ABSTRACT:
Equid alphaherpesvirus type 1 (EHV-1) is distributed worldwide and is a major agent of abortion, respiratory and neurological disease in horses. No specific treatment is available for EHV-1 infection, yet the potential of antiviral therapy has been explored. In this study we investigated the in vitro activity of Acyclovir, Ganciclovir, Foscarnet, Famciclovir, Vidarabina and Cidofovir against EHV-1. For this, the MTT test was performed, in which all the tested drugs showed no toxicity up to 200μg/mL. Subsequently, different drug concentrations were submitted to viral plaque reduction assays in cell culture. The selectivity index (SI) of the compounds was determined using the cytotoxic concentration for 50% of cells (CC50), obtained by MTT, and effective drug concentration to inhibit by 50% the number of viral plaques (EC50). Ganciclovir (SI: 490; EC50: 1.9 μg/mL) was the most efficient and safest drug against EHV-1, followed by Cidofovir (SI: 150, EC50: 5.7μg/mL), Acyclovir (SI: 37.4, EC50: 22.2μg/mL), Famciclovir (SI: 25.1, EC50: 24.5μg/mL), Vidarabine (SI: 12.2, EC50: 40.9μg/mL) and Foscarnet (SI: 6.9, EC50: 49.5 μg/mL), respectively. These results indicated that Ganciclovir (followed by Cidofovir), is a promising candidate for use in in vivo experiments.
Key words:
EHV-1; plaque assay; Acyclovir; Ganciclovir; Foscarnet; Famciclovir; Vidarabine; Cidofovir
RESUMO:
O alfaherpesvírus equino tipo 1 (EHV-1) está amplamente distribuído nos rebanhos equinos de todo o mundo e é um dos principais agentes causadores de abortos, doença respiratória e neurológica em equinos. Ainda não há tratamento específico para a infecção pelo EHV-1 em equinos, mas o potencial da terapia antiviral tem sido investigado. Neste trabalho, foi investigada a atividade anti-herpética in vitro dos fármacos Aciclovir, Ganciclovir, Foscanet, Famciclovir, Vidarabina e Cidofovir frente ao EHV-1. Para isso, foi realizado o teste de MTT, em que todas as drogas não apresentaram citotoxicidade até a dose de 200μg/mL. A seguir, diferentes concentrações dos fármacos foram submetidas ao teste de redução de placas virais em cultivo celular. O índice de seletividade (IS) dos compostos foi determinado usando a concentração citotóxica para 50% dos cultivos celulares (CC50), obtida pelo MTT, e pela concentração dos fármacos efetiva para inibir em 50% o número de placas virais (EC50). O Ganciclovir (IS: 490; EC50: 1,9μg/mL) foi o mais eficiente e seguro frente ao EHV-1, seguido pelo Cidofovir (IS: 150; EC50: 5,7 μg/mL), Aciclovir (IS: 37,4; EC50: 22,2μg/mL), Famciclovir (IS: 25,1; EC50: 24,5μg/mL), Vidarabina (IS: 12,2; EC50: 40,9μg/mL) e Foscarnet (IS: 6,9; EC50: 49,5μg/mL). Estes resultados indicam que o Ganciclovir constitui-se em um candidato para uso em experimentos in vivo.
Palavras-chave:
EHV-1; ensaio de placa; Aciclovir; Ganciclovir; Foscarnet; Famciclovir; Vidarabina; Cidofovir
INTRODUCTION:
Viruses belonging to the family Herpesviridae affect a wide range of mammals, and the subfamily Alphaherpesvirinae harbors the main herpesviruses of veterinary importance, including Equid alphaherpesvirus 1 (EHV-1) (ICTV, 2016ICTV. International Committee on Taxonomy of Viruses. Virus Taxonomy: 2016 Release. Available from: <http://ictvonline.org/virusTaxonomy.asp>. Accessed: Oct. 30, 2017.
http://ictvonline.org/virusTaxonomy.asp...
). This virus produces acute and latent infection in horses, whose reactivation and virus shedding may occur under immunosuppression and stressful situations (WALTER et al., 2013WALTER, J. et al. Clinical observations and management of a severe equine herpesvirus type 1 outbreak with abortion and encephalomyelitis. Acta Veterinaria Scandinavica, v. 55, n. 1, p. 19, 2013. Available from: <Available from: https://www-ncbi-nlm-nih-gov.ez47.periodicos.capes.gov.br/pmc/articles/PMC3630004/
>. Accessed: Oct. 23, 2017. doi: 10.1186/1751-0147-55-19.
https://www-ncbi-nlm-nih-gov.ez47.period...
).
The EHV-1 presents a worldwide distribution, and in Brazil, the first isolation occurred in 1966 in São Paulo State (NILSON & CORRÊA, 1966NILSON, M.R.; CORRÊA, W.M. Isolation of the equine abortion virus in the state of São Paulo. Arquivos do Instituto Biológico, v.33, p.23-25, 1966.). Since then, several reports of virus isolation and antibody detection have been described, demonstrating the wide distribution of EHV-1 in the country (WEIBLEN et al., 1994WEIBLEN, R. et al. Abortion due to equine herpesvirus in southern Brazil. Brazilian Journal of Medical and Biological Research, v. 27, p. 1317-1320, 1994.; HEINEMANN et al., 2002HEINEMANN, M.B. et al. Seroprevalence of equine infection anemia, equine viral arteritis and equine viral abortion in Uruará municipality, Pará state, Brazil. Brazilian Journal of Veterinary Research and Animal Science, v.39, n.1, p.50-53, 2002. Available from: <Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1413-95962002000100009&lng=en&nrm=iso&tlng=pt
>. Accessed: Oct. 27, 2017. doi: 10.1590/S1413-95962002000100009.
http://www.scielo.br/scielo.php?script=s...
