Abstract
OBJECTIVE: To evaluate the effect of low dose methotrexate alone or in combination with glucocorticoid treatment on titanium implant osseointegration. METHODS: Groups of 6-8 adult New Zealand White rabbits were treated for 18 weeks with saline (control), methotrexate, glucocorticoid, or methotrexate plus glucocorticoid. The animals received a titanium implant in the tibia at week 6. Lumbar spine and tibia bone mineral densities were analyzed before and after treatment. Histomorphometric analysis of bone cortical thickness, total bone area around the implant, and % of bone to implant contact was performed. RESULTS: After 18 weeks, the change in the bone mineral density in the lumbar spines and tibias in the methotrexate group was comparable to the control group (0.035 vs. 0.055 g/cm² and 0.021 vs. 0.041 g/cm², respectively). In contrast, both the glucocorticoid group and glucocorticoid plus methotrexate group had significant reductions at both sites. Histomorphometric analysis of the tibia in the control and methotrexate groups revealed no significant changes in cortical thickness (133 vs. 126 μm), total bone area around the implant (33 vs. 30%), or bone to implant contact (40 vs. 38%). In contrast, glucocorticoid group had significant reductions compared to controls in tibia cortical thickness (99 vs. 133 μm), total bone area around the implant (24 vs. 33%), and bone to implant contact (27 vs. 40%). Similar reductions were observed in the glucocorticoid plus methotrexate group. CONCLUSIONS: Our results demonstrate that low dose methotrexate treatment does not affect titanium implant osseointegration, suggesting that this therapy is safe for surgical procedures requiring a titanium implant.
Methotrexate; Osseointegration; Dental Implant; Glucocorticoid
BASIC RESEARCH
IRheumatology Division, Faculdade de Medicina da Universidade de São Paulo
IIPeriodontics, Dental Branch, Universidade de São Paulo, SP, Brazil
ABSTRACT
OBJECTIVE: To evaluate the effect of low dose methotrexate alone or in combination with glucocorticoid treatment on titanium implant osseointegration.
METHODS: Groups of 6-8 adult New Zealand White rabbits were treated for 18 weeks with saline (control), methotrexate, glucocorticoid, or methotrexate plus glucocorticoid. The animals received a titanium implant in the tibia at week 6. Lumbar spine and tibia bone mineral densities were analyzed before and after treatment. Histomorphometric analysis of bone cortical thickness, total bone area around the implant, and % of bone to implant contact was performed.
RESULTS: After 18 weeks, the change in the bone mineral density in the lumbar spines and tibias in the methotrexate group was comparable to the control group (0.035 vs. 0.055 g/cm2 and 0.021 vs. 0.041 g/cm2, respectively). In contrast, both the glucocorticoid group and glucocorticoid plus methotrexate group had significant reductions at both sites. Histomorphometric analysis of the tibia in the control and methotrexate groups revealed no significant changes in cortical thickness (133 vs. 126 μm), total bone area around the implant (33 vs. 30%), or bone to implant contact (40 vs. 38%). In contrast, glucocorticoid group had significant reductions compared to controls in tibia cortical thickness (99 vs. 133 μm), total bone area around the implant (24 vs. 33%), and bone to implant contact (27 vs. 40%). Similar reductions were observed in the glucocorticoid plus methotrexate group.
CONCLUSIONS: Our results demonstrate that low dose methotrexate treatment does not affect titanium implant osseointegration, suggesting that this therapy is safe for surgical procedures requiring a titanium implant.
Keywords: Methotrexate; Osseointegration; Dental Implant; Glucocorticoid.
