a16v61n6
LETTER TO THE EDITOR
Palindromic rheumatism associated with primary hypertrophic osteoarthropathy
Samuel Katsuyuki Shinjo; Mauricio Levy-Neto; Eduardo Ferreira Borba
Division of Rheumatology, São Paulo University Medical School - São Paulo/SP - Brazil. Email: samuel.shinjo@terra.com.br
INTRODUCTION
Hypertrophic osteoarthropathy (HOA) is a syndrome characterized by the triad of finger clubbing, periosteal new bone formation, skin and soft tissue changes, giving an acromegaloid look.1 It is present in 2 distinct syndromes: primary and secondary. The former is a rare inherited disorder occurring predominantly in males and not associated with any known disease process.2 The secondary syndrome is acquired and often associated with malignancy, especially lung carcinoma.2-4 Rarely, it can also be associated with nonmalignant disorders, such as chronic liver disease,5 inflammatory bowel disease, cyanotic heart disease and other forms of shunts (cavernous hemangiomas and arteriovenous fistulae,3 sarcoidosis,6,7 and pulmonary tuberculosis.8,9 If the underlying disease is correctable, the musculoskeletal manifestations may resolve, suggesting a humoral factor.10
Both types may be associated with peculiar skin changes known as pachyderma, characterized by thickened, furrowed skin. When idiopathic HOA is associated with pachyderma, it is called pachydermoperiostosis.11 In extremely rare instances, it may occur without pachyderma,2,12 possibly representing a nosologically distinct form of osteoarthropathy.12
Palindromic rheumatism is a recurrent or relapsing oligoarticular arthritis of short duration with peri- and para-articular tissue inflammation,13 resulting in no residual clinical or radiographic changes. Hench and Rosenberg first used the term in 1944 to describe 34 patients with recurrent, short-duration polyarthritis.13
In the long term, a substantial proportion of patients with palindromic rheumatism will go on to develop rheumatoid arthritis or other connective tissue diseases (eg, systemic lupus erythematosus, Wegener granulomatosis, or spondyloarthropathies), but the determinants of subsequent chronic disease have not yet to been well established.14,15 Positive rheumatoid factor in females and early involvement of the wrist and proximal interphalangeal joints predict subsequent development of rheumatoid arthritis of other connective tissue disease in patients with palindromic rheumatism.16
Besides crystal arthritis, there are several diseases that may be clinically similar to palindromic rheumatism, such as systemic autoinflammatory disorders (periodic fever syndromes), Whipple's disease, arthritis associated with hyperlipidemia, and intermittent hydrarthrosis.14
In the present work, we report an unusual case of a Brazilian male patient with palindromic rheumatism manifested in association with primary HOA without pachyderma. Disease activity was controlled with diphosphate chloroquine combined with methotrexate, leading to remission of arthritis relapse.
CASE REPORT
A 49-year-old black male civil servant had presented short (hours to 2 days) and intermittent asymmetric migratory oligoarthritis of the feet (metatarsal-phalanges), knees, and hands (wrist, metacarpal-phalanges, and proximal inter-phalanges) during the previous year. Those symptoms were not accompanied by morning stiffness, enthesitis, fever, weight loss, uveitis, intestinal, or genital-urinary alterations. His past history revealed significant digital clubbing since childhood. He reported that his grandfather also had digital clubbing. He was a chronic smoker, but not an abnormal alcohol consumer.
Physical examination showed clubbing and hypertrophic changes in fingers and toes (Figures 1A and 1B). Moreover, arthritis in the left ankle and right second and third metacarpal-phalanges were also observed. No skin thickening, lesions, or cardiopulmonary disease features were detected.
Laboratory investigation showed a persistent 3- to 4-fold increase of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels, along with a normal uric acid level, absence of antinuclear antibodies, and a 1200 IU/mL titer of rheumatoid factor. Hepatic enzymes, blood cell counts, serum calcium and phosphorus, thyroid hormones, and growth hormone were all within normal ranges.