; DIEL et al., 2006DIEL, D.G. et al. Prevalence of antibodies to influenza virus, viral arteritis and herpesvirus in horses of the Rio Grande do Sul state, Brazil. Ciência Rural, v.36, n.5, p.1467-1673, 2006. Available from: <Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-84782006000500019&lng=pt&tlng=pt
>. Accessed: Oct. 27, 2017. doi: 10.1590/S0103-84782006000500019.
http://www.scielo.br/scielo.php?script=s...
; LARA et al., 2010LARA, M.C.C.S.H. et al. Serological survey of equine herpesvirus infection in Minas Gerais state. Brazilian Journal of Veterinary Research and Animal Science, v.47, n.5, p. 352-356, 2010. Available from: <Available from: http://www.revistas.usp.br/bjvras/article/view/26815/28598
>. Accessed: Oct. 27, 2017. doi: 10.11606/is sn.1678-4456.bjvras.2010.26815.
http://www.revistas.usp.br/bjvras/articl...
).
The EHV-1 is one of the main agents involved in outbreaks of abortion in horses, and it is also associated with respiratory and neurological disease, causing important economic losses in breeding horses, mainly in pregnant and young animals (LUNN et al., 2009LUNN, D.P. et al. Equine herpesvirus-1 consensus statement. Journal of Veterinary Internal Medicine, v.23, p. 450-461, 2009. Available from: <Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1939-1676.2009.0304.x/pdf
>. Accessed: Oct. 23, 2017. doi: 10.1111/j.1939-167 6.2009.0304.x.
http://onlinelibrary.wiley.com/doi/10.11...
). The EHV-1-associated myeloencephalopathy (EHM) is a neurological condition considered emergent by the United States Department of Agriculture (USDA, 2007USDA: APHIS: VS: CEAH. Equine herpesvirus myeloencephalopathy: a potentially emerging disease, 2007. Available from: <Available from: https://www.aph is.usda.gov/anim al_health/emergingissue s/downloads/ehv1final.pdf
>. Accessed: Oct. 23, 2017.
https://www.aph is.usda.gov/anim al_heal...
). The disease may affect a large number of animals, similar to those that occurred in riding schools, racetracks and veterinary hospitals throughout North America and Europe (HENNINGER et al., 2007HENNINGER R.W. et al. Outbreak of neurologic disease caused by equine herpesvirus-1 at a university equestrian center. Journal Veterinary Internal Medicine, v. 21, p.157-165, 2007. Available from: <Available from: https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1939-1676.2007.tb02942.x
> Accessed: Oct. 23, 2017. doi: 10.1111/j.1939-1676.2007.tb02942.x.
https://onlinelibrary.wiley.com/doi/epdf...
; WALTER et al., 2013WALTER, J. et al. Clinical observations and management of a severe equine herpesvirus type 1 outbreak with abortion and encephalomyelitis. Acta Veterinaria Scandinavica, v. 55, n. 1, p. 19, 2013. Available from: <Available from: https://www-ncbi-nlm-nih-gov.ez47.periodicos.capes.gov.br/pmc/articles/PMC3630004/
>. Accessed: Oct. 23, 2017. doi: 10.1186/1751-0147-55-19.
https://www-ncbi-nlm-nih-gov.ez47.period...
). Cases of EHM and respiratory disease may occur even in vaccinated herds, in spite of vaccination since the efficacy of vaccines is questioned (PUSTERLA et al., 2009PUSTERLA, N. et al. Equine herpesvírus-1 myeloencephalopathy: A review of recent developments. The Veterinary Journal, v. 180, p. 279-289, 2009. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S1090023308002864?via%3Dihub
>. Accessed: Oct. 23, 2017. doi: 10.1016/j.tvjl.2008.08.004.
https://www.sciencedirect.com/science/ar...
). Thus, in addition to vaccination, it is necessary to implement sanitary and management measures to susceptible and/or affected animals, aiming to reduce virus introduction and dissemination in equine herds, since there is no specific treatment for EHV-1 infection (LUNN et al., 2009LUNN, D.P. et al. Equine herpesvirus-1 consensus statement. Journal of Veterinary Internal Medicine, v.23, p. 450-461, 2009. Available from: <Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1939-1676.2009.0304.x/pdf
>. Accessed: Oct. 23, 2017. doi: 10.1111/j.1939-167 6.2009.0304.x.
http://onlinelibrary.wiley.com/doi/10.11...
).
