Abstract
BACKGROUND: Failure of anastomotic healing is one of the major complications in colorectal surgery. Because histamine plays an important role in immune and inflammatory reactions, we demonstrate the effects of famotidine on the healing of colonic anastomosis in rats. METHODS: Twenty-eight Sprague-Dawley rats were used in the study. Excision and end-to-end anastomosis was performed in the distal colon of the rat. The Famotidine Group received 2 mg/kg/day famotidine; the Control Group received the same amount of saline. Bursting pressure of anastomoses and hydroxyproline content of perianastomotic tissues were evaluated on the third and seventh days following surgery. RESULTS: Bursting pressures and hydroxyproline contents for the Famotidine Group were significantly lower than the equivalent parameters for the Control Group on both the third and seventh days post-surgery. CONCLUSIONS: According to our findings, famotidine exerts detrimental effects on the anastomotic bursting pressure and hydroxyproline content of perianastomotic tissues in the colon of rats.
Colonic anastomosis; Anastomotic healing; Famotidine; Histamine
CLINICAL SCIENCE
Effects of the histamine H2 receptor antagonist famotidine on the healing of colonic anastomosis in rats
Aydin Inan; Meral Sen; Önder Sürgit; Metin Ergin; Mikdat Bozer
Fatih University School of Medicine - Ankara, Türkiye. Email: aydininan@ttmail.com. Tel: +90 312 2035555
ABSTRACT
BACKGROUND: Failure of anastomotic healing is one of the major complications in colorectal surgery. Because histamine plays an important role in immune and inflammatory reactions, we demonstrate the effects of famotidine on the healing of colonic anastomosis in rats.
METHODS: Twenty-eight Sprague-Dawley rats were used in the study. Excision and end-to-end anastomosis was performed in the distal colon of the rat. The Famotidine Group received 2 mg/kg/day famotidine; the Control Group received the same amount of saline. Bursting pressure of anastomoses and hydroxyproline content of perianastomotic tissues were evaluated on the third and seventh days following surgery.
RESULTS: Bursting pressures and hydroxyproline contents for the Famotidine Group were significantly lower than the equivalent parameters for the Control Group on both the third and seventh days post-surgery.
CONCLUSIONS: According to our findings, famotidine exerts detrimental effects on the anastomotic bursting pressure and hydroxyproline content of perianastomotic tissues in the colon of rats.
Keywords: Colonic anastomosis; Anastomotic healing; Famotidine; Histamine.
INTRODUCTION
Anastomotic leakage is a serious complication in colorectal surgery.1-3 Leakage rates vary greatly and are associated with 0 to 30% of all cases. Clinically apparent leakage rates are between 2.1 and 14.9%.1,2 Failure of anastomotic healing is associated with increased duration of hospital stay, morbidity and mortality.2-4 At least one-third of deaths following colorectal surgery are attributed to anastomotic leakage, although the reduction in the incidence of anastomotic dehiscence is due to contributing factors such as advances in perioperative care, bowel preparation, and surgical techniques.3
Healing of colon anastomoses involves a complex interaction of peptide growth factors and collagen turnover through the phases of inflammation, fibroplasias, and maturation.5-7
Histamine plays an important role in immune reactions, in the coagulation cascade, and in various related inflammatory reactions. Histamine H2 receptors accelerate cell proliferation, thereby impacting both lymphocyte and immune system response.8,9
Acid suppressive drugs (H2 receptor antagonists and proton pump inhibitors) are used for the prevention and treatment of stress-related mucosal diseases and other acid-related disorders.10
In the context of these histamine-induced effects, we decided to examine the effects of famotidine, an H2 receptor antagonist, on the healing of the anastomosis of the colon.
METHODS
Twenty-eight Sprague-Dawley male rats weighing 230-255 g were used in this experiment. A biostatistician was consulted to determine the appropriate number of rats per group. The animals had free access to water and were fed standard rodent chow throughout the experiment. Rats were housed for one week prior to surgical intervention. The Ethics Committee of Fatih University Medical School approved the experiment.
