YELA, J.L. & HOLYOAK, M. 1997. Effects of moonlight and meterological factors on light bait trap catches of Noctuid moths (Lepidoptera: Noctuidae). Environ. Entomol. 26(6):1283-1290, 10.1093/ee/26.6.1283
https://doi.org/10.1093/ee/26.6.1283...
). Sampling was conducted for three years during both the dry (June to July 2010, July 2011 and July 2012) and rainy seasons (December 2010 to February 2011, November 2011 and December 2012). The moths were collected in all plots over two non-consecutive nights in order to increase the representativeness of the fauna, totaling 12 nights of sampling in each plot (2 in each dry season and 2 in each rainy season) and 84 nights of sampling in total. On each sampling night, we set eight light traps, two in each vegetation type. Plots sampled in the same night were the most distant possible to avoid pseudoreplication. Each plot was sampled once at the new moon and once during the waning moon.
Arctiinae individuals were identified by comparison with digital images of the identified species of the V.O. Becker Collection (where the identification was confirmed by comparison with types) and through the literature (Hampson, 1898HAMPSON, G.F. 1898. Catalogue of the Lepidoptera Phalaenae in the British Museum. Order of the Trustees, London., 1900HAMPSON, G.F. 1900. Catalogue of the Lepidoptera Phalaenae in the British Museum. Order of the Trustees, London., 1901HAMPSON, G.F. 1901. Catalogue of the Lepidoptera Phalaenae in the British Museum. Order of the Trustees, London., 1914HAMPSON, G.F. 1914. Catalogue of the Lepidoptera Phalaenae in the British Museum. Order of the Trustees, London., Watson & Goodger 1986WATSON, A. & GOODGER, D.T. 1986. Catalogue of the Neotropical tiger-moths. Occas. Pap. Syst. Entomol. 1(1):1-70., Piãas-Rubio et al. 2000PIÑAS-RUBIO, F., RAB-GREEN, S., ONORE, G. & MANZANO, P.I. 2000. Mariposas del Ecuador. Familia: Arctiidae. Subfamilias: Arctiinae y Pericopinae. Pontificia Universidad Católica del Ecuador, Quito., Piãas-Rubio & Manzano 2003PIÑAS-RUBIO, F., & MANZANO, P.I. 2003. Mariposas del Ecuador. Familia: Arctiidae. Subfamilia: Ctenuchinae. Compaãía de Jesús, Quito.). All individuals were deposited in the Zoological Collection of the Federal University of Goiás (Goiânia, Brazil).
We used three non-parametric species richness estimators (first and second order Jackknife and second order Chao) to better estimate the total richness of Arctiinae in the study area and in each vegetation type. These three estimators are based on incidence (presence/ausence) of species in assemblages (Melo 2004MELO, A.S. 2004. A critique of the use of jackknife and related non-parametric techniques to estimate species richness. Community Ecol. 5(2):149-157, 10.1556/ComEc.5.2004.2.1
https://doi.org/10.1556/ComEc.5.2004.2.1...
).
Results
We recorded 5,644 Arctiinae individuals during our sampling; belonging to 149 species, 73 genera, two tribes (Arctiini and Lithosiini) and nine subtribes (Arctiina, Callimorphina, Cisthenina, Ctenuchina, Euchromiina, Eudesmiina, Lithosiina, Pericopina and Phaegopterina). Of the 149 species sampled, 117 were identified to the species level (78%), 16 at the genus level (10%) and 16 at the tribal level (10%) (Appendix 1 Appendix 1. List of Actiinae moth species sampled in four phytophysiognomies of the Emas National Park (ENP), in the dry and rainy seasons. CS means campo sujo, CC campo cerrado, CSS cerrado sensu stricto and SF semideciduous forest. Species with symbols #$* are new records for Cerrado, #$ for Goias State and # for ENP. Phytophysiognomy Season Species CS CC CSS SF Dry Rainy Arctiinae Arctiini Arctiinii sp.1 x x x x Arctiinii sp.2 x x Arctiinii sp.3 x x x Arctiinii sp.4 x x Ctenuchiini sp.1 x x x Arctiina Hypercompe mus (Oberthür, 1881)#$ x x Paracles phaeocera (Hampson, 1905)# x x x x Paracles sp.1 x x x x x x Paracles sp.2 x x Pseudalus limona Schaus, 1896# x x x x x Callimorphina Utetheisa ornatrix (Linnaeus, 1758) x x x x x x Ctenuchina Aclytia flavigutta (Walker, 1854)#$ x x x x x x Aclytia heber (Cramer, 1780)# x x x x x x Aclytia sp.1 x x x x Argyroeides braco (Herrich-Schäffer, [1855]# x x Cercopimorpha postflavia Rothschild, 1912#$ x x Correbidia calopteridia (Butler, 1878)#$ x x Correbidia sp.1 x x Delphyre discalis (Druce, 1905)# x x x x x x Delphyre dizona (Druce, 1898)# x x x x x x Episcepsis klagesi Rothschild, 1911#$ x x Episcepsis lenaeus (Cramer, 1780)# x x Episcepsis thetis (Linnaeus, 1771)#$ x x x Eucereon albidia Rothschild, 1912#$* x x x Eucereon arenosun Butler, 1877#$ x x Eucereon dorsipuncta Hampson, 1905# x x x Eucereon pseudarchias Hampson, 1898#$ x x Eucereon setosum (Sepp, [1830])#$ x x x x x Eucereon sp.1 x x x x x Heliura rhodophila (Walker, 1856)# x x Heliura tetragramma (Walker, 1854)# x x x x x x Napata leucotela Butler, 1876# x x Philoros rubriceps (Walker, 1854)# x x x x x Pseudohyaleucerea vulnerata (Butler, 1875)#$ x x Pseudosphex discoplaga (Schaus, 1905)# x x Pseudosphex fulvisphex (Druce, 1898)#$ x x x x Pseudosphex nivaca (Jones, 1914) x x x x x x Euchromiina Autochloris enagrus (Cramer, 1780)#$* x x x x Cosmosoma achemon (Fabricius, 1781)# x x x x x x Cosmosoma auge (Linnaeus, 1767)# x x x Cosmosoma nigriscens Rothschild, 1911# x x Cosmosoma rasera Jones, 1914# x x x x x Cosmosoma theuthras restrictum Butler, 1876# x x x x x x Cosmosoma sp.1 x x x x x Cosmosoma sp.2 x x Cosmosoma sp.3 x x x x x Dycladia lucetius (Stoll, 1781) x x x x x x Erruca hanga (Herrich-Schäffer, [1854])#$ x x Eurota histrio (Guérin, 1843)# x x Eurota nigricincta Hampson, 1907#$ x x Hyda basilutea (Walker, 1854)# x x x x Lepidoneiva erubescens (Butler, 1876) x x x x x x Macrocneme aurifera Hampson, 1914#$* x x x x x x Nyridela acroxantha (Perty, 1833)# x x x x Nyridela chalciope (Hübner, [1827])# x x Pheia albisigna (Walker, 1854) x x x x x x Pheia gaudens (Walker, 1856)# x x Pheia haematosticta Jones, 1908 x x x x x x Pheia haemopera Schaus, 1898 x x x x x x Pheia seraphina (Herrich-Schäffer, 1854) x x x x x x Pheia sp.1 x x x Phoenicoprocta baeri Rothschild, 1911 x x x x x Phoenicoprocta sp.1 x x x x Poliopastea plumbea Hampson, 1898#$ x x x x x Poliopastea sp.1 x x Saurita attenuata Hampson, 1905#$* x x x Sphecosoma aenetus (Schaus, 1896)#$* x x Pericopina Dysschema boisduvalli (van der Hoeven & de Vriese, 1840)# x x Dysschema sacrifica (Hübner, [1831])# x x x x Hyalurga fenestra (Linnaeus, 1758)# x x Hyalurga partita (Walker, 1854)#$* x x Phaegopterina Agaraea semivitrea Rothschild, 1909# x x Amaxia dyuna Schaus, 1896#$ x x x x x x Amaxia kennedyi (Rothschild, 1909)#$ x x Bertholdia detracta Seitz, 1921# x x Biturix diversipes (Walker, 1855)#$* x x Carales astur (Cramer, 1777)# x x Cresera affinis (Rothschild, 1909)# x x Cresera ilioides (Schaus, 1905)#$* x x Cresera optima (Butler, 1877)#$ x x x Echeta juno (Schaus, 1892)#$* x x Elysius hermia (Cramer, 1777)# x x Elysius joiceyi Talbot, 1928# x x x x Eupseudosoma grandis Rothschild, 1909#$ x x x x x Eupseudosoma involuta (Sepp, [1855])# x x x Halysidota sannionis (Rothschild, 1909)#$ x x x x x Hyperandra appendiculata (Herrich-Schäffer, [1856])# x x Hyperthaema sp.1 x x x x x Hyperthaema sp.2 x x x x Hyponerita lavinia (Druce, 1890)#$ x x Idalus agricus Dyar, 1910#$* x x x x Idalus carinosa (Schaus, 1905) x x x x x x Idalus citrina Druce, 1890#$ x x x x x x Idalus dares Druce, 1894# x x Idalus lineosus Walker, 1869# x x x x Lepidokirbyia vittipes (Walker, 1855)# x x x x x Leucanopsis rosetta (Schaus, 1896)# x x x x x Leucanopsis squalida (Herrich-Schäffer, [1855])#$ x x x Leucanopsis strigulosa (Walker, 1855)#$ x x x x x x Lophocampa annulosa (Walker, 1855)#$ x x Lophocampa atrimaculata (Hampson, 1901)#$* x x Lophocampa citrina (Sepp, [1852])# x x x x x Mazaeras francki Schaus, 1896# x x Melese incertus (Walker, 1855)# x x x Melese paranensis Dognin, 1911#$ x x x Neritos atta Schaus, 1920#$ x x x x Neritos flavimargo Joicey & Talbot, 1916 x x x Neritos hampsoni Rothschild, 1909#$ x x x x Neritos sanguipuncta Schaus, 1901# x x Pareuchaetes aurata (Butler, 1875)# x x x x x x Pelochyta arontes (Stoll, 1782)# x x Psychophasma erosa (Herrich-Schäffer, [1858])# x x x Rhipha pulcherrima (Rothschild, 1935)# x x x x x Rhipha strigosa (Walker, 1854)#$ x x x Robinsonia dewitzi Gundlach, 1881#$ x x Scaptius submarginalis (Rothschild, 1909)#$* x x Viviennea salma (Druce, 1896)#$ x x x x Lithosiini Lithosiinii sp. 1 x x x Lithosiinii sp. 2 x x x x x Lithosiinii sp. 3 x x x x Lithosiinii sp. 4 x x x Lithosiinii sp. 5 x x Lithosiinii sp. 6 x x Lithosiinii sp. 7 x x Lithosiinii sp. 8 x x x x x Lithosiinii sp. 9 x x Lithosiinii sp. 12 x x Lithosiinii sp. 14 x x x x x x Cisthenina Barsinella mirabilis Butler, 1878 x x x x Cisthene dives (Schaus, 1896)# x x x x Cisthene ruficollis (Schaus, 1896)#$ x x Cisthene subruba (Schaus, 1905)# x x x x x x Cisthene triplaga (Hampson, 1905)# x x x x x x Cisthene sp.1 x x x x x Cisthene sp.2 x x x x Cisthene sp.3 x x x Illice croesus Hampson, 1914#$ x x Illice griseola (Rothschild, 1913)#$* x x Odozana domina (Schaus, 1896) x x x x x Odozana obscura (Schaus, 1896) x x x x x x Talara grisea Schaus, 1896# x x x x x Eudesmiina Antona fallax (Butler, 1877)# x x Lithosina Agylla argentea (Walker, 1863)#$* x x x x x x Agylla marcata (Schaus, 1894)#$ x x x x Agylla sp.1 x x x x x x Apistosia judas Hübner, [1819]# x x Metalobosia diaxantha Hampson, 1914# x x x Nodozana jucunda Jones, 1914 x x x x x x Parablavia sadima (Schaus, 1896) x x x x x x ). The subtribe with the highest number of species was Phaegopterina (46 species, 30% of the total), followed by Euchromiina (30, 20%), Ctenuchina (26, 17%), Cisthenina (13, 8%), Lithosina (7, 4%), Arctiina (5, 3%), Pericopina (4, 2%), Callimorphina and Eudesmiina (both with 1 species, 0.6%) (Appendix 1 Appendix 1. List of Actiinae moth species sampled in four phytophysiognomies of the Emas National Park (ENP), in the dry and rainy seasons. CS means campo sujo, CC campo cerrado, CSS cerrado sensu stricto and SF semideciduous forest. Species with symbols #$* are new records for Cerrado, #$ for Goias State and # for ENP. Phytophysiognomy Season Species CS CC CSS SF Dry Rainy Arctiinae Arctiini Arctiinii sp.1 x x x x Arctiinii sp.2 x x Arctiinii sp.3 x x x Arctiinii sp.4 x x Ctenuchiini sp.1 x x x Arctiina Hypercompe mus (Oberthür, 1881)#$ x x Paracles phaeocera (Hampson, 1905)# x x x x Paracles sp.1 x x x x x x Paracles sp.2 x x Pseudalus limona Schaus, 1896# x x x x x Callimorphina Utetheisa ornatrix (Linnaeus, 1758) x x x x x x Ctenuchina Aclytia flavigutta (Walker, 1854)#$ x x x x x x Aclytia heber (Cramer, 1780)# x x x x x x Aclytia sp.1 x x x x Argyroeides braco (Herrich-Schäffer, [1855]# x x Cercopimorpha postflavia Rothschild, 1912#$ x x Correbidia calopteridia (Butler, 1878)#$ x x Correbidia sp.1 x x Delphyre discalis (Druce, 1905)# x x x x x x Delphyre dizona (Druce, 1898)# x x x x x x Episcepsis klagesi Rothschild, 1911#$ x x Episcepsis lenaeus (Cramer, 1780)# x x Episcepsis thetis (Linnaeus, 1771)#$ x x x Eucereon albidia Rothschild, 1912#$* x x x Eucereon arenosun Butler, 1877#$ x x Eucereon dorsipuncta Hampson, 1905# x x x Eucereon pseudarchias Hampson, 1898#$ x x Eucereon setosum (Sepp, [1830])#$ x x x x x Eucereon sp.1 x x x x x Heliura rhodophila (Walker, 1856)# x x Heliura tetragramma (Walker, 1854)# x x x x x x Napata leucotela Butler, 1876# x x Philoros rubriceps (Walker, 1854)# x x x x x Pseudohyaleucerea vulnerata (Butler, 1875)#$ x x Pseudosphex discoplaga (Schaus, 1905)# x x Pseudosphex fulvisphex (Druce, 1898)#$ x x x x Pseudosphex nivaca (Jones, 1914) x x x x x x Euchromiina Autochloris enagrus (Cramer, 1780)#$* x x x x Cosmosoma achemon (Fabricius, 1781)# x x x x x x Cosmosoma auge (Linnaeus, 1767)# x x x Cosmosoma nigriscens Rothschild, 1911# x x Cosmosoma rasera Jones, 1914# x x x x x Cosmosoma theuthras restrictum Butler, 1876# x x x x x x Cosmosoma sp.1 x x x x x Cosmosoma sp.2 x x Cosmosoma sp.3 x x x x x Dycladia lucetius (Stoll, 1781) x x x x x x Erruca hanga (Herrich-Schäffer, [1854])#$ x x Eurota histrio (Guérin, 1843)# x x Eurota nigricincta Hampson, 1907#$ x x Hyda basilutea (Walker, 1854)# x x x x Lepidoneiva erubescens (Butler, 1876) x x x x x x Macrocneme aurifera Hampson, 1914#$* x x x x x x Nyridela acroxantha (Perty, 1833)# x x x x Nyridela chalciope (Hübner, [1827])# x x Pheia albisigna (Walker, 1854) x x x x x x Pheia gaudens (Walker, 1856)# x x Pheia haematosticta Jones, 1908 x x x x x x Pheia haemopera Schaus, 1898 x x x x x x Pheia seraphina (Herrich-Schäffer, 1854) x x x x x x Pheia sp.1 x x x Phoenicoprocta baeri Rothschild, 1911 x x x x x Phoenicoprocta sp.1 x x x x Poliopastea plumbea Hampson, 1898#$ x x x x x Poliopastea sp.1 x x Saurita attenuata Hampson, 1905#$* x x x Sphecosoma aenetus (Schaus, 1896)#$* x x Pericopina Dysschema boisduvalli (van der Hoeven & de Vriese, 1840)# x x Dysschema sacrifica (Hübner, [1831])# x x x x Hyalurga fenestra (Linnaeus, 1758)# x x Hyalurga partita (Walker, 1854)#$* x x Phaegopterina Agaraea semivitrea Rothschild, 1909# x x Amaxia dyuna Schaus, 1896#$ x x x x x x Amaxia kennedyi (Rothschild, 1909)#$ x x Bertholdia detracta Seitz, 1921# x x Biturix diversipes (Walker, 1855)#$* x x Carales astur (Cramer, 1777)# x x Cresera affinis (Rothschild, 1909)# x x Cresera ilioides (Schaus, 1905)#$* x x Cresera optima (Butler, 1877)#$ x x x Echeta juno (Schaus, 1892)#$* x x Elysius hermia (Cramer, 1777)# x x Elysius joiceyi Talbot, 1928# x x x x Eupseudosoma grandis Rothschild, 1909#$ x x x x x Eupseudosoma involuta (Sepp, [1855])# x x x Halysidota sannionis (Rothschild, 1909)#$ x x x x x Hyperandra appendiculata (Herrich-Schäffer, [1856])# x x Hyperthaema sp.1 x x x x x Hyperthaema sp.2 x x x x Hyponerita lavinia (Druce, 1890)#$ x x Idalus agricus Dyar, 1910#$* x x x x Idalus carinosa (Schaus, 1905) x x x x x x Idalus citrina Druce, 1890#$ x x x x x x Idalus dares Druce, 1894# x x Idalus lineosus Walker, 1869# x x x x Lepidokirbyia vittipes (Walker, 1855)# x x x x x Leucanopsis rosetta (Schaus, 1896)# x x x x x Leucanopsis squalida (Herrich-Schäffer, [1855])#$ x x x Leucanopsis strigulosa (Walker, 1855)#$ x x x x x x Lophocampa annulosa (Walker, 1855)#$ x x Lophocampa atrimaculata (Hampson, 1901)#$* x x Lophocampa citrina (Sepp, [1852])# x x x x x Mazaeras francki Schaus, 1896# x x Melese incertus (Walker, 1855)# x x x Melese paranensis Dognin, 1911#$ x x x Neritos atta Schaus, 1920#$ x x x x Neritos flavimargo Joicey & Talbot, 1916 x x x Neritos hampsoni Rothschild, 1909#$ x x x x Neritos sanguipuncta Schaus, 1901# x x Pareuchaetes aurata (Butler, 1875)# x x x x x x Pelochyta arontes (Stoll, 1782)# x x Psychophasma erosa (Herrich-Schäffer, [1858])# x x x Rhipha pulcherrima (Rothschild, 1935)# x x x x x Rhipha strigosa (Walker, 1854)#$ x x x Robinsonia dewitzi Gundlach, 1881#$ x x Scaptius submarginalis (Rothschild, 1909)#$* x x Viviennea salma (Druce, 1896)#$ x x x x Lithosiini Lithosiinii sp. 1 x x x Lithosiinii sp. 2 x x x x x Lithosiinii sp. 3 x x x x Lithosiinii sp. 4 x x x Lithosiinii sp. 5 x x Lithosiinii sp. 6 x x Lithosiinii sp. 7 x x Lithosiinii sp. 8 x x x x x Lithosiinii sp. 9 x x Lithosiinii sp. 12 x x Lithosiinii sp. 14 x x x x x x Cisthenina Barsinella mirabilis Butler, 1878 x x x x Cisthene dives (Schaus, 1896)# x x x x Cisthene ruficollis (Schaus, 1896)#$ x x Cisthene subruba (Schaus, 1905)# x x x x x x Cisthene triplaga (Hampson, 1905)# x x x x x x Cisthene sp.1 x x x x x Cisthene sp.2 x x x x Cisthene sp.3 x x x Illice croesus Hampson, 1914#$ x x Illice griseola (Rothschild, 1913)#$* x x Odozana domina (Schaus, 1896) x x x x x Odozana obscura (Schaus, 1896) x x x x x x Talara grisea Schaus, 1896# x x x x x Eudesmiina Antona fallax (Butler, 1877)# x x Lithosina Agylla argentea (Walker, 1863)#$* x x x x x x Agylla marcata (Schaus, 1894)#$ x x x x Agylla sp.1 x x x x x x Apistosia judas Hübner, [1819]# x x Metalobosia diaxantha Hampson, 1914# x x x Nodozana jucunda Jones, 1914 x x x x x x Parablavia sadima (Schaus, 1896) x x x x x x ).
It was estimated by first order Jackknife, second order Jackknife and second order Chao, that the sample region has a richness of approximately 190, 214 and 197 species respectively (Table 1). Fourteen species (9%) were new records for the Cerrado, 47 (31%) were new records for the State of Goiás and 101 (67%) were new records for the ENP (Appendix 1 Appendix 1. List of Actiinae moth species sampled in four phytophysiognomies of the Emas National Park (ENP), in the dry and rainy seasons. CS means campo sujo, CC campo cerrado, CSS cerrado sensu stricto and SF semideciduous forest. Species with symbols #$* are new records for Cerrado, #$ for Goias State and # for ENP. Phytophysiognomy Season Species CS CC CSS SF Dry Rainy Arctiinae Arctiini Arctiinii sp.1 x x x x Arctiinii sp.2 x x Arctiinii sp.3 x x x Arctiinii sp.4 x x Ctenuchiini sp.1 x x x Arctiina Hypercompe mus (Oberthür, 1881)#$ x x Paracles phaeocera (Hampson, 1905)# x x x x Paracles sp.1 x x x x x x Paracles sp.2 x x Pseudalus limona Schaus, 1896# x x x x x Callimorphina Utetheisa ornatrix (Linnaeus, 1758) x x x x x x Ctenuchina Aclytia flavigutta (Walker, 1854)#$ x x x x x x Aclytia heber (Cramer, 1780)# x x x x x x Aclytia sp.1 x x x x Argyroeides braco (Herrich-Schäffer, [1855]# x x Cercopimorpha postflavia Rothschild, 1912#$ x x Correbidia calopteridia (Butler, 1878)#$ x x Correbidia sp.1 x x Delphyre discalis (Druce, 1905)# x x x x x x Delphyre dizona (Druce, 1898)# x x x x x x Episcepsis klagesi Rothschild, 1911#$ x x Episcepsis lenaeus (Cramer, 1780)# x x Episcepsis thetis (Linnaeus, 1771)#$ x x x Eucereon albidia Rothschild, 1912#$* x x x Eucereon arenosun Butler, 1877#$ x x Eucereon dorsipuncta Hampson, 1905# x x x Eucereon pseudarchias Hampson, 1898#$ x x Eucereon setosum (Sepp, [1830])#$ x x x x x Eucereon sp.1 x x x x x Heliura rhodophila (Walker, 1856)# x x Heliura tetragramma (Walker, 1854)# x x x x x x Napata leucotela Butler, 1876# x x Philoros rubriceps (Walker, 1854)# x x x x x Pseudohyaleucerea vulnerata (Butler, 1875)#$ x x Pseudosphex discoplaga (Schaus, 1905)# x x Pseudosphex fulvisphex (Druce, 1898)#$ x x x x Pseudosphex nivaca (Jones, 1914) x x x x x x Euchromiina Autochloris enagrus (Cramer, 1780)#$* x x x x Cosmosoma achemon (Fabricius, 1781)# x x x x x x Cosmosoma auge (Linnaeus, 1767)# x x x Cosmosoma nigriscens Rothschild, 1911# x x Cosmosoma rasera Jones, 1914# x x x x x Cosmosoma theuthras restrictum Butler, 1876# x x x x x x Cosmosoma sp.1 x x x x x Cosmosoma sp.2 x x Cosmosoma sp.3 x x x x x Dycladia lucetius (Stoll, 1781) x x x x x x Erruca hanga (Herrich-Schäffer, [1854])#$ x x Eurota histrio (Guérin, 1843)# x x Eurota nigricincta Hampson, 1907#$ x x Hyda basilutea (Walker, 1854)# x x x x Lepidoneiva erubescens (Butler, 1876) x x x x x x Macrocneme aurifera Hampson, 1914#$* x x x x x x Nyridela acroxantha (Perty, 1833)# x x x x Nyridela chalciope (Hübner, [1827])# x x Pheia albisigna (Walker, 1854) x x x x x x Pheia gaudens (Walker, 1856)# x x Pheia haematosticta Jones, 1908 x x x x x x Pheia haemopera Schaus, 1898 x x x x x x Pheia seraphina (Herrich-Schäffer, 1854) x x x x x x Pheia sp.1 x x x Phoenicoprocta baeri Rothschild, 1911 x x x x x Phoenicoprocta sp.1 x x x x Poliopastea plumbea Hampson, 1898#$ x x x x x Poliopastea sp.1 x x Saurita attenuata Hampson, 1905#$* x x x