Anti-herpetic therapy is a common practice in human medicine, yet is rarely used against animal herpesviruses. In this sense, Acyclovir has been the main drug used to treat animal viral infections, as BoHV-1, FeHV-1 and EHV-1 and EHV-4. This drug presented varied efficacy in vivo when used according to the required concentration to reduce 50% of viral plaques (EC50) (VISSANI et al., 2016VISSANI, M. A. et al. Antiviral agents against equid alphaherpesviruses: Current status and Perspectives. The Veterinary Journal , v. 207, p. 38-44, 2016. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S1090023315002543
>. Accessed: Nov. 14, 2017. doi: 10.10 16/j.tvjl.20.15.06.010.
https://www-sciencedirect-com.ez47.perio...
). Although, there are some investigations regarding to anti-EHV-1 activity in vitro using human anti-herpetics drugs (GARRÉ et al., 2007GARRÉ, B. et al. In vitro susceptibility of six isolates of equine herpesvirus 1 to acyclovir, ganciclovir, cidofovir, adefovir, PMEDAP and foscarnet. Veterinary Microbiology, v.122, p. 43-45, 2007. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0378113507000120
>. Accessed: Oct. 27, 2017. doi: 10.1016/j.vetmic.2007.01.004.
https://www-sciencedirect-com.ez47.perio...
; AZAB et al., 2010AZAB, W. et al. Characterization of a thymidine kinase-deficient mutant of equine herpesvirus 4 and in vitro susceptibility of the virus to antiviral agents. Antiviral Research, v. 85, p. 389 - 395, 2010. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0166354209005361
>. Accessed: Oct. 15, 2017. doi: 10.1016/j.antiviral.2009.11.007.
https://www-sciencedirect-com.ez47.perio...
), there is no defined guidelines on formulations available for treatment. Thus, more detailed in vitro studies are needed to verify the possibility of in vivo therapy using these drugs. Therefore, the aim of this research paper was to investigate, the susceptibility of EHV-1 in vitro to six antiviral drugs used in human medicine.
MATERIALS AND METHODS:
Experimental design
The in vitro susceptibility of EHV-1 to antiviral drugs Acyclovir (ACV), Ganciclovir (GCV), Foscarnet (PFA), Famciclovir (FAM), Vidarabine (VID) and Cidofovir (CDV) was evaluated by plaque reduction assay (PRA). Initially, the cellular toxicity of the drugs was investigated by MTT. Then, the PRA was performed testing each drug against EHV-1. Lastly, the selectivity index (SI) of each compound was determined.
Cells and virus
Vero cells (African Green Monkey kidney - ATCC CCL-81, passage 65) was used for amplification and quantification of EHV-1 and herpesvirus simplex type 1 (HSV-1), cytotoxicity and PRA. Cells were cultured in RPMI medium (Roswell Park Memorial Institute), supplemented with 10% fetal bovine serum (FBS), antibiotics (streptomycin 0.4mg/mL; penicillin 1.6mg/mL) and antifungic (amphotericin B 0.0025mg/mL). The strain EHV-1 Kentucky D (p.10, Genbank: AB279610), kindly provided by Dr. Rodrigo Franco (Instituto Butantan, SP, Brasil) was tested against the six drugs. The HSV-1 KOS strain (Dr. Paulo Michel Roehe, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brasil) was used as control of drug efficacy.
Antiviral drugs
Six commercial anti-herpetic drugs used to treat human herpetic infections were used: ACV (molecular weight - MW 225.21; Dermapelle Farmácia de Manipulação, Santa Maria, RS, BR), GCV (MW 255.2; Sigma-Aldrich, St Louis, MO, USA), PFA (MW 300.04; Sigma-Aldrich, St Louis, MO, USA), FAM (MW 321.3; Sigma-Aldrich, St Louis, MO, USA), VID (MW 285.2; Sigma-Aldrich, St Louis, MO, USA) and CDV (MW 279.1; Sigma-Aldrich, St Louis, MO, USA). Drugs were diluted to 1mg/mL in saline solution (ACV); saline solution with 0.5% dimethyl sulfoxide (DMSO) (GCV); ultrapure water (PFA, CDV e VID); or only in dimethyl sulfoxide (DMSO) (FAM).
Cytotoxicity assay
Cytotoxicity of the six drugs was determined by MTT test (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) adapted from GARRÉ et al. (2007GARRÉ, B. et al. In vitro susceptibility of six isolates of equine herpesvirus 1 to acyclovir, ganciclovir, cidofovir, adefovir, PMEDAP and foscarnet. Veterinary Microbiology, v.122, p. 43-45, 2007. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0378113507000120
>. Accessed: Oct. 27, 2017. doi: 10.1016/j.vetmic.2007.01.004.
https://www-sciencedirect-com.ez47.perio...
). For this, Vero cells (2x106 cells/ 96-well plates) were incubated at 37ºC with CO2 at 5% and, after 24h, five different dilutions of each drugs were added onto cell monolayers (1; 10; 100; 200 and 500μg/mL). After 72h of incubation, the supernatant was removed and 50μL/well of MTT was added, followed by incubation for 2h at 37ºC in a CO2 incubator at 5%. Then, the MTT was replaced by 150μL/well of DMSO, and absorbance (in optical density - OD) was measured at a wave-length of 550nm in microplate reader. Cell viability at each drug concentration was determined by the formula: .