All animals were anesthetized by subcutaneous injection of 30 mL/kg ketamine (Ketalar, Eczac1ba_1, Türkiye). The abdomen was shaved and prepared using povidone-iodine and a sterile dressing. A laparotomy was performed with a 2 cm lower midline incision. An end-to-end anastomosis was performed after excision of a 0.5 cm segment of the distal colon, 3 cm proximal to the peritoneal reflection. Ten to twelve interrupted 6/0 polypropylene (Prolene, Ethicon) inverting sutures were used in the anastomosis. The abdominal fascia was closed with 3/0 continuous polyglactine (Polysorb, Tyco) sutures, and the skin was closed with 4/0 polyglactine (Polysorb, Tyco) in a subcuticular manner.
The animals were randomly assigned to two groups. The Famotidine Group (14 rats) received 2 mg/kg/day (0.5 mL/kg/day) famotidine (Nevofam-L amp, Mustafa Nevzat), while the Control Group (14 rats) received the same amount of saline subcutaneously. This dose of famotidine is almost equivalent to double the maximal dose used clinically. The justification for this dose selection was to more clearly elicit the possible effects of famotidine. Following surgery, the rats were allowed free access to water and resumed standard feeding.
Seven rats from each group were randomly selected to assess the bursting pressure and hydroxyproline content of anastomosis on the third day following surgery. The remaining seven rats from each group underwent the same procedure on the seventh day following surgery.
After anesthesia was maintained with 30 mL/kg Ketamine, the abdomen was opened, and the whole colon was resected. Great attention was paid to preserve the adhesions. Wide resections were performed when necessary. The proximal colon was attached to a continuous pressure monitoring system with a transducer (Peta_, KMA365B) and supplied with air (1 mL/minute) with an air pump. The distal colon was occluded. The entire colon was immersed in saline, and the air pump was activated. The maximum pressure before bubbles were seen was recorded as the bursting pressure for each rat. After the bursting pressure was determined, the peri-anastomotic region was cleared. A 1-cm colon segment (0.5 cm from each side of the anastomosis) was resected for hydroxyproline determination and stored at -40°C. Hydroxyproline content determinations of the samples were performed as described by Jamall et al.11
SPSS for Windows® 10.0 was used for the statistical analysis. Differences between groups in terms of bursting pressure and hydroxyproline content were assessed using a Mann-Whitney U test. The statistical significance was set to p<0.05.
RESULTS
The effects of famotidine on healing of a colonic anastomosis were examined in rats. All rats survived the experiment, and all anastomoses were intact on both the third and seventh days post surgery.
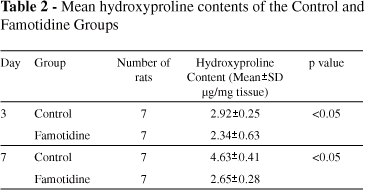
Recorded bursting pressures for the Famotidine Group were significantly lower than those for the Control Group on both the third and seventh days post surgery (Table 1). The mean hydroxyproline content was significantly lower for the Famotidine Group than for the Control Group (Table 2).
DISCUSSION
Wound healing is a complex cascade of overlapping events that depends on a number of cellular mechanisms and signaling pathways. It requires the coordinated completion of a variety of cellular activities that include phagocytosis, chemotaxis, migration, proliferation, adhesion and differentiation. The end result is synthesis and cross-linking of collagen and remodeling of the connective tissue.6,12,13 The process of anastomotic healing is similar to observations in the skin.14 The inflammatory phase is essential for healing, with hemostasis preceding inflammation.15 Any factor that disrupts one or more steps in the healing process will likely result in impaired anastomotic healing.16 Anastomotic dehiscence is a serious complication that causes significant morbidity and mortality in colorectal surgery.2,4,17
Bursting pressure and hydroxyproline determinations are measures that offer insight into the anastomosis healing process.2,18-20 Bursting pressure reveals the mechanical parameters of a colonic anastomosis and reflects growing anastomotic strength. Biochemical parameters of anastomotic healing are reflected by the collagen content in perianastomotic tissues, as determined by hydroxyproline content.19 In our experiments, we measured the bursting pressure of the anastomosis and the hydroxyproline levels of the perianastomotic tissue to determine healing rates.