Sphecosoma aenetus (Schaus, 1896)#$* x x Pericopina Dysschema boisduvalli (van der Hoeven & de Vriese, 1840)# x x Dysschema sacrifica (Hübner, [1831])# x x x x Hyalurga fenestra (Linnaeus, 1758)# x x Hyalurga partita (Walker, 1854)#$* x x Phaegopterina Agaraea semivitrea Rothschild, 1909# x x Amaxia dyuna Schaus, 1896#$ x x x x x x Amaxia kennedyi (Rothschild, 1909)#$ x x Bertholdia detracta Seitz, 1921# x x Biturix diversipes (Walker, 1855)#$* x x Carales astur (Cramer, 1777)# x x Cresera affinis (Rothschild, 1909)# x x Cresera ilioides (Schaus, 1905)#$* x x Cresera optima (Butler, 1877)#$ x x x Echeta juno (Schaus, 1892)#$* x x Elysius hermia (Cramer, 1777)# x x Elysius joiceyi Talbot, 1928# x x x x Eupseudosoma grandis Rothschild, 1909#$ x x x x x Eupseudosoma involuta (Sepp, [1855])# x x x Halysidota sannionis (Rothschild, 1909)#$ x x x x x Hyperandra appendiculata (Herrich-Schäffer, [1856])# x x Hyperthaema sp.1 x x x x x Hyperthaema sp.2 x x x x Hyponerita lavinia (Druce, 1890)#$ x x Idalus agricus Dyar, 1910#$* x x x x Idalus carinosa (Schaus, 1905) x x x x x x Idalus citrina Druce, 1890#$ x x x x x x Idalus dares Druce, 1894# x x Idalus lineosus Walker, 1869# x x x x Lepidokirbyia vittipes (Walker, 1855)# x x x x x Leucanopsis rosetta (Schaus, 1896)# x x x x x Leucanopsis squalida (Herrich-Schäffer, [1855])#$ x x x Leucanopsis strigulosa (Walker, 1855)#$ x x x x x x Lophocampa annulosa (Walker, 1855)#$ x x Lophocampa atrimaculata (Hampson, 1901)#$* x x Lophocampa citrina (Sepp, [1852])# x x x x x Mazaeras francki Schaus, 1896# x x Melese incertus (Walker, 1855)# x x x Melese paranensis Dognin, 1911#$ x x x Neritos atta Schaus, 1920#$ x x x x Neritos flavimargo Joicey & Talbot, 1916 x x x Neritos hampsoni Rothschild, 1909#$ x x x x Neritos sanguipuncta Schaus, 1901# x x Pareuchaetes aurata (Butler, 1875)# x x x x x x Pelochyta arontes (Stoll, 1782)# x x Psychophasma erosa (Herrich-Schäffer, [1858])# x x x Rhipha pulcherrima (Rothschild, 1935)# x x x x x Rhipha strigosa (Walker, 1854)#$ x x x Robinsonia dewitzi Gundlach, 1881#$ x x Scaptius submarginalis (Rothschild, 1909)#$* x x Viviennea salma (Druce, 1896)#$ x x x x Lithosiini Lithosiinii sp. 1 x x x Lithosiinii sp. 2 x x x x x Lithosiinii sp. 3 x x x x Lithosiinii sp. 4 x x x Lithosiinii sp. 5 x x Lithosiinii sp. 6 x x Lithosiinii sp. 7 x x Lithosiinii sp. 8 x x x x x Lithosiinii sp. 9 x x Lithosiinii sp. 12 x x Lithosiinii sp. 14 x x x x x x Cisthenina Barsinella mirabilis Butler, 1878 x x x x Cisthene dives (Schaus, 1896)# x x x x Cisthene ruficollis (Schaus, 1896)#$ x x Cisthene subruba (Schaus, 1905)# x x x x x x Cisthene triplaga (Hampson, 1905)# x x x x x x Cisthene sp.1 x x x x x Cisthene sp.2 x x x x Cisthene sp.3 x x x Illice croesus Hampson, 1914#$ x x Illice griseola (Rothschild, 1913)#$* x x Odozana domina (Schaus, 1896) x x x x x Odozana obscura (Schaus, 1896) x x x x x x Talara grisea Schaus, 1896# x x x x x Eudesmiina Antona fallax (Butler, 1877)# x x Lithosina Agylla argentea (Walker, 1863)#$* x x x x x x Agylla marcata (Schaus, 1894)#$ x x x x Agylla sp.1 x x x x x x Apistosia judas Hübner, [1819]# x x Metalobosia diaxantha Hampson, 1914# x x x Nodozana jucunda Jones, 1914 x x x x x x Parablavia sadima (Schaus, 1896) x x x x x x ). Cerrado sensu stricto and semideciduous forest vegetation types had the highest species richness (98), followed by campo cerrado with 86 and campo sujo with 51 (Appendix 1 Appendix 1. List of Actiinae moth species sampled in four phytophysiognomies of the Emas National Park (ENP), in the dry and rainy seasons. CS means campo sujo, CC campo cerrado, CSS cerrado sensu stricto and SF semideciduous forest. Species with symbols #$* are new records for Cerrado, #$ for Goias State and # for ENP. Phytophysiognomy Season Species CS CC CSS SF Dry Rainy Arctiinae Arctiini Arctiinii sp.1 x x x x Arctiinii sp.2 x x Arctiinii sp.3 x x x Arctiinii sp.4 x x Ctenuchiini sp.1 x x x Arctiina Hypercompe mus (Oberthür, 1881)#$ x x Paracles phaeocera (Hampson, 1905)# x x x x Paracles sp.1 x x x x x x Paracles sp.2 x x Pseudalus limona Schaus, 1896# x x x x x Callimorphina Utetheisa ornatrix (Linnaeus, 1758) x x x x x x Ctenuchina Aclytia flavigutta (Walker, 1854)#$ x x x x x x Aclytia heber (Cramer, 1780)# x x x x x x Aclytia sp.1 x x x x Argyroeides braco (Herrich-Schäffer, [1855]# x x Cercopimorpha postflavia Rothschild, 1912#$ x x Correbidia calopteridia (Butler, 1878)#$ x x Correbidia sp.1 x x Delphyre discalis (Druce, 1905)# x x x x x x Delphyre dizona (Druce, 1898)# x x x x x x Episcepsis klagesi Rothschild, 1911#$ x x Episcepsis lenaeus (Cramer, 1780)# x x Episcepsis thetis (Linnaeus, 1771)#$ x x x Eucereon albidia Rothschild, 1912#$* x x x Eucereon arenosun Butler, 1877#$ x x Eucereon dorsipuncta Hampson, 1905# x x x Eucereon pseudarchias Hampson, 1898#$ x x Eucereon setosum (Sepp, [1830])#$ x x x x x Eucereon sp.1 x x x x x Heliura rhodophila (Walker, 1856)# x x Heliura tetragramma (Walker, 1854)# x x x x x x Napata leucotela Butler, 1876# x x Philoros rubriceps (Walker, 1854)# x x x x x Pseudohyaleucerea vulnerata (Butler, 1875)#$ x x Pseudosphex discoplaga (Schaus, 1905)# x x Pseudosphex fulvisphex (Druce, 1898)#$ x x x x Pseudosphex nivaca (Jones, 1914) x x x x x x Euchromiina Autochloris enagrus (Cramer, 1780)#$* x x x x Cosmosoma achemon (Fabricius, 1781)# x x x x x x Cosmosoma auge (Linnaeus, 1767)# x x x Cosmosoma nigriscens Rothschild, 1911# x x Cosmosoma rasera Jones, 1914# x x x x x Cosmosoma theuthras restrictum Butler, 1876# x x x x x x Cosmosoma sp.1 x x x x x Cosmosoma sp.2 x x Cosmosoma sp.3 x x x x x Dycladia lucetius (Stoll, 1781) x x x x x x Erruca hanga (Herrich-Schäffer, [1854])#$ x x Eurota histrio (Guérin, 1843)# x x Eurota nigricincta Hampson, 1907#$ x x Hyda basilutea (Walker, 1854)# x x x x Lepidoneiva erubescens (Butler, 1876) x x x x x x Macrocneme aurifera Hampson, 1914#$* x x x x x x Nyridela acroxantha (Perty, 1833)# x x x x Nyridela chalciope (Hübner, [1827])# x x Pheia albisigna (Walker, 1854) x x x x x x Pheia gaudens (Walker, 1856)# x x Pheia haematosticta Jones, 1908 x x x x x x Pheia haemopera Schaus, 1898 x x x x x x Pheia seraphina (Herrich-Schäffer, 1854) x x x x x x Pheia sp.1 x x x Phoenicoprocta baeri Rothschild, 1911 x x x x x Phoenicoprocta sp.1 x x x x Poliopastea plumbea Hampson, 1898#$ x x x x x Poliopastea sp.1 x x Saurita attenuata Hampson, 1905#$* x x x Sphecosoma aenetus (Schaus, 1896)#$* x x Pericopina Dysschema boisduvalli (van der Hoeven & de Vriese, 1840)# x x Dysschema sacrifica (Hübner, [1831])# x x x x Hyalurga fenestra (Linnaeus, 1758)# x x Hyalurga partita (Walker, 1854)#$* x x Phaegopterina Agaraea semivitrea Rothschild, 1909# x x Amaxia dyuna Schaus, 1896#$ x x x x x x Amaxia kennedyi (Rothschild, 1909)#$ x x Bertholdia detracta Seitz, 1921# x x Biturix diversipes (Walker, 1855)#$* x x Carales astur (Cramer, 1777)# x x Cresera affinis (Rothschild, 1909)# x x Cresera ilioides (Schaus, 1905)#$* x x Cresera optima (Butler, 1877)#$ x x x Echeta juno (Schaus, 1892)#$* x x Elysius hermia (Cramer, 1777)# x x Elysius joiceyi Talbot, 1928# x x x x Eupseudosoma grandis Rothschild, 1909#$ x x x x x Eupseudosoma involuta (Sepp, [1855])# x x x Halysidota sannionis (Rothschild, 1909)#$ x x x x x Hyperandra appendiculata (Herrich-Schäffer, [1856])# x x Hyperthaema sp.1 x x x x x Hyperthaema sp.2 x x x x Hyponerita lavinia (Druce, 1890)#$ x x Idalus agricus Dyar, 1910#$* x x x x Idalus carinosa (Schaus, 1905) x x x x x x Idalus citrina Druce, 1890#$ x x x x x x Idalus dares Druce, 1894# x x Idalus lineosus Walker, 1869# x x x x Lepidokirbyia vittipes (Walker, 1855)# x x x x x Leucanopsis rosetta (Schaus, 1896)# x x x x x Leucanopsis squalida (Herrich-Schäffer, [1855])#$ x x x Leucanopsis strigulosa (Walker, 1855)#$ x x x x x x Lophocampa annulosa (Walker, 1855)#$ x x Lophocampa atrimaculata (Hampson, 1901)#$* x x Lophocampa citrina (Sepp, [1852])# x x x x x Mazaeras francki Schaus, 1896# x x Melese incertus (Walker, 1855)# x x x Melese paranensis Dognin, 1911#$ x x x Neritos atta Schaus, 1920#$ x x x x Neritos flavimargo Joicey & Talbot, 1916 x x x Neritos hampsoni Rothschild, 1909#$ x x x x Neritos sanguipuncta Schaus, 1901# x x Pareuchaetes aurata (Butler, 1875)# x x x x x x Pelochyta arontes (Stoll, 1782)# x x Psychophasma erosa (Herrich-Schäffer, [1858])# x x x Rhipha pulcherrima (Rothschild, 1935)# x x x x x Rhipha strigosa (Walker, 1854)#$ x x x Robinsonia dewitzi Gundlach, 1881#$ x x Scaptius submarginalis (Rothschild, 1909)#$* x x Viviennea salma (Druce, 1896)#$ x x x x Lithosiini Lithosiinii sp. 1 x x x Lithosiinii sp. 2 x x x x x Lithosiinii sp. 3 x x x x Lithosiinii sp. 4 x x x Lithosiinii sp. 5 x x Lithosiinii sp. 6 x x Lithosiinii sp. 7 x x Lithosiinii sp. 8 x x x x x Lithosiinii sp. 9 x x Lithosiinii sp. 12 x x Lithosiinii sp. 14 x x x x x x Cisthenina Barsinella mirabilis Butler, 1878 x x x x Cisthene dives (Schaus, 1896)# x x x x Cisthene ruficollis (Schaus, 1896)#$ x x Cisthene subruba (Schaus, 1905)# x x x x x x Cisthene triplaga (Hampson, 1905)# x x x x x x Cisthene sp.1 x x x x x Cisthene sp.2 x x x x Cisthene sp.3 x x x Illice croesus Hampson, 1914#$ x x Illice griseola (Rothschild, 1913)#$* x x Odozana domina (Schaus, 1896) x x x x x Odozana obscura (Schaus, 1896) x x x x x x Talara grisea Schaus, 1896# x x x x x Eudesmiina Antona fallax (Butler, 1877)# x x Lithosina Agylla argentea (Walker, 1863)#$* x x x x x x Agylla marcata (Schaus, 1894)#$ x x x x Agylla sp.1 x x x x x x Apistosia judas Hübner, [1819]# x x Metalobosia diaxantha Hampson, 1914# x x x Nodozana jucunda Jones, 1914 x x x x x x Parablavia sadima (Schaus, 1896) x x x x x x ).