These results allowed determining the cytotoxic concentration to 50% of the cells (CC50), by linear regression analysis. According to GARRÉ et al. (2007GARRÉ, B. et al. In vitro susceptibility of six isolates of equine herpesvirus 1 to acyclovir, ganciclovir, cidofovir, adefovir, PMEDAP and foscarnet. Veterinary Microbiology, v.122, p. 43-45, 2007. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0378113507000120
>. Accessed: Oct. 27, 2017. doi: 10.1016/j.vetmic.2007.01.004.
https://www-sciencedirect-com.ez47.perio...
), in the MTT test, the evaluated drug concentration is considered cytotoxic when the cell viability is less than 80% when compared to controls.
Plaque reduction assays (PRA)
The antiviral activity of the drugs against EHV-1 and HSV-1 was determined by PRA, using Vero cells seeded in six-well polystyrene plates (0.3x106 cells/well). Initially, three wells of Vero cells monolayers received 1mL of RPMI containing the different drug concentrations (0.1; 1; 10; 25; 75 and 100μg/mL) and incubated for 1h at 37ºC in a CO2 incubator at 5%. Two other wells were maintained as viral and cellular controls, receiving 1mL of RPMI. Next, the supernatant was removed, and 1mL of virus suspension containing 100 plaque forming units (PFU) of the respective virus was added. After 2h of adsorption, the inoculum was removed and cell monolayers were covered with 3mL of solid medium culture, containing RPMI, 10% fetal bovine serum and 1% low melting point agar, besides the different concentrations (μg/mL) of each drug. The plates were incubated at 37ºC in a CO2 incubator at 5%. After 72h, the solid medium was removed and the infected monolayers were fixed and stained with 2ml/well of violet crystal solution and formalin (0.65g violet crystal; 62.5ml formalin and 500ml water q.s.p.). Finally, viral plaques were counted in both treated and control cells.
The inhibitory effect of antiviral compounds was determined by the formula: .
The concentration of each effective drug to inhibit 50% of the viral plaques (EC50) and the selectivity index (SI) were calculated by linear regression analysis, considering line equation appropriate when the value R2 was equal to or greater than 0.9. To EC50, the line equation used was:
. The SI is the relationship between drug CC50 (acquired by MTT) and EC50. The value obtained in SI allowed estimating drug safety level for use in animals. The higher is the value (above 1), more significant will be the difference between the antiviral dose necessary to reduce in 50% the virus replication (EC50) and cytotoxic dose (CC50); therefore, safer the drug for use in animals (COEN & RICHMAN, 2007COEN, D.M.; RICHMAN, D.D. Antiviral agents. In: KNIPE, D.M.; HOWLEY, P.M. Fields Virology. 5.ed. Philadelphia: Williams & Wilkins, 2007. Cap. 14, p. 447-485. ; DEZENGRINI et al., 2010DEZENGRINI, R. et al. Activity of three antiviral drugs against bovine herpesviruses 1, 2 and 5 in cell culture. Pesquisa Veterinária Brasileira, v.30, n.10, p. 855-860, 2010. Available from: <Available from: http://www.scielo.br/scielo.php?pid=S0100-736X201000100000 8&script=sci_abstr act&tlng=pt
>. Accessed: Sept. 22, 2017. doi: 10.1590/S0100-736X20100 01000008.
http://www.scielo.br/scielo.php?pid=S010...
).
Data analysis
Two independent tests were conducted, in triplicate, for each experiment. The data obtained was statistically analyzed by the analysis of variance (ANOVA) through the program GraphPad Prism 6.
RESULTS AND DISCUSSION:
In the MTT tests performed with Vero cells, only the drugs Ganciclovir and Cidofovir did not present cytotoxicity at the concentration of 500μg/mL. These results are compatible with those obtained by GARRÉ et al. (2007GARRÉ, B. et al. In vitro susceptibility of six isolates of equine herpesvirus 1 to acyclovir, ganciclovir, cidofovir, adefovir, PMEDAP and foscarnet. Veterinary Microbiology, v.122, p. 43-45, 2007. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0378113507000120
>. Accessed: Oct. 27, 2017. doi: 10.1016/j.vetmic.2007.01.004.
https://www-sciencedirect-com.ez47.perio...
). However, all evaluated drugs promoted cell viability greater than 80% when 200μg/mL was used. Then, all assays were performed using lower doses than 200μg/mL of each drug.
