ABSTRACT:
The interactions between biological macromolecules have been important for biotechnology, but further understanding is needed to maximize the utility of these interactions. Calorimetric techniques provide information regarding these interactions through the thermal energy that is produced or consumed during interactions. Notable techniques include differential scanning calorimetry, which generates a thermodynamic profile from temperature scanning, and isothermal titration calorimetry that provide the thermodynamic parameters directly related to the interaction. This review described how calorimetric techniques can be used to study interactions between proteins and polysaccharides, and provided valuable insight into the thermodynamics of their interaction.
Key words:
isothermal titration calorimetry; differential scanning calorimetry; calorimetric techniques; biomolecules; proteins.
RESUMO:
As interações entre macromoléculas biológicas têm tido importante aplicação na biotecnologia, mas, para sua devida utilização, estudos mais detalhados são necessários. As técnicas calorimétricas permitem estudá-las ao serem capazes de fornecer informações referentes a essas interações através da energia térmica que é gerada ou absorvida durante o processo de interação. Dentre as técnicas que mais se destacam estão a Calorimetria Exploratória Diferencial, que é capaz de fornecer um perfil termodinâmico a partir de uma varredura de temperatura, e a Calorimetria de Titulação Isotérmica, que fornece parâmetros termodinâmicos diretamente relacionados ao processo de interação. Nesta revisão, descrevemos como essas técnicas calorimétricas podem ser efetivamente aplicadas no estudo das interações entre proteínas e polissacarídeos, com o propósito de obter informações valiosas sobre a termodinâmica da interação.
Palavras-chave:
de titulação isotérmica; calorimetria exploratória diferencial; técnicas calorimétricas; biomoléculas; proteínas
INTRODUCTION:
Characterizing the interactions between macromolecules greatly enhances understanding of biological systems and is useful for various applications in biotechnology. Macromolecular interactions can include bonds between substrates and enzymes, antigens and antibodies and smaller molecules like drugs and hormones linked to carrier proteins or receptor (ARMSTRONG et al., 2013ARMSTRONG, A. et al. Structural and thermodynamic insights into the recognition of native proteins by anti-peptide antibodies. Journal of Molecular Biology, v.425, n.11, p.2027-2038, 2013. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0022283613001460
>. Accessed: Dec. 16, 2014. doi: 10.1016/j.jmb.2013.02.031.
http://www.sciencedirect.com/science/art...
; NADEMI et al., 2013NADEMI, Z. et al. Characteristics of antibody responses in Pigeon Fanciers' lung. Molecular Immunology, v.54, n.2, p.227-232, 2013. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0161589012004828
>. Accessed: Dec. 16, 2014. doi:10.1016/j.molimm.2012.12.007.
http://www.sciencedirect.com/science/art...
; CAO et al., 2013CAO, Z. et al. Potential toxicity of sarafloxacin to catalase: Spectroscopic, ITC and molecular docking descriptions. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, v.115, p.457-463, 2013. Available from: <Available from: http://www.ncbi.nlm.nih.gov/pubmed/23871971
>. Accessed: Dec. 16, 2014. doi: 10.1016/j.saa.2013.06.093.
http://www.ncbi.nlm.nih.gov/pubmed/23871...
; RÀFOLS et al., 2014RÀFOLS, C. et al. Molecular interactions between some non-steroidal anti-inflammatory drugs (NSAID׳s) and bovine (BSA) or human (HSA) serum albumin estimated by means of isothermal titration calorimetry (ITC) and frontal analysis capillary electrophoresis (FA/CE). Talanta, v.130, p.241-250, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0039914014005165
>. Accessed: Oct. 14, 2014. doi: 10.1016/j.talanta.2014.06.060.
http://www.sciencedirect.com/science/art...
). In addition, proteins and polysaccharides are increasingly used in technological applications to form new products (DIARRASSOUBA et al., 2015DIARRASSOUBA, F. et al. Self-assembly of β-lactoglobulin and egg white lysozyme as a potential carrier for nutraceuticals. Food Chemistry, v.173, p.203-209, 2015. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0308814614015660
>. Accessed: Mar. 27, 2015. doi: 10.1016/j.foodchem.2014.10.009.
http://www.sciencedirect.com/science/art...
) and understanding how they interact will be critical to future technologies.
Almost all physical, chemical, or biological processes result in the produce or consume of thermal energy. Calorimetry, which means measuring heat, is the general term that describes all experiments in which thermal energy is measured in function of time or temperature. (WADSÖ, 1986WADSÖ, I. Bio-calorimetry. Trends in Biotechnology, v.4, n.2, p.45-51, 1986. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/0167779986901538
>. Accessed: Jun. 25, 2015. doi: 10.1016/0167-7799(86)90153-8.
http://www.sciencedirect.com/science/art...
; IUPAC, 1994IUPAC. Nomenclature of thermometric and enthalpimetric methods in chemical analysis. In: STAHL, J.W. Pure & Applied Chemistry, v.66, n.12, p.2487-2492, 1994. Available from: <Available from: http://pac.iupac.org/publications/pac/pdf/1994/pdf/6612x2487.pdf
>. Accessed: Jun. 25, 2015.
http://pac.iupac.org/publications/pac/pd...
; BROWN, 1998BROWN, M.E. Handbook of thermal analysis and calorimetry - Principles and practice. The Netherlands: Elsevier Science, 1998. V.1, 722.; GAISFORD & BRUCKTON, 2001GAISFORD, S.; BUCKTON, G. Potential applications of microcalorimetry for the study of physical processes in pharmaceuticals. Thermochimica Acta, v.380, n.2, p.185-198, 2001. Available frm: <Available frm: http://www.sciencedirect.com/science/article/pii/S0040603101006694
>. Accessed: Apr. 29, 2015. doi: 10.1016/S0040-6031(01)00669-4.
http://www.sciencedirect.com/science/art...
; RUSSEL, et al., 2009RUSSEL, M. et al. Different technique of microcalorimetry and their applications to environmental sciences: a review. Journal of American Science, (n.d.), p.194-208, 2009. Available from: <Available from: http://citeseerx.ist.psu.edu/viewdoc/download?
> Accessed: Jun. 25, 2015. doi: 10.1.1.457.2750&rep=rep1&type=pdf>.
http://citeseerx.ist.psu.edu/viewdoc/dow...
). Currently, the term microcalorimetry defines heat measurements in a microwatt range (RUSSEL et al., 2009; IUPAC, 2014IUPAC. ICTAC nomenclature of thermal analysis. In: LEVER, T. et al., Pure & Applied Chemistry v.86, n.4, p.545-553, 2014. Available from: <Available from: http://www.ictac.org/ICTAC-IUPAC-TA_Nomenclature_2014.pdf
>. Accessed: Jun. 25, 2015.
http://www.ictac.org/ICTAC-IUPAC-TA_Nome...
). Measuring the heat flow in the calorimeter gives insight into the thermodynamic, chemical, and structural properties of a molecule (BROWN, 1998BROWN, M.E. Handbook of thermal analysis and calorimetry - Principles and practice. The Netherlands: Elsevier Science, 1998. V.1, 722.).
Calorimetric techniques are advantageous because they do not depend on the physical nature of the sample, rarely require any prior treatment, and are completely non-invasive. Furthermore, obtaining continuous and real-time data is an appealing aspect of these techniques. However, the high sensitivity and the non-specificity have both benefits and drawbacks because an improper sample preparations can cause incorrect interpretations of the results (WADSÖ, 1986WADSÖ, I. Bio-calorimetry. Trends in Biotechnology, v.4, n.2, p.45-51, 1986. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/0167779986901538
>. Accessed: Jun. 25, 2015. doi: 10.1016/0167-7799(86)90153-8.
http://www.sciencedirect.com/science/art...