INTRODUCTION
Bone implants are a valuable tool for the reconstruction of tissue affected by trauma or inflammatory diseases and for orthodontic anchorage. The success of endosseous implants is dependent on osseointegration, a cicatricial process of implant/bone interaction defined histologically as direct bone apposition on the implant surface with nearly no interposition of soft tissue that leads to bone-to-implant fixation.1 Titanium is an excellent material for bone implants due to its biocompatibility, augmented resistance to corrosion, lack of toxicity on macrophages and fibroblasts, and reduced inflammatory response in peri-implant tissues.2 However, osseointegration also requires the integrity of the bone remodeling process, which is influenced by various factors. Issues affecting osseointegration include the properties and geometry of the implant, implant surface treatment and coating, host bone bed, mechanical stability and loading conditions,4 and the use of adjuvant treatments.5,6 In particular, non-steroidal anti-inflammatory therapies like selective COX-2 inhibitors,7 glucocorticoids (GC), cyclosporin A,8 and other immunosuppressants, which are drugs commonly used in rheumatologic patients, have deleterious effects on osseointegration.
Low dose methotrexate (MTX) is an antirheumatic drug widely prescribed to patients with rheumatoid arthritis (RA). However, its effect on bone metabolism remains controversial. Low doses of this drug in patients with active RA had a protective effect on bone metabolism from controlling the disease activity.9 In fact, MTX therapy has been reported to inhibit generalized bone loss in patients with RA.10 Conversely, a deleterious effect on bone metabolism has been described in RA patients11 and in animals.12 There is only a single case report on the effect of MTX on osseointegration, which describes a successful rehabilitation of a titanium implant in an elderly patient with severe osteoporosis and chronic polyarthritis whosetreatmentincludedMTX.1 In contrast, GC, which is an anti-inflammatory drug frequently prescribed in association with MTX for rheumatologic patients, was found to negatively affect osseointegration of titanium implants.14-16 The distinction of the deleterious effect of these drugs on bone metabolism is hampered by their concomitant use with multiple therapies in autoimmune diseases. Additionally, systemic conditions per se, such as RA and other inflammatory conditions, may also affect this process.17,18
In this context, experimental models provide a unique condition to discriminate between the effects induced by the disease itself and those caused by the therapy. Therefore, we have evaluated whether low dose MTX influences bone density and histomorphometric parameters of peri-implant bone healing around titanium implants placed in the tibia in a rabbit experimental model.
MATERIALS AND METHODS
Animals and treatments
All experimental procedures performed on animals were in accordance with UFAW (The Universities Federation for Animals Welfare) and the Animal Ethics Committee of COBEA (Brazilian College of Experimental Animals). This protocol was approved by the Institutional ethics committee # 453/05. Male New Zealand adult rabbits 16 weeks old and weighing 2.67+0.067 kg were divided into four groups of 6-8 rabbits each. The animals were kept in individual cages with food and water ad libitum and treated as follows: subcutaneous saline (0.3 mL, control group); intramuscular metho-trexate (3 mg/kg/week, MTX group)12, subcutaneous glucocorticoid (0.35 mg/kg methylprednisolone 3 times/ week, GC group),19 MTX (3 mg/kg/week) plus GC (0.35 mg/kg methylprednisolone 3 times/week, MTX+GC group).
Endosseous implant model
The implant surgery was performed 6 weeks after the initial drug administration. The animals were anesthetized with a mixture of xylazine (5 mg/kg) associated with ketamine (50 mg/kg) by the intramuscular route. After trichotomy, the skin was cleansed, an incision of approximately 2 cm was made, and the tibia was exposed by blunt dissection. A unicortical implant bed was prepared and a screw-type, commercially pure titanium implant with a rough surface, 8.5 mm in length and 3.75 mm in diameter (Conexão Sistema de Protese Ltda, 1-2 mm porosity), was placed such that the screw thread was completely perpendicular into the bone cortex.16 Soft tissues were replaced and sutured. A single dose of Enrofloxacin was administered just before surgery and dipyrone was given for three days afterwards.