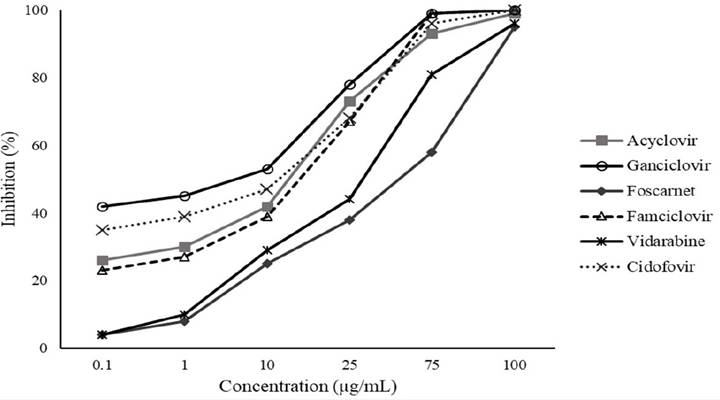
At PRA, the most effective drug against EHV-1 was GCV (EC50: 1.9), followed by CDV (EC50: 5.7), ACV (EC50: 22.2), FAM (EC50: 24.5), VID (EC50: 40.9) and PFA (EC50: 49.5), respectively (Table 1, Figure 1). Most drugs completely inhibited HSV-1 replication at concentrations 75 and 100μg/mL, except FAM that did not show complete antiviral activity at the concentration of 75μg/mL. The HSV, prototype of the subfamily Alphaherpesvirinae, is widely used as control to development of antiviral chemotherapy/drugs, because it was the first virus effectively treated using an antiviral compound (Idoxuridine) (PRUSOFF, 1959PRUSOFF, W.H. Synthesis and biological activities of iododeoxyuridine, an analog of thymidine. Biochimica et Biophysica Acta, v.32, p. 295-296, 1959. Available from: <Available from: https://wwwsciencedirectcom.ez47.periodicos.capes.gov.br/science/article/pii/0006300259905979?via%3Dihub
>. Accessed: Sept. 12, 2017. doi: 10.1016/0006-3002(59)90597-9.
https://wwwsciencedirectcom.ez47.periodi...
); and, therefore, it was used as control of the antivirals tested.
Percentage reduction in the number of Equine herpesvirus 1 (EHV-1) plaques in cell culture produced by Acyclovir, Ganciclovir, Foscarnet, Famciclovir, Vidarabine and Cidofovir.
The GCV presented lower EC50 resulting in the highest SI (Table 1). Then, it was the most effective and safest drug tested. The result obtained for this drug resembles that observed by MEULEN et al. (2006MEULEN, K.V.D. et al. In vitro comparison of antiviral drugs against feline herpesvirus 1. BioMed Central Veterinary Research, v.2, p.1-7, 2006. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1475582/
>. Accessed: Oct. 17, 2017. doi: 10.1186%2F1746-6148-2-13.
https://www.ncbi.nlm.nih.gov/pmc/article...
) and for GARRÉ et al. (2007GARRÉ, B. et al. In vitro susceptibility of six isolates of equine herpesvirus 1 to acyclovir, ganciclovir, cidofovir, adefovir, PMEDAP and foscarnet. Veterinary Microbiology, v.122, p. 43-45, 2007. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0378113507000120
>. Accessed: Oct. 27, 2017. doi: 10.1016/j.vetmic.2007.01.004.
https://www-sciencedirect-com.ez47.perio...
), who evaluated the susceptibility of six antiviral drugs against FeHV-1 and EHV-1, using feline kidney cells (CRFK) and equine embryonic lung cells (EEL), respectively. The CDV also induced notable decrease in the number of viral plaques, differing from the results obtained by GARRÉ et al. (2007)GARRÉ, B. et al. In vitro susceptibility of six isolates of equine herpesvirus 1 to acyclovir, ganciclovir, cidofovir, adefovir, PMEDAP and foscarnet. Veterinary Microbiology, v.122, p. 43-45, 2007. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0378113507000120
>. Accessed: Oct. 27, 2017. doi: 10.1016/j.vetmic.2007.01.004.
https://www-sciencedirect-com.ez47.perio...
, where this drug was not highly efficient in inhibiting virus replication; although, it was able to significantly reduce the plaque size even when very low concentrations were used. The differences observed in both studies may be related to variable susceptibility of EHV-1 isolates used in viral inhibition assays.
MEULEN et al. (2006MEULEN, K.V.D. et al. In vitro comparison of antiviral drugs against feline herpesvirus 1. BioMed Central Veterinary Research, v.2, p.1-7, 2006. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1475582/
>. Accessed: Oct. 17, 2017. doi: 10.1186%2F1746-6148-2-13.
https://www.ncbi.nlm.nih.gov/pmc/article...
) and GARRÉ et al. (2007GARRÉ, B. et al. In vitro susceptibility of six isolates of equine herpesvirus 1 to acyclovir, ganciclovir, cidofovir, adefovir, PMEDAP and foscarnet. Veterinary Microbiology, v.122, p. 43-45, 2007. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0378113507000120
>. Accessed: Oct. 27, 2017. doi: 10.1016/j.vetmic.2007.01.004.
https://www-sciencedirect-com.ez47.perio...
) showed that ACV had EC50 more than 20 times higher than GCV to FHV-1 and/or EHV-1, respectively. These results were similar to those obtained in our study, and indicated the higher efficacy of GCV compared to ACV against different virus species. Thus, GCV would be an attractive alternative to initiate in vivo experiments aiming to determine drug toxicity, bioavailability, pharmacokinetics and antiviral activity.
According to EC50 values, the activity of ACV and FAM against EHV-1 were similar, as well as reported by MAXWELL et al. (2008MAXWELL, L.K. et al. Pharmacokinetics of valacyclovir in the adult horse. Journal of Veterinary Pharmacology and Therapeutics, v.31, n.4, p. 312-20, 2008. Available from: <Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2885.2008.00957.x/full
>. Accessed: Sept. 22, 2017. doi: 10.1111/j.1365-2885.2008.00957.x.
http://onlinelibrary.wiley.com/doi/10.11...