Histamine significantly influences healing.21 Histamine is one of the regulators of mesenteric blood flow, leading to vasodilatation and increasing the regional blood flow in the mesentery22. Histamine offers homeostatic control of the circulation under both normal and pathologic conditions and causes vasodilatation of the intestine.23 H2 receptor antagonists inhibit the vasodilatory effects of histamine.22 However, blood supply is one of the determinants of anastomotic healing and is of paramount importance.24,25
Histamine also takes part in the regulation of proliferation and angiogenesis and may play an important role in the growth of both normal and malignant tissue.8 The blood supply is dependent on the formation of new blood vessels in the anastomosis. 25
An initial consequence of injury is the exposure of collagen in the vascular sub-endothelium. Platelets attach to collagen, secrete their granule constituents, and form aggregates.26 Histamine H2 receptor antagonists reduce platelet aggregation.27
Histamine participates in the regulation of immune reactions, in the coagulation cascade and in inflammatory responses and the formation of proinflammatory mediators.8,28 Histamine H2 receptors accelerate cell proliferation and affect the immune system. H2 receptor antagonists reverse the histamine-induced increases in IL-4, IL-5, and interferon-γ levels.8 IL-4 plays a role in wound fibroplasia, and interferon-γ has effects on fibroblast proliferation, both of which are components of wound healing.15,29
Cimetidine, an H2 receptor antagonist, effectively inhibits inflammation-generated increases in nitric oxide concentrations.28 It is known that nitric oxide plays role in phagocytosis and antimicrobial function and wound healing.15
Some studies have found that pretreatment with H1 antagonists improves survival in the context of shock, while H2 antagonists exacerbate mortality in low flow scenarios.22
Very few studies have explored H2 blockers in the context of colorectal anastomosis. Ranitidine has been shown to have no effect on experimental anastomotic strength, but it did result in a lower incidence of septic complications in an animal study. We cannot directly compare the results of this study to our conclusions, as hydroxyproline levels were not reported and the bursting pressure was determined in a different manner.9 In our study, famotidine exerted a significant influence on anastomotic strength and decreased the collagen content of anastomoses in rats.
In conclusion, our study demonstrates that famotidine reduces the bursting pressure of colonic anastomosis and may negatively impact the collagen content of perianastomotic tissue. This is likely a result of impairment of the positive influences of histamine on healing. Further studies are needed to explain the mechanisms of action of famotidine in the context of anastomoses and to determine whether these findings are applicable to humans.
Received for publication on January 30, 2009
Accepted for publication on March 06, 2009
-
1Schwab R, Weßendorf S, Gutcke A, Becker HP. Early bursting strength of human colon anastomoses - an in vitro study comparing current anastomotic techniques. Langenbeck's Arch Surg. 2002;386:507-11.
-
2Månsson P, Zhang XW, Jeppsson B, Thorlacius H. Anastomotic healing in the rat colon: comparison between a radiological method, breaking strength and bursting pressure. Int J Colorectal Dis. 2002;17:420-5.
-
3Egger B, Inglin R, Zeeh J, Dirsch O, Huang Y, Büchler MW. Insulin-like growth factor I and truncated keratinocyte growth factor accelarate healing of left sided colonic anastomoses. Br J Surg. 2001;88:90-8.
-
4Shashidharan M, Lin KM, Ternent CA, Smyrk TC, Thorson AG, Blatchford GJ, et al. Influence of arginine dietary supplementation on healing colonic anastomosis in the rat. Dis Colon Rectum. 1999;42:1613-7.
-
5Efron DT, Most D, Shi HP, Tantry US, Barbul A. Modulation of growth factor and cytokine expression by nitric oxide during rat colon anastomotic healing. J Gastrointest Surg. 2003;7:393-9.
-
6Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on collagen characteristics in healing dermal wounds in rats. Molec Cellular Biochem. 1998;181:71-6.
-
7Kiyama T, Tajiri T, Tokunaga A, Yoshiyuki T, Barbul A. Tacrolimus enhances colon anastomotic healing in rats. Wound Repair Regen. 2002;10:308-13.
-
8Ashida Y, Denda M, Hirao T. Histamine H1 and H2 receptor antagonists accelerate skin barrier repair and prevent epidermal hyperplasia induced by barrier disruption in a dry environment. J Invest Dermatol. 2001;116:261-5.