; GAISFORD & BRUCKTON, 2001GAISFORD, S.; BUCKTON, G. Potential applications of microcalorimetry for the study of physical processes in pharmaceuticals. Thermochimica Acta, v.380, n.2, p.185-198, 2001. Available frm: <Available frm: http://www.sciencedirect.com/science/article/pii/S0040603101006694
>. Accessed: Apr. 29, 2015. doi: 10.1016/S0040-6031(01)00669-4.
http://www.sciencedirect.com/science/art...
; RUSSEL, et al., 2009RUSSEL, M. et al. Different technique of microcalorimetry and their applications to environmental sciences: a review. Journal of American Science, (n.d.), p.194-208, 2009. Available from: <Available from: http://citeseerx.ist.psu.edu/viewdoc/download?
> Accessed: Jun. 25, 2015. doi: 10.1.1.457.2750&rep=rep1&type=pdf>.
http://citeseerx.ist.psu.edu/viewdoc/dow...
). Calorimetric techniques that are well suited for the study of macromolecular interactions include isothermal titration calorimetry (ITC) and differential scanning calorimetry (DSC). ITC measures heat flow as a function of time and DSC measures heat flow as a function of temperature (GAISFORD & BRUCKTON, 2001GAISFORD, S.; BUCKTON, G. Potential applications of microcalorimetry for the study of physical processes in pharmaceuticals. Thermochimica Acta, v.380, n.2, p.185-198, 2001. Available frm: <Available frm: http://www.sciencedirect.com/science/article/pii/S0040603101006694
>. Accessed: Apr. 29, 2015. doi: 10.1016/S0040-6031(01)00669-4.
http://www.sciencedirect.com/science/art...
). Table 1 shows different applications that use ITC and/or DSC to study interactions between proteins and polysaccharides. This review article describes each calorimetric technique and its application to understanding of interactions between proteins and polysaccharides in food systems.
Differential scanning calorimetry (DSC)
The International Union of Pure and Applied Chemistry (IUPAC, 2014IUPAC. ICTAC nomenclature of thermal analysis. In: LEVER, T. et al., Pure & Applied Chemistry v.86, n.4, p.545-553, 2014. Available from: <Available from: http://www.ictac.org/ICTAC-IUPAC-TA_Nomenclature_2014.pdf
>. Accessed: Jun. 25, 2015.
http://www.ictac.org/ICTAC-IUPAC-TA_Nome...
) defines thermal analysis as the study of the relationship between a property of the sample and its temperature when it is heated or cooled in a controlled manner. DSC is more than a calorimetric technique; it is also considered a thermal analysis, where the physical property being studied is heat (IUPAC, 1994IUPAC. Nomenclature of thermometric and enthalpimetric methods in chemical analysis. In: STAHL, J.W. Pure & Applied Chemistry, v.66, n.12, p.2487-2492, 1994. Available from: <Available from: http://pac.iupac.org/publications/pac/pdf/1994/pdf/6612x2487.pdf
>. Accessed: Jun. 25, 2015.
http://pac.iupac.org/publications/pac/pd...
). DSC studies transitions or processes that gain or lose heat as a function of temperature in other words, when a substance is subjected to a temperature change, endothermic (heat absorption) or exothermic (heat generation) processes may occur. As most biological molecules of interest undergo transformations when subjected to temperature variations, it is possible to use DSC to determine the energy involved in such processes (JOHNSON, 2013JOHNSON, C.M. Differential scanning calorimetry as a tool for protein folding and stability. Archives of Biochemistry and Biophysics, v.531, n.1-2, p.100-109, 2013. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0003986112003530
>. Accessed: Aug. 28. 2014. doi: 10.1016/j.abb.2012.09.008.
http://www.sciencedirect.com/science/art...
).
In a DSC analysis, the sample and reference are heated in a controlled way. As a result, the instrument measures the difference of heat capacity at constant pressure (Cp
) (WADSÖ, 1986WADSÖ, I. Bio-calorimetry. Trends in Biotechnology, v.4, n.2, p.45-51, 1986. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/0167779986901538
>. Accessed: Jun. 25, 2015. doi: 10.1016/0167-7799(86)90153-8.
http://www.sciencedirect.com/science/art...
; HEERKLOTZ, 2004HEERKLOTZ, H. The microcalorimetry of lipids membranes. Journal of Physics Condensed Matter, v.16, n.15, p.441-467, 2004. Available from: <Available from: http://iopscience.iop.org/0953-8984/16/15/R01
>. Accessed: Jun. 24, 2015. doi: 10.1088/0953-8984/16/15/R01.
http://iopscience.iop.org/0953-8984/16/1...
). The Cp
is defined as the ability of the sample to absorb or release energy without changing temperature. Cp
is a fundamental property derived from other thermodynamic parameters such as enthalpy change (ΔH) and entropy change (ΔS), defined as follows (equations1 by invoking the Kirchhoff equation) (PIRES et al., 2009PIRES, A.C.S. et al. Microcalorimetry a food science and engineering approach. In: COIMBRA, J.; TEIXEIRA, J.A. Engineering aspects of milk and dairy products. Boca Raton: CRC, 2009. p.201-218.; PRIVALOV, 2015PRIVALOV, P.L. Microcalorimetry of macromolecules: the physical basis of biological structures. Journal of Solution Chemistry, v.44, n.5, p.1141-1161, 2015. Available from: <Available from: http://link.springer.com/article/10.1007/s10953-015-0337-x
>. Accessed: Jul. 13, 2015. doi: 10.1007/s10953-015-0337-x.
http://link.springer.com/article/10.1007...
):
The thermodynamic properties may be evaluated in accordance with the following standard relations (equations 2 to 4) (PRIVALOV, 2015PRIVALOV, P.L. Microcalorimetry of macromolecules: the physical basis of biological structures. Journal of Solution Chemistry, v.44, n.5, p.1141-1161, 2015. Available from: <Available from: http://link.springer.com/article/10.1007/s10953-015-0337-x
>. Accessed: Jul. 13, 2015. doi: 10.1007/s10953-015-0337-x.
http://link.springer.com/article/10.1007...
):
ΔH (T) = ΔH (Tt ) - ΔCp (Tt - T) (2)
ΔS (T) = ΔH (Tt ) / Tt - ΔC p ln(Tt |T) (3)
ΔG (T) = ΔH (T) - TΔS(T) (4)
Considering the equations above, when ΔH is negative and ΔS is positive, the free energy (ΔG) is negative and the interaction is spontaneous (O'BRIEN et al., 2001O'BRIEN, R. et al. Isothermal titration calorimetry of biomolecules. In: HARDING, S.E.; CHOWDHRY, B.Z. Protein-ligand interactions: hydrodynamics and calorimetry. New York: Oxford University, 2001. p.263-286.). DSC can provide a complete thermodynamic characterization of interactions induced by temperature. Regarding proteins, the literature has shown its application in determinating the melting enthalpy change (ΔHm
) and melting temperature (Tm
) (CAO et al., 2008; DAMODARAN & AGYARE 2013DAMODARAN, S.; AGYARE, K.K. Effect of microbial transglutaminase treatment on thermal stability and pH-solubility of heat-shocked whey protein isolate., Food Hydrocolloids v.30, n.1, p.12-18, 2013. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X12000872
>. Accessed: Jun. 02, 2015. doi: 10.1016/j.foodhyd.2012.04.012.
http://www.sciencedirect.com/science/art...
; TABILO-MUNIZAGA et al., 2014TABILO-MUNIZAGA, G. et al. Effects of high hydrostatic pressure (HHP) on the protein structure and thermal stability of Sauvignon blanc wine., Food Chemistry v.155, p.214-220, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0308814614000727
>. Accessed: Jun. 02, 2015. doi: 10.1016/j.foodchem.2014.01.051.
http://www.sciencedirect.com/science/art...