). However, in horses, there is no pharmacokinetic study yet using FAM, possibly due to the high cost of the drug and the unavailability of parenteral products (MAXWELL, 2017MAXWELL, L.K. Antiherpetic drugs in equine medicine. Veterinary Clinics of North America: Equine Practice, v. 33, n.1, p. 99-125, 2017. Available from: <Available from: https://www-sciencedirectcom.ez47.periodicos.capes.gov.br/science/article/pii/S0749073916300852#tbl2
>. Accessed: Oct. 17, 2017. doi: 10.1016/j.cveq.2016.12.002.
https://www-sciencedirectcom.ez47.period...
). Though, in the study carried out by MALIK et al. (2009MALIK, R. et al. Treatment of feline herpesvirus-1 associated disease in cats with famciclovir and related drugs. Journal of Feline Medicine and Surgery, v.11, n.1, p.40-48, 2009. Available from: <Available from: http://www.jfm.sagepub.com/content/11/1/40.full.pdf
>. Accessed: Nov. 14, 2017. doi: 10.1016/j.jfms.2008.11.012.
http://www.jfm.sagepub.com/content/11/1/...
), FAM was a promising systemic drug for the treatment of ocular diseases attributed to FeHV-1, being more effective than ACV and its prodrug Valacyclovir. FAM resembles ACV in the structure, selectivity and mechanism of action. However, FAM has longer action, due to the longer half-life of its metabolites, formed after the uptake of the drug by the cells, thus decreasing the duration of the lesion (MAXWELL, 2017MAXWELL, L.K. Antiherpetic drugs in equine medicine. Veterinary Clinics of North America: Equine Practice, v. 33, n.1, p. 99-125, 2017. Available from: <Available from: https://www-sciencedirectcom.ez47.periodicos.capes.gov.br/science/article/pii/S0749073916300852#tbl2
>. Accessed: Oct. 17, 2017. doi: 10.1016/j.cveq.2016.12.002.
https://www-sciencedirectcom.ez47.period...
).
The drugs GCV and ACV replaced VID in the treatment of HSV infections, because they are metabolically less toxic and more stable (SHEN et al., 2009SHEN, W. et al. 5-O-D-VALYL ara A, A potential prodrug for improving oral biovailability of the antiviral agent vidarabine, National Institute of Health, v. 28, n.1, p. 43-55, 2009.). In equine medicine, there are few reports of use of VID against EHV-1. In this study, this drug presented moderately satisfactory results, being more efficient that PFA. The PFA reduced the number of viral plaques, but presented the highest indexes of EC50 and lower SI for the EHV-1. These results differed from those obtained by DEZENGRINI et al. (2010DEZENGRINI, R. et al. Activity of three antiviral drugs against bovine herpesviruses 1, 2 and 5 in cell culture. Pesquisa Veterinária Brasileira, v.30, n.10, p. 855-860, 2010. Available from: <Available from: http://www.scielo.br/scielo.php?pid=S0100-736X201000100000 8&script=sci_abstr act&tlng=pt
>. Accessed: Sept. 22, 2017. doi: 10.1590/S0100-736X20100 01000008.
http://www.scielo.br/scielo.php?pid=S010...
), in which PFA presented the better results against BoHV-1, BoHV-2 and BoHV-5. Against EHV-1, in vitro antiviral activity and SI presented by PFA were lower than the other drugs tested, indicating that this drug should not be considered the main alternative for therapies and in vivo studies.
Results obtained by in vitro assays are essential for the choice of drug to be used for in in vivo studies. The use of animal models is an attractive alternative for in vivo studies of antiviral drugs, mainly to determine doses, administration routes, time of treatment, toxicity and drug efficacy. Experimental infection of rabbits with EHV-1 (KANITZ et al., 2015KANITZ, F. A. et al. Respiratory and neurological disease in rabbits experimentally infected with equid herpesvirus 1. Microbial Pathogenesis, v. 87, p. 45 - 50, 2015. Available from: <Available from: https://www-sciencedirectcom.ez47.periodicos.capes.gov.br/science/article/pii/S0882401015001072
>. Accessed: May 23, 2018. doi: 10.1016/j.micpath.2015.07.007.
https://www-sciencedirectcom.ez47.period...
) resulted in infection and development of respiratory and neurological signs. In this sense, the initial evaluation in vivo of the antiviral activity of drugs such as GCV, may be performed in this animal model, as they developed respiratory disease similar to naturally infected horses.
CONCLUSION:
The obtained results in plaque reduction assay indicate that the drug Ganciclovir is the safest and most effective against EHV-1. Thus, the compound has the potential to be used in in vivo experiments.
ACKNOWLEDGMENTS
RW and EFF are Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) fellowship (Grant #304153/2014-1 and #301414/2010-6, respectively). APGM and JFC are Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) fellowship.
REFERENCES:
- AZAB, W. et al. Characterization of a thymidine kinase-deficient mutant of equine herpesvirus 4 and in vitro susceptibility of the virus to antiviral agents. Antiviral Research, v. 85, p. 389 - 395, 2010. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0166354209005361 >. Accessed: Oct. 15, 2017. doi: 10.1016/j.antiviral.2009.11.007.
