Abstracts
It is a systematic review and meta-analysis of previous observational epidemiologic studies examining the relationship between residential pesticide exposures during critical exposure time windows (preconception, pregnancy, and childhood) and childhood leukemia. Searches of Medline and other electronic databases were performed (1950-2009). Study selection, data abstraction, and quality assessment were performed by two independent reviewers. Random effects models were used to obtain summary odds ratios (ORs) and 95% confidence intervals (Cis). Of the 17 identified studies, 15 were included in the meta-analysis. Exposures during pregnancy to unspecified residential pesticides insecticides, and herbicides were positively associated with childhood leukemia. Exposures during childhood to unspecified residential pesticides and insecticides were also positively associated with childhood leukemia, but there was no association with herbicides. Positive associations were observed between childhood leukemia and residential pesticide exposures. Further work is needed to confirm previous findings based on self-report, to examine potential exposure-response relationships, and to assess specific pesticides and toxicologically related subgroups of pesticides in more detail.
Child; Environmental exposure; Leukemia; Meta-analysis; Pesticides
Trata-se de uma revisão sistemática e meta-análise de estudos epidemiológicos observacionais anteriores que examinaram a relação entre a exposição de pesticidas residenciais durante as janelas de exposição crítica do tempo (pré-concepção, gravidez e infância) e leucemia infantil. Foram realizadas pesquisas de dados em diversas bases de dados eletrônicas como Medline e outras. Dois revisores independentes realizaram o estudo de seleção, abstração de dados e avaliação da qualidade. Foram utilizados modelos de efeitos aleatórios para obtenção de razões chances (odds ratio) e intervalos de confiança de 95% (IC). Dos 17 estudos identificados, 15 foram incluídos na meta-análise. A exposição a pesticidas e inseticidas residenciais não especificados durante a infância foi positivamente associada com a leucemia infantil, mas não houve associação com herbicidas. Foram observadas associações positivas entre leucemia infantil e exposição a pesticidas residenciais. São necessários mais estudos para confirmar os resultados anteriores com base no autorrelato, para examinar possíveis relações exposição-resposta, e para a avaliação em detalhes de pesticidas específicos e subgrupos de pesticidas toxicologicamente relacionados.
Criança; Exposição ambiental; Leucemia; Meta-análise; Pesticidas
REVIEW REVISÃO
Residential pesticides and childhood leukemia: a systematic review and meta-analysis
Pesticidas residenciais e leucemia na infância: revisão sistemática e meta-análise
Michelle C. Turner; Donald T. Wigle; Daniel Krewski
McLaughlin Centre for Population Health Risk Assessment, Institute of Population Health, University of Ottawa. One Stewart St., Room 313, Ottawa, ON, Canada K1N 6N5. mturner@uottawa.ca
ABSTRACT
It is a systematic review and meta-analysis of previous observational epidemiologic studies examining the relationship between residential pesticide exposures during critical exposure time windows (preconception, pregnancy, and childhood) and childhood leukemia. Searches of Medline and other electronic databases were performed (1950-2009). Study selection, data abstraction, and quality assessment were performed by two independent reviewers. Random effects models were used to obtain summary odds ratios (ORs) and 95% confidence intervals (Cis). Of the 17 identified studies, 15 were included in the meta-analysis. Exposures during pregnancy to unspecified residential pesticides insecticides, and herbicides were positively associated with childhood leukemia. Exposures during childhood to unspecified residential pesticides and insecticides were also positively associated with childhood leukemia, but there was no association with herbicides. Positive associations were observed between childhood leukemia and residential pesticide exposures. Further work is needed to confirm previous findings based on self-report, to examine potential exposure-response relationships, and to assess specific pesticides and toxicologically related subgroups of pesticides in more detail.
Key words: Child, Environmental exposure, Leukemia, Meta-analysis, Pesticides
RESUMO
Trata-se de uma revisão sistemática e meta-análise de estudos epidemiológicos observacionais anteriores que examinaram a relação entre a exposição de pesticidas residenciais durante as janelas de exposição crítica do tempo (pré-concepção, gravidez e infância) e leucemia infantil. Foram realizadas pesquisas de dados em diversas bases de dados eletrônicas como Medline e outras. Dois revisores independentes realizaram o estudo de seleção, abstração de dados e avaliação da qualidade. Foram utilizados modelos de efeitos aleatórios para obtenção de razões chances (odds ratio) e intervalos de confiança de 95% (IC). Dos 17 estudos identificados, 15 foram incluídos na meta-análise. A exposição a pesticidas e inseticidas residenciais não especificados durante a infância foi positivamente associada com a leucemia infantil, mas não houve associação com herbicidas. Foram observadas associações positivas entre leucemia infantil e exposição a pesticidas residenciais. São necessários mais estudos para confirmar os resultados anteriores com base no autorrelato, para examinar possíveis relações exposição-resposta, e para a avaliação em detalhes de pesticidas específicos e subgrupos de pesticidas toxicologicamente relacionados.