) and regarding polysaccharide measurements, DSC has been used to identify the glass transition (Tg) (LIU et al., 2007LIU, Y. et al. Study of glass transition and enthalpy relaxation of mixtures of amorphous sucrose and amorphous tapioca starch syrup solid by differential scanning calorimetry (DSC). Journal of Food Engineering, v.81, n.3, p.599-610, 2007. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0260877407000258
>. Accessed: Jun. 22, 2015. doi: 10.1016/j.jfoodeng.2006.12.017.
http://www.sciencedirect.com/science/art...
; HOMER et al., 2014HOMER, S. et al. Determination of the thermo-mechanical properties in starch and starch/gluten systems at low moisture content - A comparison of DSC and TMA. Carbohydrate Polymers, v.108, p.1-9, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0144861714001775
>. Accessed: Oct. 03, 2014. doi: 10.1016/j.carbpol.2014.02.049.
http://www.sciencedirect.com/science/art...
). For biomolecular interactions, DSC can identify an increase or decrease in the denaturation temperature and glass transition after an interaction compared to isolated molecules (VARDHANABHUTI et al., 2009VARDHANABHUTI, B. et al. Interactions between β-lactoglobulin and dextran sulfate at near neutral pH and their effect on thermal stability., Food Hydrocolloids v.23, n.6, p.1511-1520, 2009. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X08002233
>. Accessed: Oct. 25, 2014. doi: 10.1016/j.foodhyd.2008.09.006.
http://www.sciencedirect.com/science/art...
; HERNÁNDEZ et al., 2011HERNÁNDEZ, G.H.S. et al. Phase transitions of dairy proteins, dextrans and their mixtures as a function of water interactions., Food Hydrocolloids v.25, n.5, p.1311-1318, 2011. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X10002894
>. Accessed: Jun. 22, 2015. doi: 10.1016/j.foodhyd.2010.12.006.
http://www.sciencedirect.com/science/art...
; HOMER et al., 2014; MAO et al., 2014MAO, L. et al. Evaluation of volatile characteristics in whey protein isolate-pectin mixed layer emulsions under different environmental conditions., Food Hydrocolloids v.41, p.79-85, 2014. Available from: <Available from: http://www-sciencedirectcom.ez30.periodicos.capes.gov.br/science/article/pii/S0268005X14000988
>. Accessed: Sept. 10, 2014. doi: 10.1016/j.foodhyd.2014.03.025.
http://www-sciencedirectcom.ez30.periodi...
)
Most recent equipment used in DSC are highly sensitive and also highly stable. They also have large dynamic measurement ranges (below 0°C to over 100°C, under pressure) (PRIVALOV & DRAGAN, 2007PRIVALOV, P.L.; DRAGAN, A.I. Microcalorimetry of biological macromolecules. Biophysical Chemistry, v.126, n.1-3, p.16-24, 2007. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0301462206001608
>. Accessed: Jun. 25, 2015. doi: 10.1016/j.bpc.2006.05.004.
http://www.sciencedirect.com/science/art...
). In general, calorimeters control minimum temperature variation between the reference cell containing the buffer, and the sample cell containing the molecule of interest diluted with the buffer, both subjected to the same temperature program. As the changes temperature processes that generate or absorb energy (heat) occur in the sample cell and produce a temperature difference between the two cells. Heaters around the cells, to keep the difference between cells equal to zero, respond increasing the temperature in the reference cell when the process is exothermic and increasing the temperature of the sample cell when the process is endothermic. The amount of energy necessary to maintain thermal balance within the system is proportional to the energy change occurring in the sample (PIRES et al., 2009PIRES, A.C.S. et al. Microcalorimetry a food science and engineering approach. In: COIMBRA, J.; TEIXEIRA, J.A. Engineering aspects of milk and dairy products. Boca Raton: CRC, 2009. p.201-218.; JOHNSON, 2013JOHNSON, C.M. Differential scanning calorimetry as a tool for protein folding and stability. Archives of Biochemistry and Biophysics, v.531, n.1-2, p.100-109, 2013. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0003986112003530
>. Accessed: Aug. 28. 2014. doi: 10.1016/j.abb.2012.09.008.
http://www.sciencedirect.com/science/art...
).
There is also a heat flux DSC instrument that has a single heating system. For this instrument, the temperature difference between the sample and the reference cell is logged as the direct measure of the difference between heat flow rates (BROWN, 1998BROWN, M.E. Handbook of thermal analysis and calorimetry - Principles and practice. The Netherlands: Elsevier Science, 1998. V.1, 722.). However, this method overall is less accurate because the temperature measurement is less accurate than the energy input measurements (PIRES et al., 2009PIRES, A.C.S. et al. Microcalorimetry a food science and engineering approach. In: COIMBRA, J.; TEIXEIRA, J.A. Engineering aspects of milk and dairy products. Boca Raton: CRC, 2009. p.201-218.). To perform this calorimetric technique, all parameters such as heating rate, sample concentration, pH, presence of solutes, kind of container and instrumentation must be well defined for results to be interpreted (MA & HARWALKAR, 1996MA, C.; HARWALKAR, V. Effects of medium and chemical modification on thermal characteristics of β-lactoglobulin. Journal of Thermal Analysis, v.47, p.1513-1525, 1996. Available from: <Available from: http://link.springer.com/article/10.1007/BF01992843
>. Accessed: Jan. 19, 2015. doi: 10.1007/BF01992843.
http://link.springer.com/article/10.1007...
; HOMER et al., 2014HOMER, S. et al. Determination of the thermo-mechanical properties in starch and starch/gluten systems at low moisture content - A comparison of DSC and TMA. Carbohydrate Polymers, v.108, p.1-9, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0144861714001775
>. Accessed: Oct. 03, 2014. doi: 10.1016/j.carbpol.2014.02.049.
http://www.sciencedirect.com/science/art...
).
Interactions between proteins and polysaccharides and their effect on the thermal stability of compounds have been the focus of many studies. However, results have shown variations according to the macromolecules used. Two studies evaluated the influence of Dextran in thermal stability of Bovine Serum Albumin (BSA) (ANTONOV & WOLF, 2005ANTONOV, A.Y.; WOLF, A.B. Calorimetric and structural investigation of the interaction between bovine serum albumin and high molecular weight dextran in water. Biomacromolecules, v.6, p.2980-2989, 2005. Available from: <Available from: http://pubs.acs.org/doi/pdf/10.1021/bm050279h
>. Access: Dec. 08, 2015. doi: 10.1021/bm050279h.
http://pubs.acs.org/doi/pdf/10.1021/bm05...
) and β-Lactoglobulin (β-Lg) (VARDHANABHUTI et al., 2009VARDHANABHUTI, B. et al. Interactions between β-lactoglobulin and dextran sulfate at near neutral pH and their effect on thermal stability., Food Hydrocolloids v.23, n.6, p.1511-1520, 2009. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X08002233
>. Accessed: Oct. 25, 2014. doi: 10.1016/j.foodhyd.2008.09.006.
http://www.sciencedirect.com/science/art...
) and concluded that in both cases the polysaccharide was able to reduce the thermal stability of the protein in low pH and in high concentrations of Dextran. Another study evaluated the effect of Dextran on Casein and reported that the glass transition value (Tg) of Dextran decreased while crystallization temperature increased in the presence of casein (HERNÁNDEZ et al., 2011HERNÁNDEZ, G.H.S. et al. Phase transitions of dairy proteins, dextrans and their mixtures as a function of water interactions., Food Hydrocolloids v.25, n.5, p.1311-1318, 2011. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X10002894
>. Accessed: Jun. 22, 2015. doi: 10.1016/j.foodhyd.2010.12.006.
http://www.sciencedirect.com/science/art...
). These phase transitions must be considered in food processing and storage.
When interactions between soy protein fractions (7S and 11S) and chitosan (CS) were studied during temperature increases from 30 to 120°C at the rate of 5°C/min, the measurements revealed endothermic processes indicating the denaturation point (YUAN et al., 2014YUAN, Y. et al. Associative interactions between chitosan and soy protein fractions: effects of pH, mixing ratio, heat treatment and ionic strength. Food Research International, v.55, p.207-214, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0963996913006157
>. Accessed: Oct. 25, 2014. doi: 10.1016/j.foodres.2013.11.016.
http://www.sciencedirect.com/science/art...