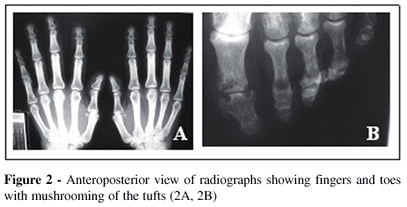
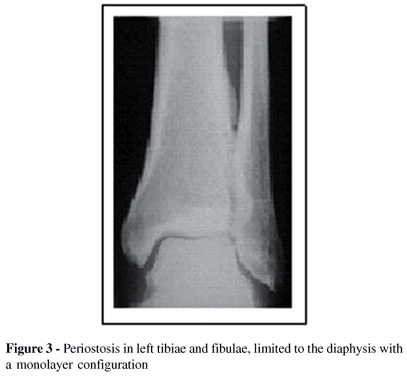
X-rays of the hands and feet showed no evidence of bone destruction. However, a marked clubbing with mushrooming of the tufts was found (Figures 2A and 2B). The chest x-ray was normal while pulmonary computerized tomography showed emphysema of the upper lung lobes. Periostosis in tibiae and fibulae, limited to the diaphysis with a monolayer configuration, were also detected (Figure 3). Echocardiography was normal.
A nonsteroidal anti-inflammatory and an antimalaria drug (diphosphate chloroquine 250 mg/day) were introduced followed by methotrexate (10 mg/week), with efficient control of arthritic crises as well as ESR and CRP levels.
DISCUSSION
We reporte an unusual case of a male patient with palindromic rheumatism manifested in association with primary HOA without pachyderma. Systemic diseases such as chronic liver disease, inflammatory bowel disease, and cyanotic heart disease and other forms of shunts, which could have contributed to characterization as a secondary HOA type, were not present.
The patient had been asymptomatic until 1 year previously, when oligoarthritis migratory manifestation commenced, without constitutional symptoms. No malignant manifestations, infectious diseases, hyperlipidemia, or medicine intake antecedence were found. Regarding connective tissue disease, radiography analysis of the extremities showed no destruction or erosions; antinuclear antibodies were negative, but titers of rheumatoid factor were elevated. Moreover, a persistent elevation of ESR and CRP levels was observed. Rheumatoid factor is frequently present in sera of patient with palindromic rheumatism, with a prevalence of between 30% and 60%, without any associated rheumatoid disease.14 The presence of rheumatoid factor has been associated with severity of arthritis crises and a high probability of development of rheumatoid arthritis during the follow-up of patients.17
Palindromic rheumatism can be evaluated by 3 clinical standards: (a) clinical remission of articular crisis; (b) persistence of recurrent articular crisis; and (c) evolution to chronic disease such as rheumatoid arthritis (one-third to one-half of cases). The latency period between the onset of palindromic rheumatism and the development of rheumatoid arthritis is variable, from several weeks to more than 10 years.14 In case present here, the patient proved positive for rheumatoid factor, although this was not clinically suggestive of rheumatoid arthritis, even through radiological images.
To date, there is no consensus on the treatment of palindromic rheumatism. One of the difficulties regarding the assessment of pharmacological action is the self-limited crisis of palindromic rheumatism. Moreover, because of the low incidence of this disease, controlled studies are difficult to carry out.18 Nonsteroid anti-inflammatory drugs and glucocorticosteroid can be used during crises, but their intake does not decrease the duration or prevent new crises. The use of antimalarials has proved to be effective in relieving palindromic rheumatism19 and decreasing progression to rheumatoid arthritis or other connective tissue diseases in patients with palindromic rheumatism.20 There are no studies on palindromic rheumatism with leflunomide or methotrexate. In our patient, since administration of nonsteroid anti-inflammatory and antimalaria drugs was inefficient in controlling the arthritic crises, we added methotrexate with good results, controlling the crises after 6 months. Moreover, laboratory analysis showed a decrease in ESR, CRP, and rheumatoid factor levels. However, it remains undefined whether this association will retard or stop possible progression to rheumatoid arthritis; this will require a longer follow-up study.