Palavras-chave: Criança, Exposição ambiental, Leucemia, Meta-análise, Pesticidas
Leukemia is the most common form of childhood cancer in Canada and the United States, accounting for > 30% of new cancer cases1,2. During 2000-2004, there were nearly 1,400 new cases of leukemia among children 0-14 years of age in Canada, with incidence rates highest among those 0-4 years of age2,3. Acute lymphoblastic leukemia (ALL) accounts for most (~ 80%) childhood leukemia cases, followed by acute myelogenous leukemia (AML)2. Although much progress in treating childhood leukemia has been achieved, treatment entails substantial morbidity, and elevated morbidity and mortality outcomes continue to be observed among survivors compared with children who have not developed the disease4,5.
Acute leukemias are heterogeneous, characterized by different genetic and chromosomal abnormalities, with differing frequency by age6. The two-step model for childhood leukemia proposes that leukemia development occurs after both a first mutation, usually a chromosomal translocation occurring in utero, and a second mutation occurring after birth6,7. Children with Down syndrome experience has an elevated risk for the disease8,9. Although a variety of environmental and chemical exposures have been suggested to play a role in the etiology of the disease, ionizing radiation remains the sole environmental risk factor established to date10. Other potential risk factors that have received some attention in the scientific literature include parental smoking and alcohol consumption, electromagnetic field exposure, hydrocarbons, socioeconomic factors, immunity and infection, and pesticides7,10-14.
Several studies examining the potential association between childhood leukemia and both parental occupational and residential pesticide exposure have been conducted over the past several decades, with positive associations observed15. Partly because of concerns surrounding potential adverse child health impacts, several Canadian provinces and municipalities have recently banned the cosmetic use of pesticides on public or private property16,17. Similar bans are also being considered elsewhere.
Residential pesticide use is associated with elevated child exposures. Use of pyrethroid insecticides in the household was found to be a significant predictor of urinary pyrethroid metabolite levels in children in a recent longitudinal study18. Child urinary concentrations of two organophosphorus pesticide metabolites (dimethyl and diethyl dialkylphosphate compounds) were found to be higher with parental garden pesticide use but not with pet treatment or indoor pesticide use in a Seattle study19.
We conducted a systematic review and meta-analysis of previous observational epidemiologic studies examining the relationship between residential pesticide exposures during critical exposure time windows (preconception, pregnancy, and childhood) and childhood leukemia and explored potential methodological and clinical sources of heterogeneity in results. Although there have been previous reviews, none have included a quantitative synthesis of the results available to date. Results of an analysis examining the association between childhood leukemia and parental occupational pesticide exposure are presented in a separate, companion review20.
Materials and methods
This systematic review and meta-analysis was conducted according to a protocol designed by M.C.T. and D.T.W.
Literature search
The search strategy was designed to identify previous observational epidemiologic studies examining the relationship between residential pesticide exposures during critical exposure time windows (preconception, pregnancy, childhood) and childhood leukemia. Preliminary searches using Ovid MEDLINE were conducted to inform the design of the final search strategy detailed below. An information specialist at the University of Ottawa was also consulted in finalizing the search strategy.
The search strategy was first developed to search the Ovid MEDLINE (1950-March week 3, 2009) and Ovid MEDLINE database of in process and other nonindexed citations (1950 to 31 March 2009) and then adapted to search the Ovid EMBASE (Excerpta Medica Database; 1980 to week 13 2009)21, TOXNET (Toxicology Data Network)22 (through 31 March 2009), OpenSigle (2009) (through 31 March 2009), and ProQuest Digital Dissertations and Theses (2009)23 (through 31 March 2009). The following medical subject headings (MeSH) and key words were used:
Exposure: exp Environmental Exposure/, exp Environmental Pollutants/, exp Pest Control/, exp Pesticides/, pesticid$.tw, herbicid$.tw, insecticid$. tw, fungicid$.tw
Population: exp Child/, exp Adolescent/, exp Infant/, child$.tw, adolescen$.tw, infant?.tw, newborn?.tw, youth.tw, teenage$.tw
Outcome: exp Hematologic Neoplasms/, exp Leukemia/, leuk?emia$.tw
Search terms were grouped according to the Boolean operators OR and AND. A complete depiction of the Ovid MEDLINE search strategy is given in Supplemental Material, Table 1 (available online at doi:10.1289/ehp.0900966.S1 via http://dx.doi.org).