). According to the authors, the interaction with formation of coacervates substantially increased ΔH and Tm
compared to the protein alone. This suggested that the coacervation between soy protein fractions and chitosan increased the thermal stability of the protein. In contrast, a different study on the thermal behavior of whey protein isolates (WPI) in the absence or presence of pectin reported that molecular interactions reduced the stability of WPI (MAO et al., 2014MAO, L. et al. Evaluation of volatile characteristics in whey protein isolate-pectin mixed layer emulsions under different environmental conditions., Food Hydrocolloids v.41, p.79-85, 2014. Available from: <Available from: http://www-sciencedirectcom.ez30.periodicos.capes.gov.br/science/article/pii/S0268005X14000988
>. Accessed: Sept. 10, 2014. doi: 10.1016/j.foodhyd.2014.03.025.
http://www-sciencedirectcom.ez30.periodi...
).
Isothermal titration calorimetry (ITC)
ITC measures the energy released during molecular interactions and is used for the qualitative and quantitative characterization of them (HAPPI EMAGA, et al., 2012HAPPI EMAGA, T. et al. Purification of pectin from apple pomace juice by using sodium caseinate and characterisation of their binding by isothermal titration calorimetry., Food Hydrocolloids v.29, p.211-218, 2012. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X12000501
>. Accessed: Aug. 28, 2014. doi: 10.1016/j.foodhyd.2012.02.019.
http://www.sciencedirect.com/science/art...
; OGNJENOVIĆ et al., 2014OGNJENOVIĆ, J. et al. Interactions of epigallo-catechin 3-gallate and ovalbumin, the major allergen of egg white., Food Chemistry v.164, p.36-43, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0308814614006979
>. Access: Oct. 15, 2014. doi: 10.1016/j.foodchem.2014.05.005.
http://www.sciencedirect.com/science/art...
). A typical interaction system involves the vacant binding site, the free ligand, and the complex at some equilibrium in solution. Understanding the interaction requires knowing the equilibrium constant for the binding process (K) and the binding stoichiometry (n). Equations 5 to 8 illustrate the relevant thermodynamic relationships (FREREY & LEWIS, 2008FREYER, M.W.; LEWIS, E.A. Isothermal titration calorimetry: experimental design, data analysis, and probing macromolecule/ligand binding and kinetic interactions. Methods in Cell Biology, v.84, p.79-113, 2008. Available from: <Available from: http://www.ncbi.nlm.nih.gov/pubmed/17964929
>. Accessed: Jan. 19, 2015. doi: 10.1016/S0091-679X(07)84004-0.
http://www.ncbi.nlm.nih.gov/pubmed/17964...
).
ΔG o = -RT ln Keq (6)
ΔG = ΔH - TΔS (8)
Where ΔG o is the standard Gibbs free energy change, R is the gas constant, and T is the temperature in Kelvin.
Among the techniques that evaluate thermodynamic interactions, only ITC provides a several thermodynamic parameters (K, n, ΔG, ΔH, and ΔS) in a single titration. It requires only small amounts of sample and does not need molecular marker which may generate interference (FREYER & LEWIS, 2008FREYER, M.W.; LEWIS, E.A. Isothermal titration calorimetry: experimental design, data analysis, and probing macromolecule/ligand binding and kinetic interactions. Methods in Cell Biology, v.84, p.79-113, 2008. Available from: <Available from: http://www.ncbi.nlm.nih.gov/pubmed/17964929
>. Accessed: Jan. 19, 2015. doi: 10.1016/S0091-679X(07)84004-0.
http://www.ncbi.nlm.nih.gov/pubmed/17964...
; RAJARATHNAM & RÖSGEN, 2014RAJARATHNAM, K.; RÖSGEN, J. Isothermal titration calorimetry of membrane proteins - Progress and challenges. Biochimica et Biophysica Acta (BBA) - Biomembranes, v.1838, n.1, p.69-77, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0005273613001697
>. Accessed: Aug. 28, 2014. doi: 10.1016/j.bbamem.2013.05.023.
http://www.sciencedirect.com/science/art...
). ΔH is related to the energy involved in molecular interactions and reflects the contribution of hydrogen bonding, electrostatic interactions, and Van der Waals forces. ΔS reflects a change in the degree of order of the system and is related to hydrophobic interactions and is the thermodynamic property that describes the way the molecules are distributed in a system (PIRES et al., 2009PIRES, A.C.S. et al. Microcalorimetry a food science and engineering approach. In: COIMBRA, J.; TEIXEIRA, J.A. Engineering aspects of milk and dairy products. Boca Raton: CRC, 2009. p.201-218.; BOU-ABDALLAH & TERPSTRA, 2012BOU-ABDALLAH, F.; TERPSTRA, T.R. The thermodynamic and binding properties of the transferrins as studied by isothermal titration calorimetry. Biochimica et Biophysica Acta (BBA) - General Subjects, v.1820, n.3, p.318-325, 2012. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0304416511001796
>. Accessed: Oct. 02, 2014. doi: 10.1016/j.bbagen.2011.07.013.
http://www.sciencedirect.com/science/art...
).
Figure 1 is a graphical representation of energy (μcal) as a function of time (s) after 18 titrations by ITC. At the beginning of the titration, the energy absorbed is greater due to interactions. Over time, the rate of energy decreases until complete saturation of binding sites is achieved. From each peak obtained in the titration, a function chart of molar ratio can also be constructed, which allows the variation of free energy (ΔG), equilibrium binding constant (K), and the stoichiometry (n) of reaction to be calculated. Greater slopes of the curve represent higher binding affinities (K) (PIRES et al., 2009PIRES, A.C.S. et al. Microcalorimetry a food science and engineering approach. In: COIMBRA, J.; TEIXEIRA, J.A. Engineering aspects of milk and dairy products. Boca Raton: CRC, 2009. p.201-218.; CERVANTES et al., 2011CERVANTES, C.F. et al. The RelA nuclear localization signal folds upon binding to IκBα. Journal Molecular Biology, v.405, n.3, p.754-764, 2011. Available from: <Available from: http://dx.doi.org/10.1016/j.jmb.2010.10.055
>. Accessed: Jan. 19. 2015. doi:10.1016/j.jmb.2010.10.055.
http://dx.doi.org/10.1016/j.jmb.2010.10....
).
Graphical representation of data generated by the ITC due to the increase in binding affinity Low (A), moderate (B) and High affinity (C). Source: CERVANTES et al. (2011CERVANTES, C.F. et al. The RelA nuclear localization signal folds upon binding to IκBα. Journal Molecular Biology, v.405, n.3, p.754-764, 2011. Available from: <Available from: http://dx.doi.org/10.1016/j.jmb.2010.10.055 >. Accessed: Jan. 19. 2015. doi:10.1016/j.jmb.2010.10.055.
http://dx.doi.org/10.1016/j.jmb.2010.10.... ).
Instrument consists of two identical cells. One is a reference, which contains only buffer solution, and the other contains the macromolecules solutions. Cells are made of material that conduct heat exceptionally well and are thermally stable, with a constant temperature variation of approximately 10-4 K (PIRES et al., 2009PIRES, A.C.S. et al. Microcalorimetry a food science and engineering approach. In: COIMBRA, J.; TEIXEIRA, J.A. Engineering aspects of milk and dairy products. Boca Raton: CRC, 2009. p.201-218.). Small aliquots of the titrant are injected through a syringe into the sample cell. When the reaction occurs, there is release (exothermic reaction) or absorption (endothermic reaction) of energy that the calorimeter detects (HEERKLOTZ & SEELIG, 2000HEERKLOTZ, H.; SEELIG, J. Titration calorimetry of surfactant-membrane partitioning and membrane solubilization. Biochimica et Biophysica Acta, v.1508, p.69-85, 2000. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0304415700000095
>. Accessed: Sept. 08, 2014. doi: 10.1016/S0304-4157(00)00009-5.
http://www.sciencedirect.com/science/art...