REFERENCES
1. Hansen-Flaschen J, Nordberg J. Clubbing and hypertrophic osteoarthropathy. Clin Chest Med. 1987;8:287-98.
2. Bhate DV, Pizarro AJ, Greenfield GB. Idiopathic hypertrophic osteoarthropathy without pachyderma. Radiology. 1978;129:379-81.
3. Schumacher HT Jr. Articular manifestation of hypertrophic pulmonary osteoarthropathy in bronchogenic carcinoma. Arthritis Rheum. 1976;19:629-36.
4. Hirakata Y, Kitamura S. Pulmonary hypertrophic osteoarthropathy and clubbing of fingers in patients with lung cancer. Nihon Kyobu Shikkan Gakkai Zasshi. 1995;33:1080-5.
5. Varju T, Lesch M, Adorjan A. Hypertrophic osteoarthropathy associated with alcoholic liver disease without cirrhosis. Clin Exp Rheumatol. 1986;4:375-8.
6. Rahbar M, Sharma OP. Hypertrophic osteoarthropathy in sarcoidosis. Sarcoidosis. 1990;7:125-7.
7. Alloway JA, Nashel DJ, Rohatgi PK. Sarcoidosis and hypertrophic osteoarthropathy. J Rheumatol. 1992;19:180-1.
8. Webb JG, Thomas P. Hypertrophic osteoarthropathy and pulmonary tuberculosis. Tubercle. 1986;67:225-8.
9. Sehgal SK, Prasad SR, Wadhwa A. Hypertrophic osteoarthropathy in pulmonary tuberculosis. Indian Pediatr. 1987;24:1161.
10. Padula SJ, Broketa G, Sampieri A, Arakawa M, Matucci-Cerinic M, Downie E et al. Increased collagen synthesis in skin fibroblasts from patients with primary hypertrophic osteoarthropathy. Evidence for trans-activational regulation of collagen transcription. Arthritis Rheum. 1994;37:1386-94.
11. Matucci-Cerinic M, Lotti T, Jajic I, Pignone A, Bussani C, Cagnoni M. The clinical spectrum of pachydermoperiostosis (primary hypertrophic osteoarthropathy). Medicine (Baltimore). 1991;70:208-14.
12. Bartolozzi G, Bernini G, Maggini M. Primary hypertrophic osteoarthropathy without pachydermia. Report of a case. Minerva Pediatr.1974;26:1173-84.
13. Hench PS, Rosenberg EF. Palindromic Rheumatism. Arch Intern Med. 1944;73:293-321.
14. Sanmarti R, Salvador G. Palindromic rheumatism and other relapsing arthritis. Best Practice and Research Clinical Rheumatology. 2004;18:647-61.
15. Guerne PA, Weisman MH. Palindromic rheumatism: part of or apart from the spectrum of rheumatoid arthritis. Am J Med. 1992;93:451-60.
16. Gonzalez-Lopez L, Gamez-Nava JI, Jhangri GS, Ramos-Remus C, Russel AS, Suarez-Almazor ME. Prognostic factors for the development of rheumatoid arthritis and other connective tissue diseases in patients with palindromic rheumatism. J Rheumatol. 1999;26:540-5.
17. Albert DA, Schumacher HR. Miscellaneous arthropathies (2004) In: Hochberg MC, Sliman AJ (eds): Rheumatology, 3ª ed. Mosby; 2004. p.1757.
18. Atra E, Goldenberg J. Reumatismo palindrômico. Res Bras Reumat. 1981;21:147-50.
19. Gonzales-Lopes L, Gamez-Nava JI, Jhangri G, Russell AS, Suarez-Almazor ME. Decreased progression to rheumatoid arthritis or other connective tissue diseases in patients with palindromic rheumatism treated with antimalarials. J Rheumatol. 2000;278:41-6.
20. Youssef W, Yan A, Russell AS. Palindromic rheumatism: a response to chloroquine. J. Rheumatol. 1991;18:35-7.
Publication Dates
-
Publication in this collection
14 Dec 2006 -
Date of issue
2006