All titles and abstracts identified were independently examined by two of us (M.C.T. and D.T.W.) in order to determine their potential suitability for inclusion in the systematic review. After this primary screen, the complete articles were obtained and the inclusion/exclusion criteria applied. Discrepancies were resolved by consensus. No language criteria were applied. Where abstracts were identified or further details required, particularly relating to the designation of pesticide exposure as residential or occupational, the corresponding author was contacted to ascertain further details of the study. In addition to searching the databases listed above, the reference lists of all included studies and journal Web sites were also hand searched; studies identified manually were evaluated in the same manner as above.
Inclusion and exclusion criteria
Original epidemiologic studies of childhood leukemia using a case-control or cohort design with an assessment of at least one index of residential/household pesticide exposure/use were included here. Reports were excluded if they were review articles, ecologic studies, case reports, cluster investigations, or studies of adults or if they examined residential exposure or proximity to agricultural pesticides. Where there were multiple publications, the most relevant report was retained (usually the most recent).
Data abstraction
After identification of all relevant studies, data abstraction was performed independently by the same two reviewers (M.C.T. and D.T.W.). A standard data abstraction form was prepared and piloted to collect relevant data related to referencing, study design, subject selection, exposure assessment, statistical analysis, and results. A single exposure index was identified for each original study and, where data were available, each combination of exposure time window and pesticide type (unspecified, insecticides, herbicides).
Quality assessment
All included studies underwent independent quality assessment by the same two reviewers (M.C.T. and D.T.W.). We used a modified version of the Downs and Black24 checklist for the assessment of the methodological quality of randomized and nonrandomized studies of health care interventions [Supplemental Material, Table 2 (doi:10.1289/ehp.0900966.S1)]. Before conducting the quality assessment, the two reviewers discussed the individual items on the checklist to clarify their interpretation. No attempt was made to blind the reviewers of the authorship or publication status of the original studies. Differences in quality assessment were resolved by consensus.
Analysis
We conducted meta-analyses using Review Manager (RevMan) version 5.0 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark). Generic inverse variance data were combined using random effects models to obtain a summary odds ratio (OR) and 95% confidence interval (CI) for the relationship between residential pesticide exposures (unspecified, insecticides, herbicides) and childhood leukemia by exposure time window (preconception, pregnancy, childhood). Heterogeneity across individual studies was quantified by the I2 statistic25. Low, moderate, or high degrees of heterogeneity may be approximated by I2 values of 25%, 50%, and 75%, respectively25. We conducted subgroup analyses according to total quality score (> median) and individual quality score components (> median for external validity and exposure measurement), study design (hospital-based or population-based case-control study), cell type (ALL, AML), location (indoor, outdoor), maternal residential pesticide use (vs. household use or exposure) only, year of publication (studies published in 2000 or later only), and publication status (studies published in the peer-reviewed literature only). Where multiple exposure indices were reported per exposure time window, pesticide type, and study, sensitivity analyses were undertaken using exposure or time window definitions different from those used in the main analysis. Finally, we also examined the impact of removing studies with extreme ORs or the highest weight in analysis, as well as removing individual studies in a sequential manner. Because of the small number of included studies, we assessed publication bias by visual inspection of inverted funnel plots, based on the main finding from all studies26.
Results
Study identification
The results of the search strategy and study selection process are detailed in Figure 1. Of the 1,776 studies identified using our search algorithm, 112 were retained from the primary screening process. Most studies were excluded during primary screening because they were irrelevant (n = 1,178), a duplicate record (n = 380), or a review article (n = 93). After the secondary screening process, 17 studies were retained (listed in Table 1). Major reasons for exclusion during secondary screening were irrelevance (n = 36), examination of occupational or residential exposure to agricultural pesticides exposure only (or unclear) (n = 27), or a letter or editorial with no results presented (n = 14).
Study characteristics
Of the 17 identified case-control studies, 6 were hospital based, 10 were population based, and 1 reported results separately for both hospital and population controls (Table 1). Studies were conducted in the United States, Canada, Mexico, Japan, France, Brazil, and Germany. Most of the studies were published in the peer-reviewed literature; however, three doctoral dissertations presented results not published elsewhere30,35,36. Although most studies examined both ALL and acute nonlymphoblastic leukemia cases among children and adolescents up to a maximum age of 19 years, one study examined infantile acute leukemia32 and another examined both ALL and AML in children with Down syndrome8.