). Number, volume, and time of injections, as well as the concentration of the samples, the cell temperature, and rotation speed must be properly adjusted. Furthermore, it is important that a control experiment is carried containing only buffer solution to correct undesired thermal effects that are related to the energy changes during dilution and mixing (PIRES et al., 2009PIRES, A.C.S. et al. Microcalorimetry a food science and engineering approach. In: COIMBRA, J.; TEIXEIRA, J.A. Engineering aspects of milk and dairy products. Boca Raton: CRC, 2009. p.201-218.; BOU-ABDALLAH & TERPSTRA, 2012BOU-ABDALLAH, F.; TERPSTRA, T.R. The thermodynamic and binding properties of the transferrins as studied by isothermal titration calorimetry. Biochimica et Biophysica Acta (BBA) - General Subjects, v.1820, n.3, p.318-325, 2012. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0304416511001796
>. Accessed: Oct. 02, 2014. doi: 10.1016/j.bbagen.2011.07.013.
http://www.sciencedirect.com/science/art...
).
Most ITC calorimeters use the power compensation method that reduces temperature in the sample cell if the reactions are exothermic, and increases temperature if they are endothermic. Energy absorbed or released during the titration will be directly proportional to the interactions (BOU-ABDALLAH & TERPSTRA, 2012BOU-ABDALLAH, F.; TERPSTRA, T.R. The thermodynamic and binding properties of the transferrins as studied by isothermal titration calorimetry. Biochimica et Biophysica Acta (BBA) - General Subjects, v.1820, n.3, p.318-325, 2012. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0304416511001796
>. Accessed: Oct. 02, 2014. doi: 10.1016/j.bbagen.2011.07.013.
http://www.sciencedirect.com/science/art...
). The most modern equipment are called "nanocalorimeters" and can precisely measure very small energy changes (<0.2mJ) and maintain a baseline of ± 0.1mW with a temperature stability of ±0.0001°C (GAISFORD & BRUCKTON, 2001GAISFORD, S.; BUCKTON, G. Potential applications of microcalorimetry for the study of physical processes in pharmaceuticals. Thermochimica Acta, v.380, n.2, p.185-198, 2001. Available frm: <Available frm: http://www.sciencedirect.com/science/article/pii/S0040603101006694
>. Accessed: Apr. 29, 2015. doi: 10.1016/S0040-6031(01)00669-4.
http://www.sciencedirect.com/science/art...
; BOU-ABDALLAH & TERPSTRA, 2012).
Several studies have demonstrated that ITC used in the study of macromolecular interactions is both noninvasive and generates a set of thermodynamic parameters. GUZEY & MCCLEMENTS (2006GUZEY, D.; MCCLEMENTS, D.J. Characterization of β-lactoglobulin-chitosan interactions in aqueous solutions: a calorimetry, light scattering, electrophoretic mobility and solubility study., Food Hydrocolloids v.20, n.1, p.124-131, 2006. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X05000676
>. Accessed: Jun, 25, 2015. doi: 10.1016/j.foodhyd.2005.03.009.
http://www.sciencedirect.com/science/art...
), showed an exothermic interaction between chitosan and β-LG in the pH range in where the polymers have opposite charge. The interaction was more exothermic at pH 6.0, with a molar ratio of about one β-LG molecule to six chitosan molecules.
HARNSILAWAT et al. (2006HARNSILAWAT, T. et al. Characterization of β-lactoglobulin-sodium alginate interactions in aqueous solutions: a calorimetry, light scattering, electrophoretic mobility and solubility study., Food Hydrocolloids v.20, n.5, p.577-585, 2006. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X05000937
>. Accessed: Dec. 08, 2015. doi: 10.1016/j.foodhyd.2005.05.005.
http://www.sciencedirect.com/science/art...
) characterized of β-LG-sodium alginate interactions and verify that the enthalpy change was highly dependent on solution pH. Similarly, the interactions between Chitosan and WPI by electrostatic bonds were dependent not only on the pH but also the ionic strength, and molar ratio (BASTOS et al., 2010BASTOS, D.S. et al. Characterization of a chitosan sample extracted from Brazilian shrimps and its application to obtain insoluble complexes with a commercial whey protein isolate., Food Hydrocolloids v.24, n.8, p.709-718, 2010. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X10000548
>. Accessed: Jun, 25, 2015. doi: 10.1016/j.foodhyd.2010.03.008.
http://www.sciencedirect.com/science/art...
).
In another study, the interaction between β-LG and gum arabic in the presence of an antioxidant were identified. The study reported a two-step interaction, an exothermic step that was mostly controlled by favorable enthalpy due to electrostatic interactions, and a second endothermic step that was driven by entropy, likely due to the release of linked water molecules. In addition, this study evaluated the influence of temperature and concluded that the contribution of enthalpy or entropy were highly dependent on temperature (ABERKANE et al., 2012ABERKANE, L. et al. Structuration mechanism of β-lactoglobulin - acacia gum assemblies in presence of quercetin. Food Hydrocolloids, v.29, n.1, p.9-20, 2012. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X12000227
>. Accessed: Oct. 02, 2014. doi: 10.1016/j.foodhyd.2012.01.010.
http://www.sciencedirect.com/science/art...
).
ITC has been used to study the thermodynamic of complex of coacervates used in developing new products and encapsulation of bioactive compounds, such as omega-3 fatty acids and vitamin D3 (WATER et al., 2014WATER, J.J. et al. Complex coacervates of hyaluronic acid and lysozyme: effect on protein structure and physical stability. European Journal of Pharmaceutics and Biopharmaceutics, v.88, n.2, p.325-331, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0939641114002720
>. Accessed: Jun. 25, 2015. doi: 10.1016/j.ejpb.2014.09.001.
http://www.sciencedirect.com/science/art...
; DONG et al., 2015DONG, D. et al. Mutual titration of soy proteins and gum arabic and the complexing behavior studied by isothermal titration calorimetry, turbidity and ternary phase boundaries., Food Hydrocolloids v.46, p.28-36, 2015. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X14004299
>. Accessed: Jan. 19, 2015. doi: 10.1016/j.foodhyd.2014.11.019.
http://www.sciencedirect.com/science/art...
; DIARRASSOUBA et al., 2015DIARRASSOUBA, F. et al. Self-assembly of β-lactoglobulin and egg white lysozyme as a potential carrier for nutraceuticals. Food Chemistry, v.173, p.203-209, 2015. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0308814614015660
>. Accessed: Mar. 27, 2015. doi: 10.1016/j.foodchem.2014.10.009.
http://www.sciencedirect.com/science/art...
; ERATTE et al., 2015ERATTE, D. et al. Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate-gum Arabic complex coacervates. Journal of Functional Foods, v.19, p.882-892, 2015 Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S1756464615000419
>. Accessed: Jun. 01, 2015. doi: 10.1016/j.jff.2015.01.037.
http://www.sciencedirect.com/science/art...
). HOSSEINI et al. (2013HOSSEINI, S.M.H. et al. Complex coacervation of β-lactoglobulin-κ-Carrageenan aqueous mixtures as affected by polysaccharide sonication., Food Chemistry v.141, n.1, p.215-222, 2013. Available from: <Available from: http://www-sciencedirect-com.ez30.periodicos.capes.gov.br/science/article/pii/S0308814613002537#
>. Accessed: Oct. 08, 2014. doi: 10.1016/j.foodchem.2013.02.090.
http://www-sciencedirect-com.ez30.period...