» https://doi.org/10.1016/j.antiviral.2009.11.007.» https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0166354209005361 - COEN, D.M.; RICHMAN, D.D. Antiviral agents. In: KNIPE, D.M.; HOWLEY, P.M. Fields Virology. 5.ed. Philadelphia: Williams & Wilkins, 2007. Cap. 14, p. 447-485.
- DEZENGRINI, R. et al. Activity of three antiviral drugs against bovine herpesviruses 1, 2 and 5 in cell culture. Pesquisa Veterinária Brasileira, v.30, n.10, p. 855-860, 2010. Available from: <Available from: http://www.scielo.br/scielo.php?pid=S0100-736X201000100000 8&script=sci_abstr act&tlng=pt >. Accessed: Sept. 22, 2017. doi: 10.1590/S0100-736X20100 01000008.
» https://doi.org/10.1590/S0100-736X20100 01000008.» http://www.scielo.br/scielo.php?pid=S0100-736X201000100000 8&script=sci_abstr act&tlng=pt - DIEL, D.G. et al. Prevalence of antibodies to influenza virus, viral arteritis and herpesvirus in horses of the Rio Grande do Sul state, Brazil. Ciência Rural, v.36, n.5, p.1467-1673, 2006. Available from: <Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-84782006000500019&lng=pt&tlng=pt >. Accessed: Oct. 27, 2017. doi: 10.1590/S0103-84782006000500019.
» https://doi.org/10.1590/S0103-84782006000500019» http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-84782006000500019&lng=pt&tlng=pt - GARRÉ, B. et al. In vitro susceptibility of six isolates of equine herpesvirus 1 to acyclovir, ganciclovir, cidofovir, adefovir, PMEDAP and foscarnet. Veterinary Microbiology, v.122, p. 43-45, 2007. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0378113507000120 >. Accessed: Oct. 27, 2017. doi: 10.1016/j.vetmic.2007.01.004.
» https://doi.org/10.1016/j.vetmic.2007.01.004.» https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S0378113507000120 - HEINEMANN, M.B. et al. Seroprevalence of equine infection anemia, equine viral arteritis and equine viral abortion in Uruará municipality, Pará state, Brazil. Brazilian Journal of Veterinary Research and Animal Science, v.39, n.1, p.50-53, 2002. Available from: <Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1413-95962002000100009&lng=en&nrm=iso&tlng=pt >. Accessed: Oct. 27, 2017. doi: 10.1590/S1413-95962002000100009.
» https://doi.org/10.1590/S1413-95962002000100009.» http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1413-95962002000100009&lng=en&nrm=iso&tlng=pt - HENNINGER R.W. et al. Outbreak of neurologic disease caused by equine herpesvirus-1 at a university equestrian center. Journal Veterinary Internal Medicine, v. 21, p.157-165, 2007. Available from: <Available from: https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1939-1676.2007.tb02942.x > Accessed: Oct. 23, 2017. doi: 10.1111/j.1939-1676.2007.tb02942.x.
» https://doi.org/10.1111/j.1939-1676.2007.tb02942.x.» https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1939-1676.2007.tb02942.x - ICTV. International Committee on Taxonomy of Viruses. Virus Taxonomy: 2016 Release. Available from: <http://ictvonline.org/virusTaxonomy.asp>. Accessed: Oct. 30, 2017.
» http://ictvonline.org/virusTaxonomy.asp - KANITZ, F. A. et al. Respiratory and neurological disease in rabbits experimentally infected with equid herpesvirus 1. Microbial Pathogenesis, v. 87, p. 45 - 50, 2015. Available from: <Available from: https://www-sciencedirectcom.ez47.periodicos.capes.gov.br/science/article/pii/S0882401015001072 >. Accessed: May 23, 2018. doi: 10.1016/j.micpath.2015.07.007.
» https://doi.org/10.1016/j.micpath.2015.07.007.» https://www-sciencedirectcom.ez47.periodicos.capes.gov.br/science/article/pii/S0882401015001072 - LARA, M.C.C.S.H. et al. Serological survey of equine herpesvirus infection in Minas Gerais state. Brazilian Journal of Veterinary Research and Animal Science, v.47, n.5, p. 352-356, 2010. Available from: <Available from: http://www.revistas.usp.br/bjvras/article/view/26815/28598 >. Accessed: Oct. 27, 2017. doi: 10.11606/is sn.1678-4456.bjvras.2010.26815.
» https://doi.org/10.11606/is sn.1678-4456.bjvras.2010.26815.» http://www.revistas.usp.br/bjvras/article/view/26815/28598 - LUNN, D.P. et al. Equine herpesvirus-1 consensus statement. Journal of Veterinary Internal Medicine, v.23, p. 450-461, 2009. Available from: <Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1939-1676.2009.0304.x/pdf >. Accessed: Oct. 23, 2017. doi: 10.1111/j.1939-167 6.2009.0304.x.
» https://doi.org/10.1111/j.1939-167 6.2009.0304.x.» http://onlinelibrary.wiley.com/doi/10.1111/j.1939-1676.2009.0304.x/pdf - MALIK, R. et al. Treatment of feline herpesvirus-1 associated disease in cats with famciclovir and related drugs. Journal of Feline Medicine and Surgery, v.11, n.1, p.40-48, 2009. Available from: <Available from: http://www.jfm.sagepub.com/content/11/1/40.full.pdf >. Accessed: Nov. 14, 2017. doi: 10.1016/j.jfms.2008.11.012.