Studies varied in size, ranging from a total of 49 leukemia cases (with 7-25 cases ever exposed, depending on exposure category and time window) in the dissertation by Dell30 up to 1,184 cases (with 25-164 exposed) in the German study by Meinert et al.40. All studies conducted to date relied on parental reports of residential pesticide exposure or use inside or outside of the home, either by themselves or by professional exterminators [Supplemental Material, Appendix 1 Appendix 1 (doi:10.1289/ehp.0900966.S1)]. Although most studies assessed use of, or exposure to, pesticides or specific pesticide subgroups (insecticides, herbicides, fungicides), some studies also attempted to collect information on pesticide names and formulations or on target organism30,35,36,39,41. Nine studies considered both residential and occupational pesticide exposures29-31,33,34,36,38,40,43, and the remaining eight studies were exclusively residential. Five studies clearly specified (or explicitly assumed) whether residential pesticide exposure during pregnancy was attributable to maternal use31,33,35,41,43 as opposed to household use or exposure.
Virtually all studies assessed pesticide exposures during separate preconception, pregnancy, and childhood time windows; however, time window definitions differed somewhat by study (Appendix 1 Appendix 1 ). Leiss and Savitz37 considered only the last 3 months of pregnancy. Ma et al.41 considered the first 3 years of age in a separate manner. Davis35 considered the first 6 months of age separately from the remainder of the childhood period. Some studies also combined results from different exposure time windows in analysis and reporting36,38,40,43.
Quality assessment
Quality scores are presented in Supplemental Material, Table 3 (doi:10.1289/ehp.0900966.S1). For hospital-based studies, total scores ranged from 7 to 12, with a median value of 9, of a possible maximum score of 20. For population-based studies, quality scores were higher, with a range of 9-14 and a median of 11. More recent studies tended to have higher quality scores. In assessing external validity, questions remained regarding the representativeness of subjects (both selected and participating), particularly for earlier hospital-based studies. Only Buckley et al.34 reported that interviewers were blind to case/control status; however, the ethics of such practices have also been questioned39. Because of the self-reported nature of exposure data, no study received a point for avoidance of bias from misclassification, since the possibility for differential misclassification remained. Only Dell30 and Ma et al.41 reported results for a clearly defined preconception exposure time window. There were few data regarding frequency or duration of pesticide use, with most studies reporting only "ever/never" use of/exposure to the pesticide of interest. Six studies attempted to examine potential exposure-response relationships8,30,34,39-41. Although confounding is difficult to assess because there are few established risk factors for childhood leukemia, most studies examined or adjusted for at least a range of sociodemographic and maternal characteristics. Four studies, however, explicitly assessed the potential confounding influence of maternal or childhood X-ray exposure28-30,33.
Publication bias
To assess the possibility of publication bias, we examined the main findings from all included studies in an inverse funnel plot [Supplemental Material, Figure 1 (doi:10.1289/ehp.0900966.S1)]. Although limited by the small number of individual studies, there was some evidence for asymmetry, with a lack of small studies found with effect sizes smaller than those from larger studies. Asymmetry may also be due to a range of other factors, including study quality, methodological differences, or the study populations per se. We attempted to identify all relevant original studies possible, including three doctoral dissertations30,35,36 and two studies published in a language other than English28,29.
Data synthesis
Of the 17 identified studies, we excluded two from the quantitative data synthesis due to a lack of CIs27 or a unique study population (Down syndrome cases only)8. Supplemental Material, Appendix 2 (doi:10.1289/ehp.0900966.S1) lists the individual studies included in each overall and subgroup analysis by exposure time window and pesticide type.
Preconceptional household use of unspecified residential indoor (summary OR = 1.53; 95% CI, 0.98-2.39; I2 = 0%) and outdoor (OR = 1.69; 95% CI, 1.03-2.77; I2 = 0%) pesticides was positively associated with childhood leukemia based on the two available studies30,41. We also found a significant positive association with preconceptional residential insecticide use (OR = 1.92; 95% CI, 1.34-2.74; I2 = 0%) in the same two studies.