) submitted the κ-carrageenan biopolymer (KC) and β-LG on ultrasound, and reported that KC-BLG interactions were exothermic with negative and favorable enthalpy and negative and unfavorable entropy. A significant reduction in the affinity constant of the formation of complex coacervates suggested a conformational change. Interactions between lactoferrin and β-LG isoforms were also identified. In this case, ITC revealed an exothermic interaction with contributions from both enthalpy and entropy contributions. The study also reported that the interaction involved at least two steps requiring two independent binding sites (TAVARES et al., 2015TAVARES, G.M. et al. Selective coacervation between lactoferrin and the two isoforms of β-lactoglobulin., Food Hydrocolloids v.48, p.238-247, 2015. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X15000880
>. Accessed: Jun. 23, 2015. doi: 10.1016/j.foodhyd.2015.02.027.
http://www.sciencedirect.com/science/art...
).
The simultaneous use of the two calorimetric techniques was performed by WATER et al. (2014WATER, J.J. et al. Complex coacervates of hyaluronic acid and lysozyme: effect on protein structure and physical stability. European Journal of Pharmaceutics and Biopharmaceutics, v.88, n.2, p.325-331, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0939641114002720
>. Accessed: Jun. 25, 2015. doi: 10.1016/j.ejpb.2014.09.001.
http://www.sciencedirect.com/science/art...
). The authors evaluated the formation of complex coacervates between hyaluronic acid and lysozyme. They reported using ITC, that the interaction was driven entropically, characterized by a slightly favorable binding enthalpy and a highly favorable entropic contribution. Nonetheless, DSC showed a reduction of Tm
of about 1°C after the complex was formed. Thus, formation of the complex did not affect the secondary structure of the protein and did not negatively impact its thermal stability.
CONCLUSION:
Proteins and polysaccharides are part of most food matrices. Understanding their thermodynamic behavior and interactions with other macromolecules is essential for food applications. Calorimetric techniques provided information regarding these interactions through the thermal energy that is produced or consumed during interactions. We have presented examples of the efficiency and applicability of calorimetry in studies of the interactions between macromolecules, and highlighted the importance of thermodynamic parameters in the interpretation of the results obtained. Simultaneous use of DSC and ITC are important tools that can provide a better understanding of the macromolecular interactions that occur during the processing and storage of food.
ACKNOWLEDGEMENTS
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) for the financial support.
REFERENCES:
- ABERKANE, L. et al. Structuration mechanism of β-lactoglobulin - acacia gum assemblies in presence of quercetin. Food Hydrocolloids, v.29, n.1, p.9-20, 2012. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X12000227 >. Accessed: Oct. 02, 2014. doi: 10.1016/j.foodhyd.2012.01.010.
» https://doi.org/10.1016/j.foodhyd.2012.01.010.» http://www.sciencedirect.com/science/article/pii/S0268005X12000227 - ANTONOV, A.Y.; WOLF, A.B. Calorimetric and structural investigation of the interaction between bovine serum albumin and high molecular weight dextran in water. Biomacromolecules, v.6, p.2980-2989, 2005. Available from: <Available from: http://pubs.acs.org/doi/pdf/10.1021/bm050279h >. Access: Dec. 08, 2015. doi: 10.1021/bm050279h.
» https://doi.org/10.1021/bm050279h.» http://pubs.acs.org/doi/pdf/10.1021/bm050279h - ARMSTRONG, A. et al. Structural and thermodynamic insights into the recognition of native proteins by anti-peptide antibodies. Journal of Molecular Biology, v.425, n.11, p.2027-2038, 2013. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0022283613001460 >. Accessed: Dec. 16, 2014. doi: 10.1016/j.jmb.2013.02.031.
» https://doi.org/10.1016/j.jmb.2013.02.031.» http://www.sciencedirect.com/science/article/pii/S0022283613001460 - BASTOS, D.S. et al. Characterization of a chitosan sample extracted from Brazilian shrimps and its application to obtain insoluble complexes with a commercial whey protein isolate., Food Hydrocolloids v.24, n.8, p.709-718, 2010. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X10000548 >. Accessed: Jun, 25, 2015. doi: 10.1016/j.foodhyd.2010.03.008.
» https://doi.org/10.1016/j.foodhyd.2010.03.008.» http://www.sciencedirect.com/science/article/pii/S0268005X10000548 - BOU-ABDALLAH, F.; TERPSTRA, T.R. The thermodynamic and binding properties of the transferrins as studied by isothermal titration calorimetry. Biochimica et Biophysica Acta (BBA) - General Subjects, v.1820, n.3, p.318-325, 2012. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0304416511001796 >. Accessed: Oct. 02, 2014. doi: 10.1016/j.bbagen.2011.07.013.
» https://doi.org/10.1016/j.bbagen.2011.07.013.» http://www.sciencedirect.com/science/article/pii/S0304416511001796 - BROWN, M.E. Handbook of thermal analysis and calorimetry - Principles and practice. The Netherlands: Elsevier Science, 1998. V.1, 722.
- CAO, Z. et al. Potential toxicity of sarafloxacin to catalase: Spectroscopic, ITC and molecular docking descriptions. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, v.115, p.457-463, 2013. Available from: <Available from: http://www.ncbi.nlm.nih.gov/pubmed/23871971 >. Accessed: Dec. 16, 2014. doi: 10.1016/j.saa.2013.06.093.
» https://doi.org/10.1016/j.saa.2013.06.093.» http://www.ncbi.nlm.nih.gov/pubmed/23871971 - CERVANTES, C.F. et al. The RelA nuclear localization signal folds upon binding to IκBα. Journal Molecular Biology, v.405, n.3, p.754-764, 2011. Available from: <Available from: http://dx.doi.org/10.1016/j.jmb.2010.10.055 >. Accessed: Jan. 19. 2015. doi:10.1016/j.jmb.2010.10.055.
» https://doi.org/10.1016/j.jmb.2010.10.055» http://dx.doi.org/10.1016/j.jmb.2010.10.055 - DAMODARAN, S.; AGYARE, K.K. Effect of microbial transglutaminase treatment on thermal stability and pH-solubility of heat-shocked whey protein isolate., Food Hydrocolloids v.30, n.1, p.12-18, 2013. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X12000872 >. Accessed: Jun. 02, 2015. doi: 10.1016/j.foodhyd.2012.04.012.
» https://doi.org/10.1016/j.foodhyd.2012.04.012.» http://www.sciencedirect.com/science/article/pii/S0268005X12000872 - DIARRASSOUBA, F. et al. Self-assembly of β-lactoglobulin and egg white lysozyme as a potential carrier for nutraceuticals. Food Chemistry, v.173, p.203-209, 2015. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0308814614015660 >. Accessed: Mar. 27, 2015. doi: 10.1016/j.foodchem.2014.10.009.
» https://doi.org/10.1016/j.foodchem.2014.10.009.» http://www.sciencedirect.com/science/article/pii/S0308814614015660 - DONG, D. et al. Mutual titration of soy proteins and gum arabic and the complexing behavior studied by isothermal titration calorimetry, turbidity and ternary phase boundaries., Food Hydrocolloids v.46, p.28-36, 2015. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X14004299 >. Accessed: Jan. 19, 2015. doi: 10.1016/j.foodhyd.2014.11.019.
» https://doi.org/10.1016/j.foodhyd.2014.11.019.» http://www.sciencedirect.com/science/article/pii/S0268005X14004299 - ERATTE, D. et al. Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate-gum Arabic complex coacervates. Journal of Functional Foods, v.19, p.882-892, 2015 Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S1756464615000419 >. Accessed: Jun. 01, 2015. doi: 10.1016/j.jff.2015.01.037.
» https://doi.org/10.1016/j.jff.2015.01.037.» http://www.sciencedirect.com/science/article/pii/S1756464615000419 - FREYER, M.W.; LEWIS, E.A. Isothermal titration calorimetry: experimental design, data analysis, and probing macromolecule/ligand binding and kinetic interactions. Methods in Cell Biology, v.84, p.79-113, 2008. Available from: <Available from: http://www.ncbi.nlm.nih.gov/pubmed/17964929 >. Accessed: Jan. 19, 2015. doi: 10.1016/S0091-679X(07)84004-0.