» https://doi.org/10.1016/j.jfms.2008.11.012.» http://www.jfm.sagepub.com/content/11/1/40.full.pdf - MAXWELL, L.K. et al. Pharmacokinetics of valacyclovir in the adult horse. Journal of Veterinary Pharmacology and Therapeutics, v.31, n.4, p. 312-20, 2008. Available from: <Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2885.2008.00957.x/full >. Accessed: Sept. 22, 2017. doi: 10.1111/j.1365-2885.2008.00957.x.
» https://doi.org/10.1111/j.1365-2885.2008.00957.x.» http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2885.2008.00957.x/full - MAXWELL, L.K. Antiherpetic drugs in equine medicine. Veterinary Clinics of North America: Equine Practice, v. 33, n.1, p. 99-125, 2017. Available from: <Available from: https://www-sciencedirectcom.ez47.periodicos.capes.gov.br/science/article/pii/S0749073916300852#tbl2 >. Accessed: Oct. 17, 2017. doi: 10.1016/j.cveq.2016.12.002.
» https://doi.org/10.1016/j.cveq.2016.12.002.» https://www-sciencedirectcom.ez47.periodicos.capes.gov.br/science/article/pii/S0749073916300852#tbl2 - MEULEN, K.V.D. et al. In vitro comparison of antiviral drugs against feline herpesvirus 1. BioMed Central Veterinary Research, v.2, p.1-7, 2006. Available from: <Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1475582/ >. Accessed: Oct. 17, 2017. doi: 10.1186%2F1746-6148-2-13.
» https://doi.org/10.1186%2F1746-6148-2-13.» https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1475582/ - NILSON, M.R.; CORRÊA, W.M. Isolation of the equine abortion virus in the state of São Paulo. Arquivos do Instituto Biológico, v.33, p.23-25, 1966.
- PRUSOFF, W.H. Synthesis and biological activities of iododeoxyuridine, an analog of thymidine. Biochimica et Biophysica Acta, v.32, p. 295-296, 1959. Available from: <Available from: https://wwwsciencedirectcom.ez47.periodicos.capes.gov.br/science/article/pii/0006300259905979?via%3Dihub >. Accessed: Sept. 12, 2017. doi: 10.1016/0006-3002(59)90597-9.
» https://doi.org/10.1016/0006-3002(59)90597-9.» https://wwwsciencedirectcom.ez47.periodicos.capes.gov.br/science/article/pii/0006300259905979?via%3Dihub - PUSTERLA, N. et al. Equine herpesvírus-1 myeloencephalopathy: A review of recent developments. The Veterinary Journal, v. 180, p. 279-289, 2009. Available from: <Available from: https://www.sciencedirect.com/science/article/pii/S1090023308002864?via%3Dihub >. Accessed: Oct. 23, 2017. doi: 10.1016/j.tvjl.2008.08.004.
» https://doi.org/10.1016/j.tvjl.2008.08.004.» https://www.sciencedirect.com/science/article/pii/S1090023308002864?via%3Dihub - SHEN, W. et al. 5-O-D-VALYL ara A, A potential prodrug for improving oral biovailability of the antiviral agent vidarabine, National Institute of Health, v. 28, n.1, p. 43-55, 2009.
- USDA: APHIS: VS: CEAH. Equine herpesvirus myeloencephalopathy: a potentially emerging disease, 2007. Available from: <Available from: https://www.aph is.usda.gov/anim al_health/emergingissue s/downloads/ehv1final.pdf >. Accessed: Oct. 23, 2017.
» https://www.aph is.usda.gov/anim al_health/emergingissue s/downloads/ehv1final.pdf - VISSANI, M. A. et al. Antiviral agents against equid alphaherpesviruses: Current status and Perspectives. The Veterinary Journal , v. 207, p. 38-44, 2016. Available from: <Available from: https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S1090023315002543 >. Accessed: Nov. 14, 2017. doi: 10.10 16/j.tvjl.20.15.06.010.
» https://doi.org/10.10 16/j.tvjl.20.15.06.010.» https://www-sciencedirect-com.ez47.periodicos.capes.gov.br/science/article/pii/S1090023315002543 - WALTER, J. et al. Clinical observations and management of a severe equine herpesvirus type 1 outbreak with abortion and encephalomyelitis. Acta Veterinaria Scandinavica, v. 55, n. 1, p. 19, 2013. Available from: <Available from: https://www-ncbi-nlm-nih-gov.ez47.periodicos.capes.gov.br/pmc/articles/PMC3630004/ >. Accessed: Oct. 23, 2017. doi: 10.1186/1751-0147-55-19.
» https://doi.org/10.1186/1751-0147-55-19.» https://www-ncbi-nlm-nih-gov.ez47.periodicos.capes.gov.br/pmc/articles/PMC3630004/ - WEIBLEN, R. et al. Abortion due to equine herpesvirus in southern Brazil. Brazilian Journal of Medical and Biological Research, v. 27, p. 1317-1320, 1994.
-
0
CR-2018-0085.R1
Publication Dates
-
Publication in this collection
2018
History
-
Received
07 Feb 2018 -
Accepted
11 Oct 2018 -
Reviewed
09 Nov 2018