Densitometric evaluation
Bone density was measured by dual-energy X-ray absorptiometry (DXA) with a densitometer (QDR 2000 Hologic, Waltham, MA) in high-resolution mode using the ''small animals'' software supplied by the equipment manufacturer. The technique was standardized by positioning the anesthetized rabbits such that the lumbar spine (vertebrae L4-L5) and the proximal portion of the tibia that was not operated on (right) was analyzed. The region of interest was defined as the same where the implant was inserted but in the contralateral tibia. The initial assessment was performed on the first day of the experiment and the final at end of the treatment (week 18). Results are expressed as mean + SE of bone mineral density (BMD) variation (ABMD = final BMD - initial BMD).
Analysis of bone parameters
After the animals were euthanized (week 18), the left tibia (implanted) and right tibia (non-operated) were removed and fixed in 4% neutral buffered formalin for 14 days. The specimens were prepared for non-decalcified histology.20 Briefly, pieces were washed in running water for 24 hours, dehydrated in an ascending series of ethanol (40% to 100%), and subsequently embedded in methyl metacrylate blocks. Sections (80 microns) were obtained and stained with toluidine blue as described previously.16
Bone cortical thickness, tibia size (diameter), ratio of bone to implant contact, total bone area of the tibia section, and peri-implant bone density were analyzed by light microscopy. Images were captured and digitalized with Image Pro Plus 6 (Media Cybernetics, Bethesda, MD). The cortical thickness (μm) was determined as the mean of multiple measurements of the non-operated right tibia taken at 30 mm intervals along the tibia perimeter.
Osseointegration was observed via light microscopy as direct bone (toluidine blue-stained) deposition on the implant surface without any other detectable tissue interposed. The total bone area of the tibia section was evaluated by software that determines the area of bone tissue (BT) stained with toluidin blue present in the total area [tibia + implant (TA, mm2)] and expresses it as the percentage of total bone area = BT/TA x100.24 The ratio of bone to implant contact (BIC) was calculated from the measurement of the total perimeter of the implant (TPI) and all the osseointegrated spaces around the implant (OSI), both of which were obtained manually, and expressed as percent bone to implant contact (% of BIC = OSI/TPI x 100). Two specialists blinded to the treatments acquired all the bone parameter data.
STATISTICAL ANALYSIS
Bone parameter results were analyzed by repeated measures ANOVA and compared with the Newman-Keuls test (when normal distribution was detected). The ratio of bone to implant contact was expressed as the median and the comparisons between groups were carried out with the Mann-Whitney U test. Based on the ratio of bone to implant contact difference between groups, the observed power of analysis was 77% (β risk =23%). Results are expressed as mean + SE and the chosen level of significance was 0.05.
RESULTS
General outcomes
Gain of body weight was comparable among the groups (control, 0.82 + 0.15 kg; MTX, 1.18 + 0.24 kg; GC, 0.58 + 0.10 kg and GC+MTX, 0.65 + 0.22 kg, p = 0.105). No adverse gastrointestinal effects (vomiting or diarrhea) were observed. Mortality, infection, and wound dehiscence was not recorded with this protocol.
Bone mass density variation
Initial bone mass densities were similar among the experimental groups for the lumbar spine (control, 0.269+ 0.008 g/cm2;MTX,0.274+ 0.011 g/cm2; GC, 0.268+ 0.010 g/cm2; GC+MTX, 0.284 + 0.011 g/cm2, p = 0.674) and tibia (control, 0.371 + 0.012 g/cm2;MTX,0.385±0.008 g/cm2; GC, 0.387+ 0.011 g/cm2;GC+MTX, 0.387+0.011 g/cm2, p = 0.641).