» http://www.ncbi.nlm.nih.gov/pubmed/17964929 - GAISFORD, S.; BUCKTON, G. Potential applications of microcalorimetry for the study of physical processes in pharmaceuticals. Thermochimica Acta, v.380, n.2, p.185-198, 2001. Available frm: <Available frm: http://www.sciencedirect.com/science/article/pii/S0040603101006694 >. Accessed: Apr. 29, 2015. doi: 10.1016/S0040-6031(01)00669-4.
» https://doi.org/10.1016/S0040-6031(01)00669-4.» http://www.sciencedirect.com/science/article/pii/S0040603101006694 - GUZEY, D.; MCCLEMENTS, D.J. Characterization of β-lactoglobulin-chitosan interactions in aqueous solutions: a calorimetry, light scattering, electrophoretic mobility and solubility study., Food Hydrocolloids v.20, n.1, p.124-131, 2006. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X05000676 >. Accessed: Jun, 25, 2015. doi: 10.1016/j.foodhyd.2005.03.009.
» https://doi.org/10.1016/j.foodhyd.2005.03.009.» http://www.sciencedirect.com/science/article/pii/S0268005X05000676 - HAPPI EMAGA, T. et al. Purification of pectin from apple pomace juice by using sodium caseinate and characterisation of their binding by isothermal titration calorimetry., Food Hydrocolloids v.29, p.211-218, 2012. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X12000501 >. Accessed: Aug. 28, 2014. doi: 10.1016/j.foodhyd.2012.02.019.
» https://doi.org/10.1016/j.foodhyd.2012.02.019.» http://www.sciencedirect.com/science/article/pii/S0268005X12000501 - HARNSILAWAT, T. et al. Characterization of β-lactoglobulin-sodium alginate interactions in aqueous solutions: a calorimetry, light scattering, electrophoretic mobility and solubility study., Food Hydrocolloids v.20, n.5, p.577-585, 2006. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X05000937 >. Accessed: Dec. 08, 2015. doi: 10.1016/j.foodhyd.2005.05.005.
» https://doi.org/10.1016/j.foodhyd.2005.05.005.» http://www.sciencedirect.com/science/article/pii/S0268005X05000937 - HEERKLOTZ, H.; SEELIG, J. Titration calorimetry of surfactant-membrane partitioning and membrane solubilization. Biochimica et Biophysica Acta, v.1508, p.69-85, 2000. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0304415700000095 >. Accessed: Sept. 08, 2014. doi: 10.1016/S0304-4157(00)00009-5.
» https://doi.org/10.1016/S0304-4157(00)00009-5.» http://www.sciencedirect.com/science/article/pii/S0304415700000095 - HEERKLOTZ, H. The microcalorimetry of lipids membranes. Journal of Physics Condensed Matter, v.16, n.15, p.441-467, 2004. Available from: <Available from: http://iopscience.iop.org/0953-8984/16/15/R01 >. Accessed: Jun. 24, 2015. doi: 10.1088/0953-8984/16/15/R01.
» https://doi.org/10.1088/0953-8984/16/15/R01.» http://iopscience.iop.org/0953-8984/16/15/R01 - HERNÁNDEZ, G.H.S. et al. Phase transitions of dairy proteins, dextrans and their mixtures as a function of water interactions., Food Hydrocolloids v.25, n.5, p.1311-1318, 2011. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X10002894 >. Accessed: Jun. 22, 2015. doi: 10.1016/j.foodhyd.2010.12.006.
» https://doi.org/10.1016/j.foodhyd.2010.12.006.» http://www.sciencedirect.com/science/article/pii/S0268005X10002894 - HOMER, S. et al. Determination of the thermo-mechanical properties in starch and starch/gluten systems at low moisture content - A comparison of DSC and TMA. Carbohydrate Polymers, v.108, p.1-9, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0144861714001775 >. Accessed: Oct. 03, 2014. doi: 10.1016/j.carbpol.2014.02.049.
» https://doi.org/10.1016/j.carbpol.2014.02.049.» http://www.sciencedirect.com/science/article/pii/S0144861714001775 - HOSSEINI, S.M.H. et al. Complex coacervation of β-lactoglobulin-κ-Carrageenan aqueous mixtures as affected by polysaccharide sonication., Food Chemistry v.141, n.1, p.215-222, 2013. Available from: <Available from: http://www-sciencedirect-com.ez30.periodicos.capes.gov.br/science/article/pii/S0308814613002537# >. Accessed: Oct. 08, 2014. doi: 10.1016/j.foodchem.2013.02.090.
» https://doi.org/10.1016/j.foodchem.2013.02.090.» http://www-sciencedirect-com.ez30.periodicos.capes.gov.br/science/article/pii/S0308814613002537# - IUPAC. Nomenclature of thermometric and enthalpimetric methods in chemical analysis. In: STAHL, J.W. Pure & Applied Chemistry, v.66, n.12, p.2487-2492, 1994. Available from: <Available from: http://pac.iupac.org/publications/pac/pdf/1994/pdf/6612x2487.pdf >. Accessed: Jun. 25, 2015.
» http://pac.iupac.org/publications/pac/pdf/1994/pdf/6612x2487.pdf - IUPAC. ICTAC nomenclature of thermal analysis. In: LEVER, T. et al., Pure & Applied Chemistry v.86, n.4, p.545-553, 2014. Available from: <Available from: http://www.ictac.org/ICTAC-IUPAC-TA_Nomenclature_2014.pdf >. Accessed: Jun. 25, 2015.
» http://www.ictac.org/ICTAC-IUPAC-TA_Nomenclature_2014.pdf - JOHNSON, C.M. Differential scanning calorimetry as a tool for protein folding and stability. Archives of Biochemistry and Biophysics, v.531, n.1-2, p.100-109, 2013. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0003986112003530 >. Accessed: Aug. 28. 2014. doi: 10.1016/j.abb.2012.09.008.
» https://doi.org/10.1016/j.abb.2012.09.008.» http://www.sciencedirect.com/science/article/pii/S0003986112003530 - LIU, Y. et al. Study of glass transition and enthalpy relaxation of mixtures of amorphous sucrose and amorphous tapioca starch syrup solid by differential scanning calorimetry (DSC). Journal of Food Engineering, v.81, n.3, p.599-610, 2007. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0260877407000258 >. Accessed: Jun. 22, 2015. doi: 10.1016/j.jfoodeng.2006.12.017.
» https://doi.org/10.1016/j.jfoodeng.2006.12.017.» http://www.sciencedirect.com/science/article/pii/S0260877407000258 - MA, C.; HARWALKAR, V. Effects of medium and chemical modification on thermal characteristics of β-lactoglobulin. Journal of Thermal Analysis, v.47, p.1513-1525, 1996. Available from: <Available from: http://link.springer.com/article/10.1007/BF01992843 >. Accessed: Jan. 19, 2015. doi: 10.1007/BF01992843.
» https://doi.org/10.1007/BF01992843.» http://link.springer.com/article/10.1007/BF01992843 - MAO, L. et al. Evaluation of volatile characteristics in whey protein isolate-pectin mixed layer emulsions under different environmental conditions., Food Hydrocolloids v.41, p.79-85, 2014. Available from: <Available from: http://www-sciencedirectcom.ez30.periodicos.capes.gov.br/science/article/pii/S0268005X14000988 >. Accessed: Sept. 10, 2014. doi: 10.1016/j.foodhyd.2014.03.025.