Observed (Obs. rich.) and extrapolated richness (first order Jackknife, second order Jackknife and second order Chao) and percentage of extrapolated richness sampled in each phytophysiognomy (Phyto) and also in the total study area (ENP). In the first column, CS means campo sujo, CC campo cerrado, CSS cerrado sensu stricto, SF semideciduous forest and ENP, Emas National Park.
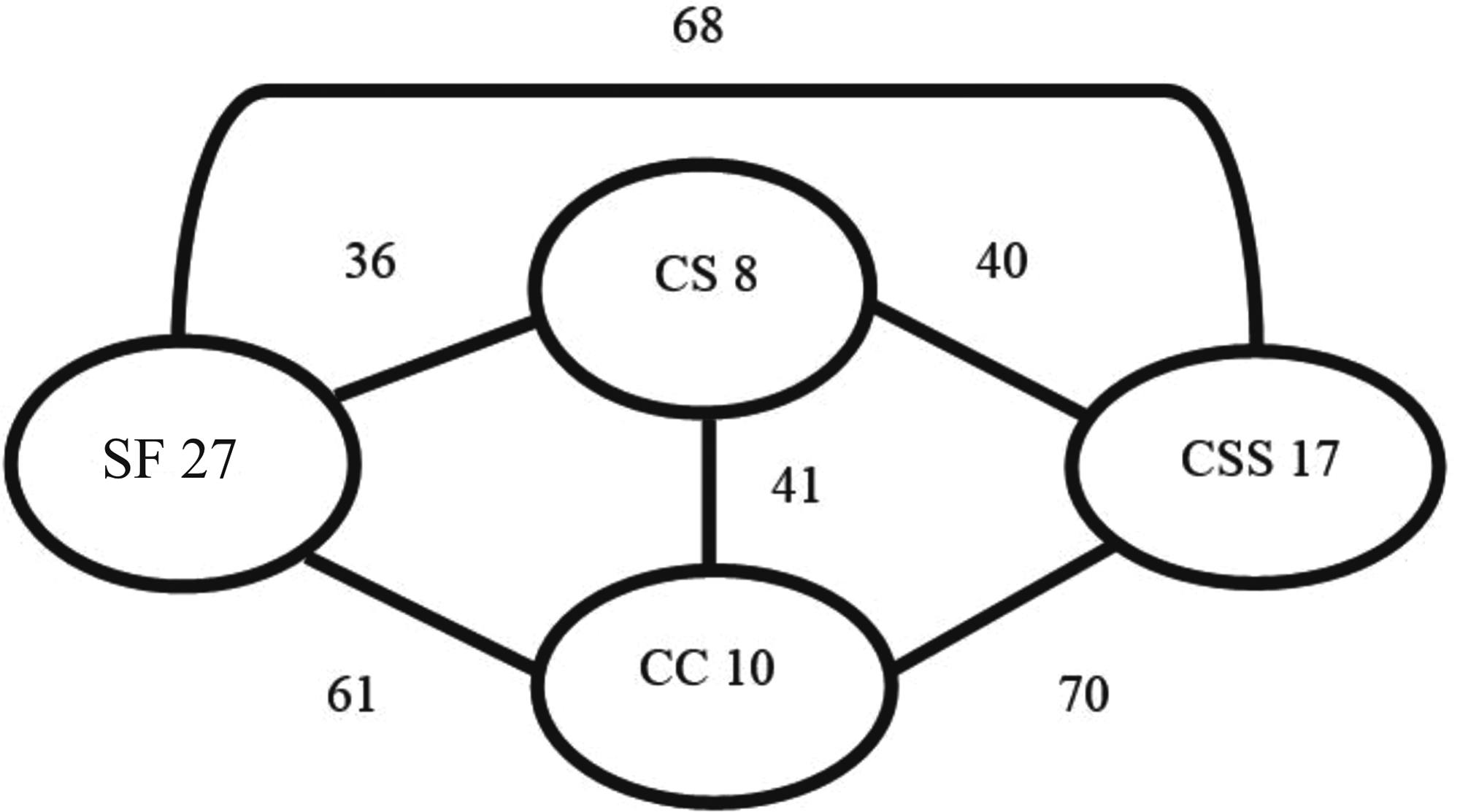
About 41% of the sampled species (n = 62) occurred in only one vegetation type, 16% (25 species) occurred in two vegetation types, 18% (27 species) in three and 23% (35 species) occurred in all vegetation types (Appendix 1 Appendix 1. List of Actiinae moth species sampled in four phytophysiognomies of the Emas National Park (ENP), in the dry and rainy seasons. CS means campo sujo, CC campo cerrado, CSS cerrado sensu stricto and SF semideciduous forest. Species with symbols #$* are new records for Cerrado, #$ for Goias State and # for ENP. Phytophysiognomy Season Species CS CC CSS SF Dry Rainy Arctiinae Arctiini Arctiinii sp.1 x x x x Arctiinii sp.2 x x Arctiinii sp.3 x x x Arctiinii sp.4 x x Ctenuchiini sp.1 x x x Arctiina Hypercompe mus (Oberthür, 1881)#$ x x Paracles phaeocera (Hampson, 1905)# x x x x Paracles sp.1 x x x x x x Paracles sp.2 x x Pseudalus limona Schaus, 1896# x x x x x Callimorphina Utetheisa ornatrix (Linnaeus, 1758) x x x x x x Ctenuchina Aclytia flavigutta (Walker, 1854)#$ x x x x x x Aclytia heber (Cramer, 1780)# x x x x x x Aclytia sp.1 x x x x Argyroeides braco (Herrich-Schäffer, [1855]# x x Cercopimorpha postflavia Rothschild, 1912#$ x x Correbidia calopteridia (Butler, 1878)#$ x x Correbidia sp.1 x x Delphyre discalis (Druce, 1905)# x x x x x x Delphyre dizona (Druce, 1898)# x x x x x x Episcepsis klagesi Rothschild, 1911#$ x x Episcepsis lenaeus (Cramer, 1780)# x x Episcepsis thetis (Linnaeus, 1771)#$ x x x Eucereon albidia Rothschild, 1912#$* x x x Eucereon arenosun Butler, 1877#$ x x Eucereon dorsipuncta Hampson, 1905# x x x Eucereon pseudarchias Hampson, 1898#$ x x Eucereon setosum (Sepp, [1830])#$ x x x x x Eucereon sp.1 x x x x x Heliura rhodophila (Walker, 1856)# x x Heliura tetragramma (Walker, 1854)# x x x x x x Napata leucotela Butler, 1876# x x Philoros rubriceps (Walker, 1854)# x x x x x Pseudohyaleucerea vulnerata (Butler, 1875)#$ x x Pseudosphex discoplaga (Schaus, 1905)# x x Pseudosphex fulvisphex (Druce, 1898)#$ x x x x Pseudosphex nivaca (Jones, 1914) x x x x x x Euchromiina Autochloris enagrus (Cramer, 1780)#$* x x x x Cosmosoma achemon (Fabricius, 1781)# x x x x x x Cosmosoma auge (Linnaeus, 1767)# x x x Cosmosoma nigriscens Rothschild, 1911# x x Cosmosoma rasera Jones, 1914# x x x x x Cosmosoma theuthras restrictum Butler, 1876# x x x x x x Cosmosoma sp.1 x x x x x Cosmosoma sp.2 x x Cosmosoma sp.3 x x x x x Dycladia lucetius (Stoll, 1781) x x x x x x Erruca hanga (Herrich-Schäffer, [1854])#$ x x Eurota histrio (Guérin, 1843)# x x Eurota nigricincta Hampson, 1907#$ x x Hyda basilutea (Walker, 1854)# x x x x Lepidoneiva erubescens (Butler, 1876) x x x x x x Macrocneme aurifera Hampson, 1914#$* x x x x x x Nyridela acroxantha (Perty, 1833)# x x x x Nyridela chalciope (Hübner, [1827])# x x Pheia albisigna (Walker, 1854) x x x x x x Pheia gaudens (Walker, 1856)# x x Pheia haematosticta Jones, 1908 x x x x x x Pheia haemopera Schaus, 1898 x x x x x x Pheia seraphina (Herrich-Schäffer, 1854) x x x x x x Pheia sp.1 x x x Phoenicoprocta baeri Rothschild, 1911 x x x x x Phoenicoprocta sp.1 x x x x Poliopastea plumbea Hampson, 1898#$ x x x x x Poliopastea sp.1 x x Saurita attenuata Hampson, 1905#$* x x x Sphecosoma aenetus (Schaus, 1896)#$* x x Pericopina Dysschema boisduvalli (van der Hoeven & de Vriese, 1840)# x x Dysschema sacrifica (Hübner, [1831])# x x x x Hyalurga fenestra (Linnaeus, 1758)# x x Hyalurga partita (Walker, 1854)#$* x x Phaegopterina Agaraea semivitrea Rothschild, 1909# x x Amaxia dyuna Schaus, 1896#$ x x x x x x Amaxia kennedyi (Rothschild, 1909)#$ x x Bertholdia detracta Seitz, 1921# x x Biturix diversipes (Walker, 1855)#$* x x Carales astur (Cramer, 1777)# x x Cresera affinis (Rothschild, 1909)# x x Cresera ilioides (Schaus, 1905)#$* x x Cresera optima (Butler, 1877)#$ x x x Echeta juno (Schaus, 1892)#$* x x Elysius hermia (Cramer, 1777)# x x Elysius joiceyi Talbot, 1928# x x x x Eupseudosoma grandis Rothschild, 1909#$ x x x x x Eupseudosoma involuta (Sepp, [1855])# x x x Halysidota sannionis (Rothschild, 1909)#$ x x x x x Hyperandra appendiculata (Herrich-Schäffer, [1856])# x x Hyperthaema sp.1 x x x x x Hyperthaema sp.2 x x x x Hyponerita lavinia (Druce, 1890)#$ x x Idalus agricus Dyar, 1910#$* x x x x Idalus carinosa (Schaus, 1905) x x x x x x Idalus citrina Druce, 1890#$ x x x x x x Idalus dares Druce, 1894# x x Idalus lineosus Walker, 1869# x x x x Lepidokirbyia vittipes (Walker, 1855)# x x x x x Leucanopsis rosetta (Schaus, 1896)# x x x x x Leucanopsis squalida (Herrich-Schäffer, [1855])#$ x x x Leucanopsis strigulosa (Walker, 1855)#$ x x x x x x Lophocampa annulosa (Walker, 1855)#$ x x Lophocampa atrimaculata (Hampson, 1901)#$* x x Lophocampa citrina (Sepp, [1852])# x x x x x Mazaeras francki Schaus, 1896# x x Melese incertus (Walker, 1855)# x x x Melese paranensis Dognin, 1911#$ x x x Neritos atta Schaus, 1920#$ x x x x Neritos flavimargo Joicey & Talbot, 1916 x x x Neritos hampsoni Rothschild, 1909#$ x x x x Neritos sanguipuncta Schaus, 1901# x x Pareuchaetes aurata (Butler, 1875)# x x x x x x Pelochyta arontes (Stoll, 1782)# x x Psychophasma erosa (Herrich-Schäffer, [1858])# x x x Rhipha pulcherrima (Rothschild, 1935)# x x x x x Rhipha strigosa (Walker, 1854)#$ x x x Robinsonia dewitzi Gundlach, 1881#$ x x Scaptius submarginalis (Rothschild, 1909)#$* x x Viviennea salma (Druce, 1896)#$ x x x x Lithosiini Lithosiinii sp. 1 x x x Lithosiinii sp. 2 x x x x x Lithosiinii sp. 3 x x x x Lithosiinii sp. 4 x x x Lithosiinii sp. 5 x x Lithosiinii sp. 6 x x Lithosiinii sp. 7 x x Lithosiinii sp. 8 x x x x x Lithosiinii sp. 9 x x Lithosiinii sp. 12 x x Lithosiinii sp. 14 x x x x x x Cisthenina Barsinella mirabilis Butler, 1878 x x x x Cisthene dives (Schaus, 1896)# x x x x Cisthene ruficollis (Schaus, 1896)#$ x x Cisthene subruba (Schaus, 1905)# x x x x x x Cisthene triplaga (Hampson, 1905)# x x x x x x Cisthene sp.1 x x x x x Cisthene sp.2 x x x x Cisthene sp.3 x x x Illice croesus Hampson, 1914#$ x x Illice griseola (Rothschild, 1913)#$* x x Odozana domina (Schaus, 1896) x x x x x Odozana obscura (Schaus, 1896) x x x x x x Talara grisea Schaus, 1896# x x x x x Eudesmiina Antona fallax (Butler, 1877)# x x Lithosina Agylla argentea (Walker, 1863)#$* x x x x x x Agylla marcata (Schaus, 1894)#$ x x x x Agylla sp.1 x x x x x x Apistosia judas Hübner, [1819]# x x Metalobosia diaxantha Hampson, 1914# x x x Nodozana jucunda Jones, 1914 x x x x x x Parablavia sadima (Schaus, 1896) x x x x x x ). The semideciduous forest had the highest number of exclusive species (27), followed by cerrado sensu stricto (17), campo cerrado (10) and campo sujo (eight) (Figure 2, Appendix 1 Appendix 1. List of Actiinae moth species sampled in four phytophysiognomies of the Emas National Park (ENP), in the dry and rainy seasons. CS means campo sujo, CC campo cerrado, CSS cerrado sensu stricto and SF semideciduous forest. Species with symbols #$* are new records for Cerrado, #$ for Goias State and # for ENP. Phytophysiognomy Season Species CS CC CSS SF Dry Rainy Arctiinae Arctiini Arctiinii sp.1 x x x x Arctiinii sp.2 x x Arctiinii sp.3 x x x Arctiinii sp.4 x x Ctenuchiini sp.1 x x x Arctiina Hypercompe mus (Oberthür, 1881)#$ x x Paracles phaeocera (Hampson, 1905)# x x x x Paracles sp.1 x x x x x x Paracles sp.2 x x Pseudalus limona Schaus, 1896# x x x x x Callimorphina Utetheisa ornatrix (Linnaeus, 1758) x x x x x x Ctenuchina Aclytia flavigutta (Walker, 1854)#$ x x x x x x Aclytia heber (Cramer, 1780)# x x x x x x Aclytia sp.1 x x x x Argyroeides braco (Herrich-Schäffer, [1855]# x x Cercopimorpha postflavia Rothschild, 1912#$ x x Correbidia calopteridia (Butler, 1878)#$ x x Correbidia sp.1 x x Delphyre discalis (Druce, 1905)# x x x x x x Delphyre dizona (Druce, 1898)# x x x x x x Episcepsis klagesi Rothschild, 1911#$ x x Episcepsis lenaeus (Cramer, 1780)# x x Episcepsis thetis (Linnaeus, 1771)#$ x x x Eucereon albidia Rothschild, 1912#$* x x x Eucereon arenosun Butler, 1877#$ x x Eucereon dorsipuncta Hampson, 1905# x x x Eucereon pseudarchias Hampson, 1898#$ x x Eucereon setosum (Sepp, [1830])#$ x x x x x Eucereon sp.1 x x x x x Heliura rhodophila (Walker, 1856)# x x Heliura tetragramma (Walker, 1854)# x x x x x x Napata leucotela Butler, 1876# x x Philoros rubriceps (Walker, 1854)# x x x x x Pseudohyaleucerea vulnerata (Butler, 1875)#$ x x Pseudosphex discoplaga (Schaus, 1905)# x x Pseudosphex fulvisphex (Druce, 1898)#$ x x x x Pseudosphex nivaca (Jones, 1914) x x x x x