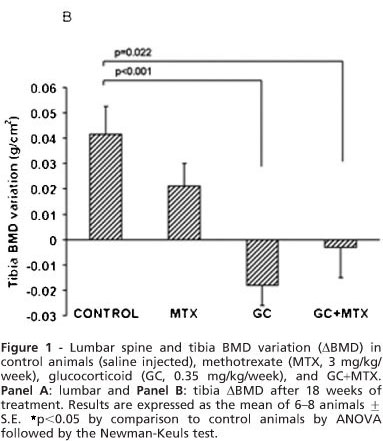
After 18 weeks, control animals had a positive lumbar BMD variation (ABMD, 0.055 + 0.009 g/cm2 ) comparable to the MTX group (0.035 + 0.015 g/cm2, p = 0.280, Fig. 1A). However, there was a significant reduction of ABMD in the GC group (-0.004 + 0.012 g/cm2, p = 0.003) and GC+MTX group (-0.003 + 0.012 g/cm2, p = 0.003, Fig. 1A). Tibia ABMD were also positive and similar in control and MTX groups (0.041 + 0.011 g/cm2 vs. 0.021 + 0.009 g/cm2, p = 0.190), but significantly decreased in GC (-0.018+ 0.008 g/cm2, p<0.001) and GC+MTX groups (-0.003+ 0.012 g/cm2, p = 0.022, Fig. 1B).
Histomorphometric analysis
Histomorphometric analysis at 18 weeks revealed that the cortical thickness was comparable in the control and MTX groups (133.08 + 2.36 vs. 126.24 + 2.42 mm, p = 0.071). In contrast, at the final evaluation GC (98.81 + 2.28 mm, p<0.001) and GC+MTX groups (96.41 + 3.12 mm, p<0.001) had significant reductions. In addition, the percentage of bone tissue around the implant in control and MTX groups was similar (33.16+ 1.29 vs. 30.13+ 1.04%, p= 0.097) whereas GC (24.40+ 1.51%, p<0.001) and GC+MTX (25.65+ 1.63%, p= 0.005) groups had significantly lower percentages of bone tissue around the implant compared.
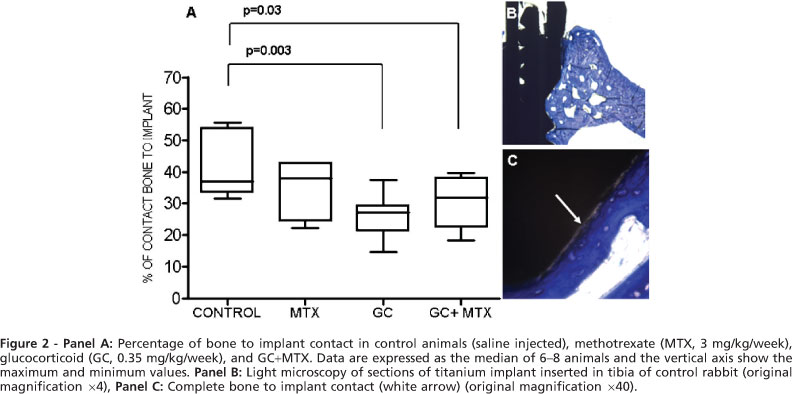
The effects of these treatments on the percent of bone to implant contact (BIC) are summarized in Fig. 2A. Again, control and MTX groups had comparable values of osseointegration [median 39.56% (31.63-55.57%) and 37.99% (22.34-42.97%), respectively, p = 0.101], while GC [27.12% (14.53-37.45%), p = 0.003] and GC+MTX groups [31.94% (18.43-46.55%), p= 0.03] reduced values. The osseointegration of titanium implants in rabbit tibia was observed as direct bone (toluidine blue stained) deposition on the implant surface without any other detectable tissue interposed (Fig. 2B & C).
DISCUSSION
The present study is the first to provide compelling evidence that low dose methotrexate does not have a deleterious effect on titanium implant osseointegration. Patients with inflammatory diseases are often treated with MTX and novel biological therapies are frequently prescribed in combination with this drug.21 The increase in quality of life for rheumatologic patients achieved in the last decade22 may increase the demand for arthroplasty and prosthodontic treatments.