» https://doi.org/10.1016/j.foodhyd.2014.03.025.» http://www-sciencedirectcom.ez30.periodicos.capes.gov.br/science/article/pii/S0268005X14000988 - NADEMI, Z. et al. Characteristics of antibody responses in Pigeon Fanciers' lung. Molecular Immunology, v.54, n.2, p.227-232, 2013. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0161589012004828 >. Accessed: Dec. 16, 2014. doi:10.1016/j.molimm.2012.12.007.
» https://doi.org/10.1016/j.molimm.2012.12.007» http://www.sciencedirect.com/science/article/pii/S0161589012004828 - O'BRIEN, R. et al. Isothermal titration calorimetry of biomolecules. In: HARDING, S.E.; CHOWDHRY, B.Z. Protein-ligand interactions: hydrodynamics and calorimetry. New York: Oxford University, 2001. p.263-286.
- OGNJENOVIĆ, J. et al. Interactions of epigallo-catechin 3-gallate and ovalbumin, the major allergen of egg white., Food Chemistry v.164, p.36-43, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0308814614006979 >. Access: Oct. 15, 2014. doi: 10.1016/j.foodchem.2014.05.005.
» https://doi.org/10.1016/j.foodchem.2014.05.005.» http://www.sciencedirect.com/science/article/pii/S0308814614006979 - PIRES, A.C.S. et al. Microcalorimetry a food science and engineering approach. In: COIMBRA, J.; TEIXEIRA, J.A. Engineering aspects of milk and dairy products. Boca Raton: CRC, 2009. p.201-218.
- PRIVALOV, P.L. Microcalorimetry of macromolecules: the physical basis of biological structures. Journal of Solution Chemistry, v.44, n.5, p.1141-1161, 2015. Available from: <Available from: http://link.springer.com/article/10.1007/s10953-015-0337-x >. Accessed: Jul. 13, 2015. doi: 10.1007/s10953-015-0337-x.
» https://doi.org/10.1007/s10953-015-0337-x.» http://link.springer.com/article/10.1007/s10953-015-0337-x - PRIVALOV, P.L.; DRAGAN, A.I. Microcalorimetry of biological macromolecules. Biophysical Chemistry, v.126, n.1-3, p.16-24, 2007. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0301462206001608 >. Accessed: Jun. 25, 2015. doi: 10.1016/j.bpc.2006.05.004.
» https://doi.org/10.1016/j.bpc.2006.05.004.» http://www.sciencedirect.com/science/article/pii/S0301462206001608 - RÀFOLS, C. et al. Molecular interactions between some non-steroidal anti-inflammatory drugs (NSAID׳s) and bovine (BSA) or human (HSA) serum albumin estimated by means of isothermal titration calorimetry (ITC) and frontal analysis capillary electrophoresis (FA/CE). Talanta, v.130, p.241-250, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0039914014005165 >. Accessed: Oct. 14, 2014. doi: 10.1016/j.talanta.2014.06.060.
» https://doi.org/10.1016/j.talanta.2014.06.060.» http://www.sciencedirect.com/science/article/pii/S0039914014005165 - RAJARATHNAM, K.; RÖSGEN, J. Isothermal titration calorimetry of membrane proteins - Progress and challenges. Biochimica et Biophysica Acta (BBA) - Biomembranes, v.1838, n.1, p.69-77, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0005273613001697 >. Accessed: Aug. 28, 2014. doi: 10.1016/j.bbamem.2013.05.023.
» https://doi.org/10.1016/j.bbamem.2013.05.023.» http://www.sciencedirect.com/science/article/pii/S0005273613001697 - RUSSEL, M. et al. Different technique of microcalorimetry and their applications to environmental sciences: a review. Journal of American Science, (n.d.), p.194-208, 2009. Available from: <Available from: http://citeseerx.ist.psu.edu/viewdoc/download? > Accessed: Jun. 25, 2015. doi: 10.1.1.457.2750&rep=rep1&type=pdf>.
» https://doi.org/10.1.1.457.2750&rep=rep1&type=pdf>.» http://citeseerx.ist.psu.edu/viewdoc/download? - SPADA, J.C. et al. Interactions between soy protein from water-soluble soy extract and polysaccharides in solutions with polydextrose., Carbohydrate Polymers v.134, p.119-127, 2015. Available from: <Available from: http://sciencedirect.com/science/article/pii/S0144861715007043 >. Accessed: Sept. 23, 2015. doi: 10.1016/j.carbpol.2015.07.075.
» https://doi.org/10.1016/j.carbpol.2015.07.075.» http://sciencedirect.com/science/article/pii/S0144861715007043 - TABILO-MUNIZAGA, G. et al. Effects of high hydrostatic pressure (HHP) on the protein structure and thermal stability of Sauvignon blanc wine., Food Chemistry v.155, p.214-220, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0308814614000727 >. Accessed: Jun. 02, 2015. doi: 10.1016/j.foodchem.2014.01.051.
» https://doi.org/10.1016/j.foodchem.2014.01.051.» http://www.sciencedirect.com/science/article/pii/S0308814614000727 - TAVARES, G.M. et al. Selective coacervation between lactoferrin and the two isoforms of β-lactoglobulin., Food Hydrocolloids v.48, p.238-247, 2015. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X15000880 >. Accessed: Jun. 23, 2015. doi: 10.1016/j.foodhyd.2015.02.027.
» https://doi.org/10.1016/j.foodhyd.2015.02.027» http://www.sciencedirect.com/science/article/pii/S0268005X15000880 - THOMSON, J.A.; LADBURY, J.E. Isothermal titration calorimetry: a tuturial. In: LADBURY, J.E.; DOYLE, M.L. Biocalorimetry 2: applications of calorimetry in the biological sciences. Chichester: John Wiley and Sons, 2004. p.37-58.
- VARDHANABHUTI, B. et al. Interactions between β-lactoglobulin and dextran sulfate at near neutral pH and their effect on thermal stability., Food Hydrocolloids v.23, n.6, p.1511-1520, 2009. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0268005X08002233 >. Accessed: Oct. 25, 2014. doi: 10.1016/j.foodhyd.2008.09.006.
» https://doi.org/10.1016/j.foodhyd.2008.09.006.» http://www.sciencedirect.com/science/article/pii/S0268005X08002233 - WADSÖ, I. Bio-calorimetry. Trends in Biotechnology, v.4, n.2, p.45-51, 1986. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/0167779986901538 >. Accessed: Jun. 25, 2015. doi: 10.1016/0167-7799(86)90153-8.
» https://doi.org/10.1016/0167-7799(86)90153-8.» http://www.sciencedirect.com/science/article/pii/0167779986901538 - WATER, J.J. et al. Complex coacervates of hyaluronic acid and lysozyme: effect on protein structure and physical stability. European Journal of Pharmaceutics and Biopharmaceutics, v.88, n.2, p.325-331, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0939641114002720 >. Accessed: Jun. 25, 2015. doi: 10.1016/j.ejpb.2014.09.001.
» https://doi.org/10.1016/j.ejpb.2014.09.001.» http://www.sciencedirect.com/science/article/pii/S0939641114002720 - YUAN, Y. et al. Associative interactions between chitosan and soy protein fractions: effects of pH, mixing ratio, heat treatment and ionic strength. Food Research International, v.55, p.207-214, 2014. Available from: <Available from: http://www.sciencedirect.com/science/article/pii/S0963996913006157 >. Accessed: Oct. 25, 2014. doi: 10.1016/j.foodres.2013.11.016.
» https://doi.org/10.1016/j.foodres.2013.11.016.» http://www.sciencedirect.com/science/article/pii/S0963996913006157
-
1
CR-2015-1313.R1
Publication Dates
-
Publication in this collection
Aug 2016
History
-
Received
17 Sept 2015 -
Accepted
14 Jan 2016 -
Reviewed
16 May 2016

 (1)
(1)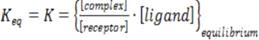 (5)
(5) (7)
(7)

 Thumbnail
Thumbnail
 Thumbnail
Thumbnail
 Thumbnail
Thumbnail