x Euchromiina Autochloris enagrus (Cramer, 1780)#$* x x x x Cosmosoma achemon (Fabricius, 1781)# x x x x x x Cosmosoma auge (Linnaeus, 1767)# x x x Cosmosoma nigriscens Rothschild, 1911# x x Cosmosoma rasera Jones, 1914# x x x x x Cosmosoma theuthras restrictum Butler, 1876# x x x x x x Cosmosoma sp.1 x x x x x Cosmosoma sp.2 x x Cosmosoma sp.3 x x x x x Dycladia lucetius (Stoll, 1781) x x x x x x Erruca hanga (Herrich-Schäffer, [1854])#$ x x Eurota histrio (Guérin, 1843)# x x Eurota nigricincta Hampson, 1907#$ x x Hyda basilutea (Walker, 1854)# x x x x Lepidoneiva erubescens (Butler, 1876) x x x x x x Macrocneme aurifera Hampson, 1914#$* x x x x x x Nyridela acroxantha (Perty, 1833)# x x x x Nyridela chalciope (Hübner, [1827])# x x Pheia albisigna (Walker, 1854) x x x x x x Pheia gaudens (Walker, 1856)# x x Pheia haematosticta Jones, 1908 x x x x x x Pheia haemopera Schaus, 1898 x x x x x x Pheia seraphina (Herrich-Schäffer, 1854) x x x x x x Pheia sp.1 x x x Phoenicoprocta baeri Rothschild, 1911 x x x x x Phoenicoprocta sp.1 x x x x Poliopastea plumbea Hampson, 1898#$ x x x x x Poliopastea sp.1 x x Saurita attenuata Hampson, 1905#$* x x x Sphecosoma aenetus (Schaus, 1896)#$* x x Pericopina Dysschema boisduvalli (van der Hoeven & de Vriese, 1840)# x x Dysschema sacrifica (Hübner, [1831])# x x x x Hyalurga fenestra (Linnaeus, 1758)# x x Hyalurga partita (Walker, 1854)#$* x x Phaegopterina Agaraea semivitrea Rothschild, 1909# x x Amaxia dyuna Schaus, 1896#$ x x x x x x Amaxia kennedyi (Rothschild, 1909)#$ x x Bertholdia detracta Seitz, 1921# x x Biturix diversipes (Walker, 1855)#$* x x Carales astur (Cramer, 1777)# x x Cresera affinis (Rothschild, 1909)# x x Cresera ilioides (Schaus, 1905)#$* x x Cresera optima (Butler, 1877)#$ x x x Echeta juno (Schaus, 1892)#$* x x Elysius hermia (Cramer, 1777)# x x Elysius joiceyi Talbot, 1928# x x x x Eupseudosoma grandis Rothschild, 1909#$ x x x x x Eupseudosoma involuta (Sepp, [1855])# x x x Halysidota sannionis (Rothschild, 1909)#$ x x x x x Hyperandra appendiculata (Herrich-Schäffer, [1856])# x x Hyperthaema sp.1 x x x x x Hyperthaema sp.2 x x x x Hyponerita lavinia (Druce, 1890)#$ x x Idalus agricus Dyar, 1910#$* x x x x Idalus carinosa (Schaus, 1905) x x x x x x Idalus citrina Druce, 1890#$ x x x x x x Idalus dares Druce, 1894# x x Idalus lineosus Walker, 1869# x x x x Lepidokirbyia vittipes (Walker, 1855)# x x x x x Leucanopsis rosetta (Schaus, 1896)# x x x x x Leucanopsis squalida (Herrich-Schäffer, [1855])#$ x x x Leucanopsis strigulosa (Walker, 1855)#$ x x x x x x Lophocampa annulosa (Walker, 1855)#$ x x Lophocampa atrimaculata (Hampson, 1901)#$* x x Lophocampa citrina (Sepp, [1852])# x x x x x Mazaeras francki Schaus, 1896# x x Melese incertus (Walker, 1855)# x x x Melese paranensis Dognin, 1911#$ x x x Neritos atta Schaus, 1920#$ x x x x Neritos flavimargo Joicey & Talbot, 1916 x x x Neritos hampsoni Rothschild, 1909#$ x x x x Neritos sanguipuncta Schaus, 1901# x x Pareuchaetes aurata (Butler, 1875)# x x x x x x Pelochyta arontes (Stoll, 1782)# x x Psychophasma erosa (Herrich-Schäffer, [1858])# x x x Rhipha pulcherrima (Rothschild, 1935)# x x x x x Rhipha strigosa (Walker, 1854)#$ x x x Robinsonia dewitzi Gundlach, 1881#$ x x Scaptius submarginalis (Rothschild, 1909)#$* x x Viviennea salma (Druce, 1896)#$ x x x x Lithosiini Lithosiinii sp. 1 x x x Lithosiinii sp. 2 x x x x x Lithosiinii sp. 3 x x x x Lithosiinii sp. 4 x x x Lithosiinii sp. 5 x x Lithosiinii sp. 6 x x Lithosiinii sp. 7 x x Lithosiinii sp. 8 x x x x x Lithosiinii sp. 9 x x Lithosiinii sp. 12 x x Lithosiinii sp. 14 x x x x x x Cisthenina Barsinella mirabilis Butler, 1878 x x x x Cisthene dives (Schaus, 1896)# x x x x Cisthene ruficollis (Schaus, 1896)#$ x x Cisthene subruba (Schaus, 1905)# x x x x x x Cisthene triplaga (Hampson, 1905)# x x x x x x Cisthene sp.1 x x x x x Cisthene sp.2 x x x x Cisthene sp.3 x x x Illice croesus Hampson, 1914#$ x x Illice griseola (Rothschild, 1913)#$* x x Odozana domina (Schaus, 1896) x x x x x Odozana obscura (Schaus, 1896) x x x x x x Talara grisea Schaus, 1896# x x x x x Eudesmiina Antona fallax (Butler, 1877)# x x Lithosina Agylla argentea (Walker, 1863)#$* x x x x x x Agylla marcata (Schaus, 1894)#$ x x x x Agylla sp.1 x x x x x x Apistosia judas Hübner, [1819]# x x Metalobosia diaxantha Hampson, 1914# x x x Nodozana jucunda Jones, 1914 x x x x x x Parablavia sadima (Schaus, 1896) x x x x x x ). Campo cerrado and cerrado sensu stricto had more shared species and campo sujo and semideciduous forest had the least shared species (Figure 2, Appendix 1 Appendix 1. List of Actiinae moth species sampled in four phytophysiognomies of the Emas National Park (ENP), in the dry and rainy seasons. CS means campo sujo, CC campo cerrado, CSS cerrado sensu stricto and SF semideciduous forest. Species with symbols #$* are new records for Cerrado, #$ for Goias State and # for ENP. Phytophysiognomy Season Species CS CC CSS SF Dry Rainy Arctiinae Arctiini Arctiinii sp.1 x x x x Arctiinii sp.2 x x Arctiinii sp.3 x x x Arctiinii sp.4 x x Ctenuchiini sp.1 x x x Arctiina Hypercompe mus (Oberthür, 1881)#$ x x Paracles phaeocera (Hampson, 1905)# x x x x Paracles sp.1 x x x x x x Paracles sp.2 x x Pseudalus limona Schaus, 1896# x x x x x Callimorphina Utetheisa ornatrix (Linnaeus, 1758) x x x x x x Ctenuchina Aclytia flavigutta (Walker, 1854)#$ x x x x x x Aclytia heber (Cramer, 1780)# x x x x x x Aclytia sp.1 x x x x Argyroeides braco (Herrich-Schäffer, [1855]# x x Cercopimorpha postflavia Rothschild, 1912#$ x x Correbidia calopteridia (Butler, 1878)#$ x x Correbidia sp.1 x x Delphyre discalis (Druce, 1905)# x x x x x x Delphyre dizona (Druce, 1898)# x x x x x x Episcepsis klagesi Rothschild, 1911#$ x x Episcepsis lenaeus (Cramer, 1780)# x x Episcepsis thetis (Linnaeus, 1771)#$ x x x Eucereon albidia Rothschild, 1912#$* x x x Eucereon arenosun Butler, 1877#$ x x Eucereon dorsipuncta Hampson, 1905# x x x Eucereon pseudarchias Hampson, 1898#$ x x Eucereon setosum (Sepp, [1830])#$ x x x x x Eucereon sp.1 x x x x x Heliura rhodophila (Walker, 1856)# x x Heliura tetragramma (Walker, 1854)# x x x x x x Napata leucotela Butler, 1876# x x Philoros rubriceps (Walker, 1854)# x x x x x Pseudohyaleucerea vulnerata (Butler, 1875)#$ x x Pseudosphex discoplaga (Schaus, 1905)# x x Pseudosphex fulvisphex (Druce, 1898)#$ x x x x Pseudosphex nivaca (Jones, 1914) x x x x x x Euchromiina Autochloris enagrus (Cramer, 1780)#$* x x x x Cosmosoma achemon (Fabricius, 1781)# x x x x x x Cosmosoma auge (Linnaeus, 1767)# x x x Cosmosoma nigriscens Rothschild, 1911# x x Cosmosoma rasera Jones, 1914# x x x x x Cosmosoma theuthras restrictum Butler, 1876# x x x x x x Cosmosoma sp.1 x x x x x Cosmosoma sp.2 x x Cosmosoma sp.3 x x x x x Dycladia lucetius (Stoll, 1781) x x x x x x Erruca hanga (Herrich-Schäffer, [1854])#$ x x Eurota histrio (Guérin, 1843)# x x Eurota nigricincta Hampson, 1907#$ x x Hyda basilutea (Walker, 1854)# x x x x Lepidoneiva erubescens (Butler, 1876) x x x x x x Macrocneme aurifera Hampson, 1914#$* x x x x x x Nyridela acroxantha (Perty, 1833)# x x x x Nyridela chalciope (Hübner, [1827])# x x Pheia albisigna (Walker, 1854) x x x x x x Pheia gaudens (Walker, 1856)# x x Pheia haematosticta Jones, 1908 x x x x x x Pheia haemopera Schaus, 1898 x x x x x x Pheia seraphina (Herrich-Schäffer, 1854) x x x x x x Pheia sp.1 x x x Phoenicoprocta baeri Rothschild, 1911 x x x x x Phoenicoprocta sp.1 x x x x Poliopastea plumbea Hampson, 1898#$ x x x x x Poliopastea sp.1 x x Saurita attenuata Hampson, 1905#$* x x x Sphecosoma aenetus (Schaus, 1896)#$* x x Pericopina Dysschema boisduvalli (van der Hoeven & de Vriese, 1840)# x x Dysschema sacrifica (Hübner, [1831])# x x x x Hyalurga fenestra (Linnaeus, 1758)# x x Hyalurga partita (Walker, 1854)#$* x x Phaegopterina Agaraea semivitrea Rothschild, 1909# x x Amaxia dyuna Schaus, 1896#$ x x x x x x Amaxia kennedyi (Rothschild, 1909)#$ x x Bertholdia detracta Seitz, 1921# x x Biturix diversipes (Walker, 1855)#$* x x Carales astur (Cramer, 1777)# x x Cresera affinis (Rothschild, 1909)# x x Cresera ilioides (Schaus, 1905)#$* x x Cresera optima (Butler, 1877)#$ x x x Echeta juno (Schaus, 1892)#$* x x Elysius hermia (Cramer, 1777)# x x Elysius joiceyi Talbot, 1928# x x x x Eupseudosoma grandis Rothschild, 1909#$ x x x x x Eupseudosoma involuta (Sepp, [1855])# x x x Halysidota sannionis (Rothschild, 1909)#$ x x x x x Hyperandra appendiculata (Herrich-Schäffer, [1856])# x x Hyperthaema sp.1 x x x x x Hyperthaema sp.2 x x x x Hyponerita lavinia (Druce, 1890)#$ x x Idalus agricus Dyar, 1910#$* x x x x Idalus carinosa (Schaus, 1905) x x x x x x Idalus citrina Druce, 1890#$ x x x x x x Idalus dares Druce, 1894# x x Idalus lineosus Walker, 1869# x x x x Lepidokirbyia vittipes (Walker, 1855)# x x x x x Leucanopsis rosetta (Schaus, 1896)# x x x x x Leucanopsis squalida (Herrich-Schäffer, [1855])#$ x x x Leucanopsis strigulosa (Walker, 1855)#$ x x x x x x Lophocampa annulosa (Walker, 1855)#$ x x Lophocampa atrimaculata (Hampson, 1901)#$* x x Lophocampa citrina (Sepp, [1852])# x x x x x Mazaeras francki Schaus, 1896# x x Melese incertus (Walker, 1855)# x x x Melese paranensis Dognin, 1911#$ x x x Neritos atta Schaus, 1920#$ x x x x Neritos flavimargo Joicey & Talbot, 1916 x x x Neritos hampsoni Rothschild, 1909#$ x x x x Neritos sanguipuncta Schaus, 1901# x x Pareuchaetes aurata (Butler, 1875)# x x x x x x Pelochyta arontes (Stoll, 1782)# x x Psychophasma erosa (Herrich-Schäffer, [1858])# x x x Rhipha pulcherrima (Rothschild, 1935)# x x x x x Rhipha strigosa (Walker, 1854)#$ x x x Robinsonia dewitzi Gundlach, 1881#$ x x Scaptius submarginalis (Rothschild, 1909)#$* x x Viviennea salma (Druce, 1896)#$ x x x x Lithosiini Lithosiinii sp. 1 x x x Lithosiinii sp. 2 x x x x x Lithosiinii sp. 3 x x x x Lithosiinii sp. 4 x x x Lithosiinii sp. 5 x x Lithosiinii sp. 6 x x Lithosiinii sp. 7 x x Lithosiinii sp. 8 x x x x x Lithosiinii sp. 9 x x Lithosiinii sp. 12 x x Lithosiinii sp. 14 x x x x x x Cisthenina Barsinella mirabilis Butler, 1878 x x x x Cisthene dives (Schaus, 1896)# x x x x Cisthene ruficollis (Schaus, 1896)#$ x x Cisthene subruba (Schaus, 1905)# x x x x x x Cisthene triplaga (Hampson, 1905)# x x x x x x Cisthene sp.1 x x x x x Cisthene sp.2 x x x x Cisthene sp.3 x x x Illice croesus Hampson, 1914#$ x x Illice griseola (Rothschild, 1913)#$* x x Odozana domina (Schaus, 1896) x x x x x Odozana obscura (Schaus, 1896) x x x x x x Talara grisea Schaus, 1896# x x x x x Eudesmiina Antona fallax (Butler, 1877)# x x Lithosina Agylla argentea (Walker, 1863)#$* x x x x x x Agylla marcata (Schaus, 1894)#$ x x x x Agylla sp.1 x x x x x x Apistosia judas Hübner, [1819]# x x Metalobosia diaxantha Hampson, 1914# x x x Nodozana jucunda Jones, 1914 x x x x x x Parablavia sadima (Schaus, 1896) x x x x x x ). Most species that occurred in three vegetation types (88.8%) were shared between campo cerrado, cerrado sensu stricto, and semideciduous forest.
Venn diagram indicating the number of species that were sampled only in a vegetation type (numbers within the ellipses) and the number of shared species between vegetation types (numbers next to the lines). Campo sujo (CS), campo cerrado (CC), cerrado sensu stricto (CSS) and semideciduous forest (SF).
Some species ocurred in only one season (58 species ocurred only in the dry and 30 species only in the rainy season) and the others species in both seasons (N = 61). The dry showed more species (N = 119) than the rainy season (N = 91) (Appendix 1 Appendix 1. List of Actiinae moth species sampled in four phytophysiognomies of the Emas National Park (ENP), in the dry and rainy seasons. CS means campo sujo, CC campo cerrado, CSS cerrado sensu stricto and SF semideciduous forest. Species with symbols #$* are new records for Cerrado, #$ for Goias State and # for ENP. Phytophysiognomy Season Species CS CC CSS SF Dry Rainy Arctiinae Arctiini Arctiinii sp.1 x x x x Arctiinii sp.2 x x Arctiinii sp.3 x x x Arctiinii sp.4 x x Ctenuchiini sp.1 x x x Arctiina Hypercompe mus (Oberthür, 1881)#$ x x Paracles phaeocera (Hampson, 1905)# x x x x Paracles sp.1 x x x x x x Paracles sp.2 x x Pseudalus limona Schaus, 1896# x x x x x Callimorphina Utetheisa ornatrix (Linnaeus, 1758) x x x x x x Ctenuchina Aclytia flavigutta (Walker, 1854)#$ x x x x x x Aclytia heber (Cramer, 1780)# x x x x x x Aclytia sp.1 x x x x Argyroeides braco (Herrich-Schäffer, [1855]# x x Cercopimorpha postflavia Rothschild, 1912#$ x x Correbidia calopteridia (Butler, 1878)#$ x x Correbidia sp.1 x x Delphyre discalis (Druce, 1905)# x x x x x x Delphyre dizona (Druce, 1898)# x x x x x x Episcepsis klagesi Rothschild, 1911#$ x x Episcepsis lenaeus (Cramer, 1780)# x x Episcepsis thetis (Linnaeus, 1771)#$ x x x Eucereon albidia Rothschild, 1912#$* x x x Eucereon arenosun Butler, 1877#$ x x Eucereon dorsipuncta Hampson, 1905# x x x Eucereon pseudarchias Hampson, 1898#$ x x Eucereon setosum (Sepp, [1830])#$ x x x x x Eucereon sp.1 x x x x x Heliura rhodophila (Walker, 1856)# x x Heliura tetragramma (Walker, 1854)# x x x x x x Napata leucotela Butler, 1876# x x Philoros rubriceps (Walker, 1854)# x x x x x Pseudohyaleucerea vulnerata (Butler, 1875)#$ x x Pseudosphex discoplaga (Schaus, 1905)# x x Pseudosphex fulvisphex (Druce, 1898)#$ x x x x Pseudosphex nivaca (Jones, 1914) x x x x x x Euchromiina Autochloris enagrus (Cramer, 1780)#$* x x x x Cosmosoma achemon (Fabricius, 1781)# x x x x x x Cosmosoma auge (Linnaeus, 1767)# x x x Cosmosoma nigriscens Rothschild, 1911# x x Cosmosoma rasera Jones, 1914# x x x x x Cosmosoma theuthras restrictum Butler, 1876# x x x x x x Cosmosoma sp.1 x x x x x Cosmosoma sp.2 x x Cosmosoma sp.3 x x x x x Dycladia lucetius (Stoll, 1781) x x x x x x Erruca hanga (Herrich-Schäffer, [1854])#$ x x Eurota histrio (Guérin, 1843)# x x Eurota nigricincta Hampson, 1907#$ x x Hyda basilutea (Walker, 1854)# x x x x Lepidoneiva erubescens (Butler, 1876) x x x x x x Macrocneme aurifera Hampson, 1914#$* x x x x x x Nyridela acroxantha (Perty, 1833)# x x x x Nyridela chalciope (Hübner, [1827])# x x Pheia albisigna (Walker, 1854) x x x x x x Pheia gaudens (Walker, 1856)# x x Pheia haematosticta Jones, 1908 x x x x x x Pheia haemopera Schaus, 1898 x x x x x x Pheia seraphina (Herrich-Schäffer, 1854) x x x x x x Pheia sp.1 x x x Phoenicoprocta baeri Rothschild, 1911 x x x x x Phoenicoprocta sp.1 x x x x Poliopastea plumbea Hampson, 1898#$ x x x x x Poliopastea sp.1 x x Saurita attenuata Hampson, 1905#$* x x x Sphecosoma aenetus (Schaus, 1896)#$* x x Pericopina Dysschema boisduvalli (van der Hoeven & de Vriese, 1840)# x x Dysschema sacrifica (Hübner, [1831])# x x x x Hyalurga fenestra (Linnaeus, 1758)# x x Hyalurga partita (Walker, 1854)#$* x x Phaegopterina Agaraea semivitrea Rothschild, 1909# x x Amaxia dyuna Schaus, 1896#$ x x x x x x Amaxia kennedyi (Rothschild, 1909)#$ x x Bertholdia detracta Seitz, 1921# x x Biturix diversipes (Walker, 1855)#$* x x Carales astur (Cramer, 1777)# x x Cresera affinis (Rothschild, 1909)# x x Cresera ilioides (Schaus, 1905)#$* x x Cresera optima (Butler, 1877)#$ x x x Echeta juno (Schaus, 1892)#$* x x Elysius hermia (Cramer, 1777)# x x Elysius joiceyi Talbot, 1928# x x x x Eupseudosoma grandis Rothschild, 1909#$ x x x x x Eupseudosoma involuta (Sepp, [1855])# x x x Halysidota sannionis (Rothschild, 1909)#$ x x x x x Hyperandra appendiculata (Herrich-Schäffer, [1856])# x x Hyperthaema sp.1 x x x x x Hyperthaema sp.2 x x x x Hyponerita lavinia (Druce, 1890)#$ x x Idalus agricus Dyar, 1910#$* x x x x Idalus carinosa (Schaus, 1905) x x x x x x Idalus citrina Druce, 1890#$ x x x x x x Idalus dares Druce, 1894# x x Idalus lineosus Walker, 1869# x x x x Lepidokirbyia vittipes (Walker, 1855)# x x x x x Leucanopsis rosetta (Schaus, 1896)# x x x x x Leucanopsis squalida (Herrich-Schäffer, [1855])#$ x x x Leucanopsis strigulosa (Walker, 1855)#$ x x x x x x Lophocampa annulosa (Walker, 1855)#$ x x Lophocampa atrimaculata (Hampson, 1901)#$* x x Lophocampa citrina (Sepp, [1852])# x x x x x Mazaeras francki Schaus, 1896# x x Melese incertus (Walker, 1855)# x x x Melese paranensis Dognin, 1911#$ x x x Neritos atta Schaus, 1920#$ x x x x Neritos flavimargo Joicey & Talbot, 1916 x x x Neritos hampsoni Rothschild, 1909#$ x x x x Neritos sanguipuncta Schaus, 1901# x x Pareuchaetes aurata (Butler, 1875)# x x x x x x Pelochyta arontes (Stoll, 1782)# x x Psychophasma erosa (Herrich-Schäffer, [1858])# x x x Rhipha pulcherrima (Rothschild, 1935)# x x x x x Rhipha strigosa (Walker, 1854)#$ x x x Robinsonia dewitzi Gundlach, 1881#$ x x Scaptius submarginalis (Rothschild, 1909)#$* x x Viviennea salma (Druce, 1896)#$ x x x x Lithosiini Lithosiinii sp. 1 x x x Lithosiinii sp. 2 x x x x x Lithosiinii sp. 3 x x x x Lithosiinii sp. 4 x x x Lithosiinii sp. 5 x x Lithosiinii sp. 6 x x Lithosiinii sp. 7 x x Lithosiinii sp. 8 x x x x x Lithosiinii sp. 9 x x Lithosiinii sp. 12 x x Lithosiinii sp. 14 x x x x x x Cisthenina Barsinella mirabilis Butler, 1878 x x x x Cisthene dives (Schaus, 1896)# x x x x Cisthene ruficollis (Schaus, 1896)#$ x x Cisthene subruba (Schaus, 1905)# x x x x x x Cisthene triplaga (Hampson, 1905)# x x x x x x Cisthene sp.1 x x x x x Cisthene sp.2 x x x x Cisthene sp.3 x x x Illice croesus Hampson, 1914#$ x x Illice griseola (Rothschild, 1913)#$* x x Odozana domina (Schaus, 1896) x x x x x Odozana obscura (Schaus, 1896) x x x x x x Talara grisea Schaus, 1896# x x x x x Eudesmiina Antona fallax (Butler, 1877)# x x Lithosina Agylla argentea (Walker, 1863)#$* x x x x x x Agylla marcata (Schaus, 1894)#$ x x x x Agylla sp.1 x x x x x x Apistosia judas Hübner, [1819]# x x Metalobosia diaxantha Hampson, 1914# x x x Nodozana jucunda Jones, 1914 x x x x x x Parablavia sadima (Schaus, 1896) x x x x x x ).