The advantage of the present study's design using normal rabbits is that we were able to discriminate between the effects of MTX therapy and those of inflammatory disease on bone metabolism. Analysis of intracellular signaling mechanisms in osteoclasts has demonstrated that various immunomodulatory molecules play a role in the control of bone metabolism in RA.23 In addition, there is growing acceptance of simultaneous therapy in RA with multiple drugs associated with GC24 and the present model provides a clear analysis of the effects of each drug on the osseointegration process.
Another strength of our study is that we administered doses equivalent to what is routinely prescribed in the clinical setting12 and we treated animals with tibias of almost complete (94%) length.25 This allowed us to minimize complications, since long-term therapy with high cumulative MTX-doses in children is associated with osteopathy, which is characterized by osteopenia, zones of calcification, growth arrest lines, and fractures.26 This complication seems to be due to intracellular accumulation of MTX and formation of methotrexate-polyglutamates in the rapidly growing skeletal structures of infants.27 MTX-osteopathy in adult rheumatic disease patients under low dose therapy is uncommon and restricted to a few case reports.11,28 The causal relationship is still under debate since these five patients had other important risk factors for osteoporotic fractures, emphasizing the relevance of the present study.29 In addition, the observation period in the present study was an appropriate exposure time to the drug considering the comparative life spans of rabbits and humans,30,31 which is relevant since MTX-osteopathy in adults is associated with prolonged periods of therapy.28,32
Our data confirms and extends previous observations that low dose MTX does not have a negative effect on BMD in patients with RA and psoriasis.32,33 The complete exclusion of disease interference in our model allows for an accurate conclusion regarding the lack of a deleterious effect of this drug on bone mass at either cortical or trabecular sites. In addition, reproduction of the clinical conditions often observed in rheumatologic patients revealed that MTX had no effect on BMD when associated with GC. The reductions recorded in the GC and GC+MTX groups are comparable and are attributable to the GC treatment since this drug can cause rapid bone loss, decreased bone formation, and increased bone resorption.34
Our results reveal that MTX treatment preserves cortical thickness, percent of bone around the implant, and percent of bone to implant contact (BIC), all of which are histological criteria for osseointegration, in spite of the limited number of animals. In contrast to zoledronic acid,18 MTX was unable to reverse the deleterious effect of GC. In fact, the previously reported protective effect of MTX is only seen under inflammatory conditions when several cytokines and other chemical mediators such as PGE2, which affect bone cells, were involved.35
Together our data suggest that low dose MTX therapy should be maintained in individuals requiring implant surgery since this drug does not affect the osseointegration process.
ACKNOWLEDGMENTS
The authors are grateful to Maria Aurora Gomes da Silva and Maria de Fátima de Almeida for their skillful technical assistance and to Dr. Walcy R Teodoro and Dr. Vanda Jorgetti for helpful discussions of histological analysis. This study was supported in part by grants from Conexão Sistema de Prótese Ltda, FAPESP #06/57383-5 (SBVM), Conselho Nacional de Ciência e Tecnologia (CNPQ), #300559/2009-7 (RMRP), and # 301411/2009-3 (EB), and Federico Foundation (EB).
Received for publication on February 9, 2011; Accepted for publication on March 2, 2011; First review completed on February 15, 2011
E-mail: bcarvas@hotmail.com Tel.: 55 11 3061-7200
- 1. Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC. Biology of implant osseointegration. J Musculoskelet Neuronal Interact. 2009;9:61-71.
- 2. Ellerbe DM, Frodel JL. Comparison of implant materials used in maxillofacial rigid internal fixation. Otolaryngol Clin North Am. 1995;28:365-72.
- 3. Marco F, Milena F, Gianluca G, Vittoria O. Periimplant osteogenesis in health and osteoporosis. Micron. 2005;36:630-44, doi: 10.1016/j.micron. 2005.07.008.
- 4. Soballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop Scand. Suppl 1993; 255:1-58.
- 5. Eberhardt C, Habermann B, Müller S, Schwarz M, Bauss F, Kurth AH. The bisphosphonate ibandronate accelerates osseointegration of hydro-xyapatite-coated cementless implants in an animal model. J Orthop Sci. 2007;12:61-6, doi: 10.1007/s00776-006-1081-2.