-
9Apostolidis SA, Michalopoulos AA, Papadopoulos VN, Paramythiotis D, Zatagias A, Gigigs P, et al. Effect of ranitidine on healing of normal and transfusion-suppressed experimental anastomoses. Tech Coloproctol. 2004;8S:S104-107.
-
10Pisegna JR. Pharmacology of acid suppression in the hospital setting: focus on proton pump inhibition. Crit Care Med. 2002;30S:S356-S361.
-
11Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70-5.
-
12Khalili TM, Navarro RA, Middleton Y, Marguiles DR. Early postoperative enteral feeding increases anastomotic strength in a peritonitis model. Am J Surg. 2001;182:621-4.
-
13Chintra P, Sajithlal GB, Chandrakasan G. Influence of aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol. 1998;59:195-201.
-
14Mast BA. Healing in other tissues. Surg Clin N Am. 1997;77:529-47.
-
15Witte MB, Barbul A. General principles of wound healing. Surg Clin N Am. 1997;77:509-28.
-
16Hendriks JMH, Hubens G, Wuyts FL, Vermeulen P, Hubens A, Eyskens E. Experimental study of intraperitoneal suramin on the healing of colonic anastomoses. Br J Surg. 1999;86:1171-5.
-
17Stumpf M, Cao W, Klinge U, Klosterhalfen B, Kasperk R, Schumpelick V. Collagen distribution and expression of matrix metalloproteinases 1 and 13 in patients with anastomotic leakage after large-bowel surgery. Langenbeck's Arch Surg. 2002;386:502-6.
-
18Kiyama T, Onda M, Tokunaga A, Yoshiyuki T, Barbul A. Effect of early postoperative feeding on the healing of colonic anastomoses in the presence of intra-abdominal sepsis in rats. Dis Colon Rectum. 2000;43S:S54-58.
-
19Hendricks T, Mastboom WJB. Healing of experimental intestinal anastomoses. Dis Colon Rectum. 1990;33:891-901.
-
20Seyer-Hansen M, Andreassen TT, Cristensen H, Oxlund H. Effect of experimental diabetes and growth hormone administration on the strength of colonic anastomoses in rats. Eur Surg Res. 1999;31:419-8.
-
21Franzén L, Ghassemifar R, Malcherek P. Experimental mast cell activation improves connective tissue repair in the perforated rat mesentery. Agents Actions. 1991;33:371-7.
-
22Shahinian HK, Ferguson MK, Michelassi F. Effect of histamine receptor antagonists on rat mesenteric microcirculation. J Surg Res. 1987;42:703-7.
-
23Johnston BM, Owen DAA. Histamine, histamine antagonists and regional blood flow. European J Pharmacol. 1997;44:355-63.
-
24Thornton FJ, Barbul A. Healing in the gastrointestinal tract. Surg Clin N Am. 1997;77:549-73.
-
25Mall JW, Schwemk W, Philipp AW, Büttemeyer R, Pollmann C. Intraperitoneal administration of the angiogenesis inhibitor thalidomide does not impair anastomotic healing following large bowel resection in a rabbit model. World J Surg. 2003;27:1119-23.
-
26Saxena SP, McNichol A, Brandes LJ, Becker AB, Cerrard JM. A role of intracellular histamine in collagen-induced platelet aggregation. Blood. 1990;75:407-14.
-
27Horton MA, Amos RJ, Jones RJ. The effect of histamine H2 receptor antagonists on platelet aggregation in man. Scand J Haematol. 1993;31:15-9.
-
28Hunter RP, Short CR, McClure JR, Koch CE, Keowen ML, Vansteenhouse JL, et al. Cimetidine inhibits nitric oxide associated nitrate production in a soft-tissue inflammation model in the horse. J Vet Pharmacol Therap.1999;22:136-47.
-
29Henry G, Garner WL. Inflammatory mediators in wound healing. Surg Clin N Am. 2003;83:483-507.
Publication Dates
-
Publication in this collection
30 June 2009 -
Date of issue
June 2009
History
-
Accepted
06 Mar 2009 -
Received
30 Jan 2009