Discussion
The ENP Arctiinae fauna represents approximately 20% of the species recorded for the Cerrado (Ferro et al. 2010FERRO, V.G., MELO, A.S. & DINIZ, I.R. 2010. Richness of tiger moths (Lepidoptera: Arctiidae) in the Brazilian Cerrado: how much do we know? Zoologia. 27(5):725-731, 10.1590/S1984-46702010000500009
https://doi.org/10.1590/S1984-4670201000...
) and 10% of species recorded from Brazil (Ferro & Diniz 2010FERRO, V.G. & DINIZ, I.R. 2010. Riqueza e composição de mariposas Arctiidae (Lepidoptera) no Cerrado. In Cerrado, Conhecimento científico quantitativo como subsídio para ações de conservação (I.R. Diniz, J. Marinho-Filho, R.B. Machado & R.B. Cavalcanti, eds). Thesaurus, Brasília, p. 255-313.). The previous Cerrado Arctiinae richness (Ferro & Diniz 2010FERRO, V.G. & DINIZ, I.R. 2010. Riqueza e composição de mariposas Arctiidae (Lepidoptera) no Cerrado. In Cerrado, Conhecimento científico quantitativo como subsídio para ações de conservação (I.R. Diniz, J. Marinho-Filho, R.B. Machado & R.B. Cavalcanti, eds). Thesaurus, Brasília, p. 255-313.) is replaced by 737 species, with the addition of 14 new records for the biome. The fauna of the ENP is the second richest locality in the Cerrado, after Brasilia (222 species, Ferro & Diniz 2010FERRO, V.G. & DINIZ, I.R. 2010. Riqueza e composição de mariposas Arctiidae (Lepidoptera) no Cerrado. In Cerrado, Conhecimento científico quantitativo como subsídio para ações de conservação (I.R. Diniz, J. Marinho-Filho, R.B. Machado & R.B. Cavalcanti, eds). Thesaurus, Brasília, p. 255-313.). The richness observed in the ENP (149 species) was similar to the other intensively sampled areas in the Cerrado, such as Vilhena (136) and Chapada dos Guimarães (129) (Ferro & Diniz 2010FERRO, V.G. & DINIZ, I.R. 2010. Riqueza e composição de mariposas Arctiidae (Lepidoptera) no Cerrado. In Cerrado, Conhecimento científico quantitativo como subsídio para ações de conservação (I.R. Diniz, J. Marinho-Filho, R.B. Machado & R.B. Cavalcanti, eds). Thesaurus, Brasília, p. 255-313.), and Rain Forest sites, such as São José dos Ausentes (121) (Ferro & Romanowski 2012FERRO, V.G. & ROMANOWSKI, H.P. 2012. Diversity and composition of tiger moths (Lepidoptera: Arctiidae) in an area of Atlantic Forest in southern Brazil: is the fauna more diverse in the grassland or in the forest? Zoologia. 29(1):7-18, 10.1590/S1984-46702012000100002
https://doi.org/10.1590/S1984-4670201200...
), La Selva Biological Station (148) (Brehm 2007BREHM, G. 2007. Contrasting patterns of vertical stratification in two moth families in a Costa Rican lowland rain forest. Basic Appl. Ecol. 8(1):44-54, 10.1016/j.baae.2006.02.002
https://doi.org/10.1016/j.baae.2006.02.0...
), and São Bento do Sul (162) (Ferro et al. 2012FERRO, V.G., RESENDE, I.M.H. & DUARTE, M. 2012. The Arctiinae moths (Lepidoptera: Erebidae) of Santa Catarina state, Brazil. Biota Neotrop. 12(4):166-180, 10.1590/S1676-06032012000400018
https://doi.org/10.1590/S1676-0603201200...
). However, Hilt & Fiedler (2005)HILT, N. & FIEDLER, K. 2005. Diversity and composition of Arctiidae moth ensembles along a successional gradient in the Ecuadorian Andes. Divers. Distrib. 11(5):387-398, 10.1111/j.1366-9516.2005.00167.x
https://doi.org/10.1111/j.1366-9516.2005...
observed a significantly greater tiger moth richness in Ecuador (287 species).
This high richness, the large number of new distribution records (including 14 for the Cerrado), and the existence of at least another 40 species in the study area (according to the first order Jackknife estimator), reinforces the importance of conserving the ENP and its surroundings. Despite being well preserved, the ENP is a large fragment surrounded by extensive monocultures of soybean, corn, cotton and sugarcane matrix. These monocultures can act as a barrier to the dispersal of individuals and hence cause problems associated with small, isolated populations, such as inbreeding, genetic drift and increased susceptibility to future stochastic events. Furthermore, the use of insecticides on these crops can cause increased mortality of insects at the edges of the park, affecting, for example, pollination of entomophilous plants. Moreover, the invasion of alien species such as Brachiaria can reduce the natural vegetation due to competition among species (Almeida-Neto et al. 2010ALMEIDA-NETO, M., PRADO, P.I., KUBOTA, U., BARIANI, J.M., AGUIRRE, G.H. & LEWINSOHN, T.M. 2010. Invasive grasses and native Asteraceae in the Brazilian Cerrado. Plant. Ecol. 209(1):109-122, 10.1007/s11258-010-9727-8
https://doi.org/10.1007/s11258-010-9727-...
), for example, which results in a lower availability of host plants. All of these factors can affect the assemblages of insect herbivores and pollinators. Thus, among the priority actions for the conservation of the ENP (and its surroundings) Arctiinae fauna are the creation of new protected areas in their surroundings and the creation of ecological corridors between protected areas for the fauna of the region (Rodrigues et al. 2002RODRIGUES, F.H.G., SILVEIRA, L., JÁCOMO, A.T.A., CARMIGNOTTO, A.P., BEZERRA, A.M.R., COELHO, D.C., GARBOGINI, H., PAGNOZZI, J. & HASS, A. 2002. Composition and characterization of the mammal fauna of Emas National Park, Goiás, Brasil. Rev. Bras. Zool. 19(2):589-600, 10.1590/S0101-81752002000200015
https://doi.org/10.1590/S0101-8175200200...
).
The semideciduous forest had the highest number of exclusive species in relation to other vegetation types. This result can be explained by the fact that this vegetation type has a different microclimate, plant species and soil types than cerrado sensu lato (Oliveira-Filho & Ratter 2002OLIVEIRA-FILHO, A.T. & RATTER, J.A. 2002. Vegetation physiognomies and woody flora of the Cerrado biome. In The Cerrados of Brazil. Ecology and natural history of a Neotropical savanna (P.S. Oliveira & R.J. Marquis, eds). Columbia University Press, New York, p. 91-120., Ruggiero et al. 2002RUGGIERO, P.G.C., BATALHA, M.A., PIVELLO, V.R. & MEIRELLES, S.T. 2002. Soil-vegetation relationships in cerrado (Brazilian savanna) and semideciduous forest, Southeastern Brazil. Plant Ecol. 160(1):1-16, 10.1023/A:1015819219386
https://doi.org/10.1023/A:1015819219386...
).
Both seasons presented exclusive species, but this was more evident in the dry season. The dry presented more Arctiinae species than the rainy season, as found for other Lepidoptera species (Morais et al. 1999MORAIS, H.C., DINIZ, I.R. & SILVA, D.M.S. 1999. Caterpillar seasonality in a central Brazilian cerrado. Rev. Biol. Trop. 47(4):1025-1033., Pinheiro et al. 2002PINHEIRO, F., DINIZ, I.R., COELHO, D. & BANDEIRA, M.P.S. 2002. Seasonal pattern of insect abundance in the Brazilian cerrado. Austral Ecol. 27:132-136, 10.1046/j.1442-9993.2002.01165.x
https://doi.org/10.1046/j.1442-9993.2002...
). In Cerrado biome, the dry winter season is marked by adverse conditions, like low humidity and cold temperature (Ramos-Neto & Pivello 2000RAMOS-NETO, M.B. & PIVELLO, V.R. 2000. Lightning fires in a Brazilian savanna National Park: rethinking management strategies. Environ. Manage. 26(6):675-684, 10.1007/s002670010124
https://doi.org/10.1007/s002670010124...
). Also, the vegetation faces a water deficit and a reduction in nutritional quality in this season (Ramos-Neto & Pivello 2000RAMOS-NETO, M.B. & PIVELLO, V.R. 2000. Lightning fires in a Brazilian savanna National Park: rethinking management strategies. Environ. Manage. 26(6):675-684, 10.1007/s002670010124
https://doi.org/10.1007/s002670010124...
, Pinheiro et al. 2002PINHEIRO, F., DINIZ, I.R., COELHO, D. & BANDEIRA, M.P.S. 2002. Seasonal pattern of insect abundance in the Brazilian cerrado. Austral Ecol. 27:132-136, 10.1046/j.1442-9993.2002.01165.x
https://doi.org/10.1046/j.1442-9993.2002...
) and it can affect the Arctiinae moths, as they depend on the plants, both in larval (herbivorous) and in adult (pollinator) phases. Thus, we believe that the more Arctiinae species found in the winter dry season must be due to a temporarily enemy-free space (Jeffries & Lawton 1984JEFFRIES, M.J. & LAWTON, J.H. 1984. Enemy free space and the structure of ecological communities. Biol. J. Linn. Soc. 23:269-286, 10.1111/j.1095-8312.1984.tb00145.x
https://doi.org/10.1111/j.1095-8312.1984...
, Morais et al. 1999MORAIS, H.C., DINIZ, I.R. & SILVA, D.M.S. 1999. Caterpillar seasonality in a central Brazilian cerrado. Rev. Biol. Trop. 47(4):1025-1033.). In this period, the predators and parasitoids should be less abundant than in the rainy season (Morais et al. 1999MORAIS, H.C., DINIZ, I.R. & SILVA, D.M.S. 1999. Caterpillar seasonality in a central Brazilian cerrado. Rev. Biol. Trop. 47(4):1025-1033.) and it should enable more Arctiinae species to coexist in the severe dry season.
The Cerrado biome has diminished in recent decades mainly due to agricultural expansion (Klink & Machado 2005KLINK, C.A. & MACHADO, R.B. 2005. Conservation of the Brazilian Cerrado. Conserv. Biol. 19(3):707-713, 10.1111/j.1523-1739.2005.00702.x
https://doi.org/10.1111/j.1523-1739.2005...