- 6. Basarir K, Erdemli B, Can A, Erdemli E, Zeyrek T. Osseointegration in arthroplasty: can simvastatin promote bone response to implants? Int Orthop. 2009;33:855-9, doi: 10.1007/s00264-007-0490-y.
- 7. Pablos AB, Ramalho SA, König B Jr, Furuse C, de Araujo VC, Cury PR. Effect of meloxicam and diclofenac sodium on peri-implant bone healing in rats. J Periodontol. 2008;79:300-6, doi: 10.1902/jop.2008.070301.
- 8. Sakakura CE, Marcantonio E Jr, Wenzel A, Scaf G. Influence of cyclosporin A on quality of bone around integrated dental implants: a radiographic study in rabbits. Clin Oral Implants Res. 2007;8:34-9, doi: 10.1111/j.1600-0501.2006.01253.x.
- 9. Miedany YM, Abubakr IH, Baddini M. Effect of low dose methotrexate on markers of bone metabolism in patients with rheumatoid arthritis. J Rheumatol. 1998;25:2083-7.
- 10. Torikai E, Kageyama Y, Takahashi M, Nagano A. The effect of methotrexate on bone metabolism markers in patients with rheumatoid arthritis. Mod Rheumatol. 2006;16:350-4, doi: 10.1007/s10165-006-0517-z.
- 11. Van der Bijl AE, Zijlstra TR, Engelage AH, Posthuma BJ, van Veen GJ. Three patients with a fracture during methotrexate use, possibly due to methotrexate osteopathy. Ned Tijdschr Geneeskd. 2008;152:2357-60.
- 12. May KP, West SG, McDermott MT, Huffer WE. The effect of low-dose methotrexate on bone metabolism and histomorphometry in rats. Arthritis Rheum. 1994;37:201-6, doi: 10.1002/art.1780370208.
- 13. Eder A, Watzek G. Treatment of a patient with severe osteoporosis and chronic polyarthritis with fixed implant- supported prosthesis: A case report. Int J Oral Maxillofac Implants. 1999;14:587-90.
- 14. Werner SB, Tessler J, Guglielmotti MB, Cabrini RL. Effect of dexametha-sone on osseointegration: a preliminary experimental study. J Oral Implantol. 1996;22:216-9.
- 15. Fujimoto T, Niimi A, Sawai T, Ueda M. Effects of steroid-induced osteoporosis on osseointegration of titanium implants. Int J Oral Maxillofac Implants. 1998;13:183-9.
- 16. Carvas JS, Pereira RM, Caparbo VF, Fuller P, Silveira CA, Lima LA, et al. A single dose of zoledronic acid reverses the deleterious effects of glucocorticoids on titanium implant osseointegration. Osteoporos Int. 2010;21:1723-9, doi: 10.1007/s00198-009-1125-5.
- 17. Rosenqvist R, Bylander B, Knutson K, Rydholm U, Rooser B, Egund N, et al. Loosening of the porous coating of bicompartmental prostheses in patients with rheumatoid arthritis. J Bone Joint Surg Am. 1986;68:538-42.
- 18. Mombelli A, Cionca N. Systemic diseases affecting osseointegration therapy. Clin Oral Implants Res. 2006;17:97-103, doi: 10.1111/j.1600-0501. 2006.01354.x.
- 19. Luppen CA, Blake CA, Ammirati KM, Stevens ML, Seeherman HJ, Wozney JM, et al. Recombinant human bone morphogenetic protein-2 enhances osteotomy healing in glucocorticoid- treated rabbits. J Bone Miner Res. 2002; 17:301-10, doi: 10.1359/jbmr.2002.17.2.301.
- 20. He FM, Yang GL, Li YN, Wang XX, Zhao SF. Early bone response to sandblasted, dual acid-etched and H2O2/HCl treated titanium implants: an experimental study in the rabbit. Int J Oral Maxillofac Surg. 2009;38:677-81, doi: 10.1016/j.ijom.2009.03.716.
- 21. Tang B, Rahman M, Waters HC, Callegari P. Treatment persistence with adalimumab, etanercept, or infliximab in combination with methotrexate and the effects on health care costs in patients with rheumatoid arthritis. Clin Ther. 2008;30:1375-84, doi: 10.1016/S0149-2918(08)80063-X.
- 22. Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2007;9:237-51.
- 23. Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5:667-76, doi: 10.1038/nrrheum.2009. 217.
- 24. Mottonen TT, Hannonen PJ, Boers M. Combination DMARD therapy including corticosteroids in early rheumatoid arthritis. Clin Exp Rheumatol. 1999;6Suppl 18:59-65.
- 25. Masoud I, Shapiro F, Kent R, Moses A. A longitudinal study of the growth of the New Zealand white rabbit: cumulative and biweekly incremental growth rates for body length, body weight, femoral length, and tibia length. J Orthop Res. 1986;4:221-31, doi: 10.1002/jor.1100040211.
- 26. Van Leeuwen BL, VerkerkE GJ, Hartel RM, Sluiter WJ, Kamps WA, Jansen HW, et al. Chemotherapy decreases epiphyseal strength and increases bone fracture risk. Clin Orthop Relat Res. 2003;413:243-54, doi: 10.1097/01.blo.0000073348.50837.f2.
- 27. Meister B, Gassner I, Streif W, Dengg K, Fink FM. Methotrexate osteopathy in infants with tumors of the central nervous system. Med Pediatr Oncol. 1994; 23:493-6, doi: 10.1002/mpo.2950230608.
- 28. Preston SJ, Diamond T, Scott A, Laurent MR. Methotrexate osteopathy in rheumatic disease. Ann Rheum Dis. 1993;52:582-5, doi: 10.1136/ard.52.8. 582.
- 29. Maenaut K, Westhovens R, Dequeker J. Methotrexate Osteopathy, does it exist? J Rheumatol. 1996;23:2156-9.
- 30. Dressler MR, Bulter DL, Boivin GP. Effects of age on the repair ability of mesenchymal stem cells in rabbit tendon. J Orthop Res. 2005;23:287-93, doi: 10.1016/j.orthres.2004.06.017.
- 31. Von Holst D, Huzelmeyer H, Kaetzke P, Khaschei M, Schonheiter R. Social rank, stress, fitness, and life expectancy in wild rabbits. Naturwissenschaften. 1999;86:388-93, doi: 10.1007/s001140050638.
- 32. Zonneveld IM, Bakker WK, Dijkstra PF, Bos JD, Van Soesbergen RM, Dinant HJ. Methotrexate Osteopathy in long-term, low- dose methotrex-ate treatment for psoriasis and rheumatoid arthritis. Arch Dermatol. 1996;32:184-7, doi: 10.1001/archderm.132.2.184.
- 33. Cranney AB, McKendry RJ, Wells GA, Ooi DS, Kanigsberg ND, Kraag GR, et al. The effect of low dose methotrexate on bone density. J Rheumatol. 2001; 28:2395-9.
- 34. Silverman SL, Lane NE. Glucocorticoid-induced osteoporosis. Curr Osteoporos Rep 2009;7:23-6, doi: 10.1007/s11914-009-0005-4.
- 35. Yoshida M, Kanno Y, Ishisaki A, Tokuda H, Hirade K, Nakajima K, et al. Methotrexate suppresses inflammatory agonist induced interleukin 6 synthesis in osteoblasts. J Rheumatol. 2005;32:787-95.
No deleterious effect of low dose methotrexate on titanium implant osseointegration in a rabbit model
Publication Dates
-
Publication in this collection
21 July 2011 -
Date of issue
2011
History
-
Reviewed
02 Mar 2011 -
Received
09 Feb 2011 -
Accepted
15 Feb 2011