). Many species may have been lost in this process, including species not yet known to science. According to Ferro et al. (2010)FERRO, V.G., MELO, A.S. & DINIZ, I.R. 2010. Richness of tiger moths (Lepidoptera: Arctiidae) in the Brazilian Cerrado: how much do we know? Zoologia. 27(5):725-731, 10.1590/S1984-46702010000500009
https://doi.org/10.1590/S1984-4670201000...
, much of the biome has not yet been inventoried. Thus, studies that generate lists of species, especially in places rarely or never sampled regarding the fauna, are urgent and very important to understand the biodiversity. Furthermore, these data improve our estimation of the geographical distribution and the status (e.g. rare, endemic, threatened) of species. These data, therefore, can be analyzed by niche modeling and guide future conservation strategies, such as the location of new conservation units. However, these estimates will be much more accurate if natural history data are included and if the species identification is correct.
List of Actiinae moth species sampled in four phytophysiognomies of the Emas National Park (ENP), in the dry and rainy seasons. CS means campo sujo, CC campo cerrado, CSS cerrado sensu stricto and SF semideciduous forest. Species with symbols #$* are new records for Cerrado, #$ for Goias State and # for ENP.
Acknowledgments
Vanessa Grandolfo, Taynara Nascimento (in memoriam), Luiz Rezende, Luciano Sgarbi, Carolina Caiado, Adriano Jaskulski, Murilo Menezes, Karen Neves, Amanda Honório, Verônica Bernardino, Ângela Soares, Vidal Cobos, Hauanny Rodrigues, Silas Bittencourt, Balwer Pimentel, Letícia Gomes, Matheus Alves and Tainã Cardoso helped in field sampling. Dr. Victor Becker helped in species identification. This research was funded by Site 13 (Emas National Park) of the Brazilian Long Term Ecological Research Network (CNPq, grant 558187/2009-9) and SISBIOTA-Brasil (RedeLep; CNPq, grant 563332/2010-7). CM receives a student fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- ALMEIDA-NETO, M., PRADO, P.I., KUBOTA, U., BARIANI, J.M., AGUIRRE, G.H. & LEWINSOHN, T.M. 2010. Invasive grasses and native Asteraceae in the Brazilian Cerrado. Plant. Ecol. 209(1):109-122, 10.1007/s11258-010-9727-8
» https://doi.org/10.1007/s11258-010-9727-8 - BINI, L.M., DINIZ-FILHO, J.A.F., RANGEL, T.F.L.V.B., BASTOS, R.P. & PINTO, M.P. 2006. Chalenging Wallacean and Linnean shortfalls: knowledge gradients and conservation planning in a biodiversity hotspot. Diversity Distrib. 12:475-482, 10.1111/j.1366-9516.2006.00286.x
» https://doi.org/10.1111/j.1366-9516.2006.00286.x - BREHM, G. 2007. Contrasting patterns of vertical stratification in two moth families in a Costa Rican lowland rain forest. Basic Appl. Ecol. 8(1):44-54, 10.1016/j.baae.2006.02.002
» https://doi.org/10.1016/j.baae.2006.02.002 - FERRO, V.G. & DINIZ, I.R. 2007. Composition of the Arctiidae species (Insecta, Lepidoptera) in Cerrado areas. Rev. Bras. Zool. 24(3):635-646, 10.1590/S0101-81752007000300015
» https://doi.org/10.1590/S0101-81752007000300015 - FERRO, V.G. & DINIZ, I.R. 2010. Riqueza e composição de mariposas Arctiidae (Lepidoptera) no Cerrado. In Cerrado, Conhecimento científico quantitativo como subsídio para ações de conservação (I.R. Diniz, J. Marinho-Filho, R.B. Machado & R.B. Cavalcanti, eds). Thesaurus, Brasília, p. 255-313.
- FERRO, V.G., MELO, A.S. & DINIZ, I.R. 2010. Richness of tiger moths (Lepidoptera: Arctiidae) in the Brazilian Cerrado: how much do we know? Zoologia. 27(5):725-731, 10.1590/S1984-46702010000500009
» https://doi.org/10.1590/S1984-46702010000500009 - FERRO, V.G., RESENDE, I.M.H. & DUARTE, M. 2012. The Arctiinae moths (Lepidoptera: Erebidae) of Santa Catarina state, Brazil. Biota Neotrop. 12(4):166-180, 10.1590/S1676-06032012000400018
» https://doi.org/10.1590/S1676-06032012000400018 - FERRO, V.G. & ROMANOWSKI, H.P. 2012. Diversity and composition of tiger moths (Lepidoptera: Arctiidae) in an area of Atlantic Forest in southern Brazil: is the fauna more diverse in the grassland or in the forest? Zoologia. 29(1):7-18, 10.1590/S1984-46702012000100002
» https://doi.org/10.1590/S1984-46702012000100002 - FRANÇA, H., RAMOS-NETO, M.B. & SETZER, A. 2007. O fogo no Parque Nacional das Emas. Ministério do Meio Ambiente, Brasília.
- HAMPSON, G.F. 1898. Catalogue of the Lepidoptera Phalaenae in the British Museum. Order of the Trustees, London.
- HAMPSON, G.F. 1900. Catalogue of the Lepidoptera Phalaenae in the British Museum. Order of the Trustees, London.
- HAMPSON, G.F. 1901. Catalogue of the Lepidoptera Phalaenae in the British Museum. Order of the Trustees, London.
- HAMPSON, G.F. 1914. Catalogue of the Lepidoptera Phalaenae in the British Museum. Order of the Trustees, London.
- HAUSER, C.L. & BOPPRÉ, M. 1997. A revision of the Afrotropical taxa of the genus Amerila Walker (Lepidoptera: Arctiidae). Syst. Entomol. 22(1):1-44, 10.1046/j.1365-3113.1997.d01-21.x
» https://doi.org/10.1046/j.1365-3113.1997.d01-21.x - HEPPNER, J.B. 1991. Faunal regions and the diversity of Lepidoptera. Trop. Lepid. 2(1):1-85.
- HILT, N. & FIEDLER, K. 2005. Diversity and composition of Arctiidae moth ensembles along a successional gradient in the Ecuadorian Andes. Divers. Distrib. 11(5):387-398, 10.1111/j.1366-9516.2005.00167.x
» https://doi.org/10.1111/j.1366-9516.2005.00167.x - HILT, N. & FIEDLER, K. 2006. Arctiid moth ensembles along a successional gradient in the Ecuadorian montane rain forest zone: how different are subfamilies and tribes? J. Biogeogr. 33(1):108-120, 10.1111/j.1365-2699.2005.01360.x
» https://doi.org/10.1111/j.1365-2699.2005.01360.x - JEFFRIES, M.J. & LAWTON, J.H. 1984. Enemy free space and the structure of ecological communities. Biol. J. Linn. Soc. 23:269-286, 10.1111/j.1095-8312.1984.tb00145.x
» https://doi.org/10.1111/j.1095-8312.1984.tb00145.x - KLINK, C.A. & MACHADO, R.B. 2005. Conservation of the Brazilian Cerrado. Conserv. Biol. 19(3):707-713, 10.1111/j.1523-1739.2005.00702.x
» https://doi.org/10.1111/j.1523-1739.2005.00702.x - LEWINSOHN, T.M., FREITAS, A.V.L. & PRADO, P.I. 2005. Conservation of terrestrial invertebrates and their habitats in Brazil. Conserv. Biol. 19:640-645, 10.1111/j.1523-1739.2005.00682.x
» https://doi.org/10.1111/j.1523-1739.2005.00682.x - MELO, A.S. 2004. A critique of the use of jackknife and related non-parametric techniques to estimate species richness. Community Ecol. 5(2):149-157, 10.1556/ComEc.5.2004.2.1
» https://doi.org/10.1556/ComEc.5.2004.2.1 - MORAIS, H.C., DINIZ, I.R. & SILVA, D.M.S. 1999. Caterpillar seasonality in a central Brazilian cerrado. Rev. Biol. Trop. 47(4):1025-1033.
- MORENO, C., CIANCIARUSO, M.V., SGARBI, L.F. & FERRO, V.G. 2014. Richness and composition of tiger moths (Erebidae: Arctiinae) in a Neotropical savanna: are heterogeneous habitats richer in species? Nat. Conserv. 12(2):138-143, 10.1016/j.ncon.2014.09.006
» https://doi.org/10.1016/j.ncon.2014.09.006 - MUIRHEAD-THOMPSON, R.C. 1991. Trap responses of flying insects. Academic Press, London.
- MYERS, N., MITTERMEIER, R.A., MITTERMEIER, C.G., FONSECA, G.A.B. & KENT, J. 2000. Biodiversity hotspots for conservation priorities. Nature. 403(6772):853-858, 10.1038/35002501
» https://doi.org/10.1038/35002501 - OLIVEIRA-FILHO, A.T. & RATTER, J.A. 2002. Vegetation physiognomies and woody flora of the Cerrado biome. In The Cerrados of Brazil. Ecology and natural history of a Neotropical savanna (P.S. Oliveira & R.J. Marquis, eds). Columbia University Press, New York, p. 91-120.
- PIÑAS-RUBIO, F., & MANZANO, P.I. 2003. Mariposas del Ecuador. Familia: Arctiidae. Subfamilia: Ctenuchinae. Compaãía de Jesús, Quito.
- PIÑAS-RUBIO, F., RAB-GREEN, S., ONORE, G. & MANZANO, P.I. 2000. Mariposas del Ecuador. Familia: Arctiidae. Subfamilias: Arctiinae y Pericopinae. Pontificia Universidad Católica del Ecuador, Quito.
- PINHEIRO, F., DINIZ, I.R., COELHO, D. & BANDEIRA, M.P.S. 2002. Seasonal pattern of insect abundance in the Brazilian cerrado. Austral Ecol. 27:132-136, 10.1046/j.1442-9993.2002.01165.x
» https://doi.org/10.1046/j.1442-9993.2002.01165.x - RAMOS-NETO, M.B. & PIVELLO, V.R. 2000. Lightning fires in a Brazilian savanna National Park: rethinking management strategies. Environ. Manage. 26(6):675-684, 10.1007/s002670010124
» https://doi.org/10.1007/s002670010124 - RODRIGUES, F.H.G., SILVEIRA, L., JÁCOMO, A.T.A., CARMIGNOTTO, A.P., BEZERRA, A.M.R., COELHO, D.C., GARBOGINI, H., PAGNOZZI, J. & HASS, A. 2002. Composition and characterization of the mammal fauna of Emas National Park, Goiás, Brasil. Rev. Bras. Zool. 19(2):589-600, 10.1590/S0101-81752002000200015
» https://doi.org/10.1590/S0101-81752002000200015 - RUGGIERO, P.G.C., BATALHA, M.A., PIVELLO, V.R. & MEIRELLES, S.T. 2002. Soil-vegetation relationships in cerrado (Brazilian savanna) and semideciduous forest, Southeastern Brazil. Plant Ecol. 160(1):1-16, 10.1023/A:1015819219386
» https://doi.org/10.1023/A:1015819219386 - SILVEIRA NETO, S. & SILVEIRA, A. C. 1969. Armadilha luminosa modelo "Luiz de Queiroz". O Solo 61:19-21.
- SINGER, M.S. & BERNAYS, E.A. 2009. Specialized generalists: Behavioral and evolutionary ecology of polyphagous wooly bear caterpillars. In Tiger Moths and Wolly Bears, Behavior, Ecology and Evolution of the Arctiidae (W.E. Conner, ed.). Oxford University Press, New York, p. 103-114.
- WATSON, A. & GOODGER, D.T. 1986. Catalogue of the Neotropical tiger-moths. Occas. Pap. Syst. Entomol. 1(1):1-70.
- WELLER, S., DACOSTA, M., SIMMONS, R., DITTMAR, K. & WHITING, M. 2009. Evolution and taxonomic confusion in Arctiidae. In Tiger Moths and Wolly Bears, Behavior, Ecology and Evolution of the Arctiidae (W.E. Conner, ed.). Oxford University Press, New York, p. 11-30.
- YELA, J.L. & HOLYOAK, M. 1997. Effects of moonlight and meterological factors on light bait trap catches of Noctuid moths (Lepidoptera: Noctuidae). Environ. Entomol. 26(6):1283-1290, 10.1093/ee/26.6.1283
» https://doi.org/10.1093/ee/26.6.1283 - ZAHIRI, R., HOLLOWAY, J.D., KITCHING, I.J., LAFONTAINE, J.D., MUTANEN, M. & WAHLBERG, N. 2012. Molecular phylogenetics of Erebidae (Lepidoptera, Noctuoidea). Syst. Entomol. 37(1):102-124, 10.1111/j.1365-3113.2011.00607.x
» https://doi.org/10.1111/j.1365-3113.2011.00607.x
Publication Dates
-
Publication in this collection
June 2016
History
-
Received
09 Apr 2015 -
Reviewed
23 Feb 2016 -
Accepted
16 May 2016