Exposure to unspecified residential pesticides during pregnancy had a significant and positive association with childhood leukemia when combining results from 11 studies (OR = 1.54; 95% CI, 1.13-2.11; I2 = 66%), although the combined estimate had substantial heterogeneity (Figure 2A, Table 2). The magnitude of the positive association increased somewhat and heterogeneity was reduced when we examined ALL only (OR = 2.04; 95% CI, 1.54-2.68; I2 = 19%), indoor use of unspecified pesticides (OR = 1.86; 95% CI, 1.25-2.77; I2 = 9%), maternal use of unspecified pesticides (OR = 2.07; 95% CI, 1.62-2.64; I2 = 19%), and studies published in the peer-reviewed literature only (OR = 1.81; 95% CI, 1.37-2.39; I2 = 36%). We observed the largest OR for studies published in or since the year 2000 (OR = 2.17; 95% CI, 1.85-2.53; I2 = 0%).
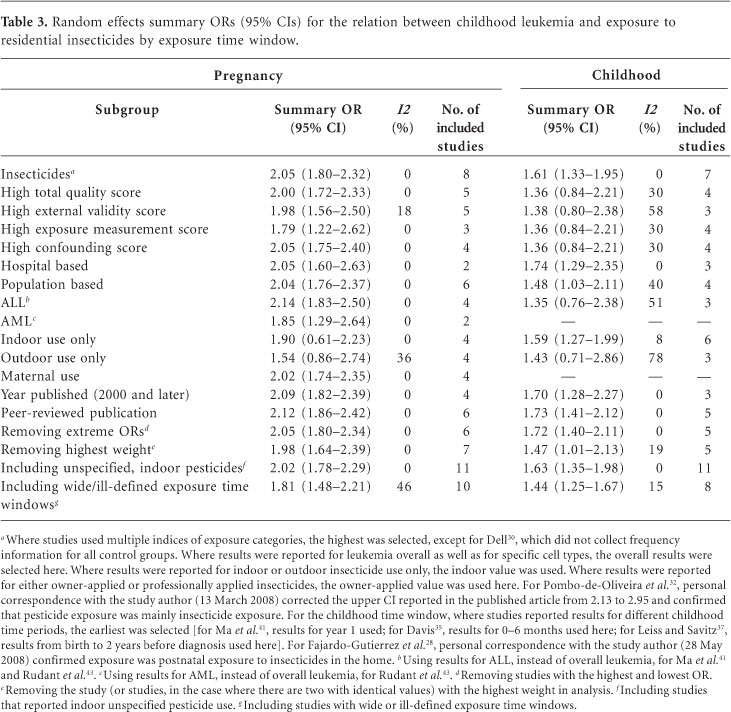
Exposure to residential insecticides during pregnancy was associated with a significant increase in risk of childhood leukemia, when combining the results from eight studies (OR = 2.05; 95% CI, 1.80-2.32; I2 = 0%), with little evidence of heterogeneity (Figure 2B, Table 3). The summary OR changed little according to total study quality, individual quality components, hospital-versus population-based design, or publication status. The association was somewhat stronger for studies of ALL (OR = 2.14; 95% CI, 1.83-2.50; I2 = 0%) compared with AML (OR = 1.85; 95% CI, 1.29-2.64; I2 = 0%) and for indoor use (OR = 1.90; 95% CI, 0.61-2.23; I2 = 0%) compared with outdoor use (OR = 1.54; 95% CI, 0.86-2.74; I2 = 36%), although they are based on fewer studies.
Exposure to residential herbicides during pregnancy also had a significant positive association with childhood leukemia (OR = 1.61; 95% CI, 1.20-2.16; I2 = 0%) when combining the results from five studies (Figure 2C, Table 4). Again, we observed little difference in the summary OR according to study quality, study design, or publication status. The combined relative risk estimate increased somewhat for ALL (OR = 1.73; 95% CI, 1.28-2.35; I2 = 0).
Results for the pregnancy exposure time window were fairly robust to sensitivity analyses: removing studies with extreme ORs or with the highest weight or including additional studies with wide or ill-defined exposure time windows. However, removing the study of AML by Steinbuch36 from the unspecified pesticide analysis did result in a somewhat stronger association (OR = 1.74; 95% CI, 1.36-2.24; I2 = 31%).
Results for residential pesticide exposure during childhood were somewhat similar but were attenuated compared with those for pregnancy (Figure 3A, Table 2). We found a significant positive association between residential unspecified pesticide exposure during childhood and childhood leukemia (OR = 1.38; 95% CI, 1.12-1.70; I2 = 4%), with little heterogeneity. The magnitude of the association was somewhat stronger for indoor use (OR = 1.56; 95% CI, 1.02-2.39; I2 = 7%), studies published in or since the year 2000 (OR = 1.55; 95% CI, 1.14-2.12; I2 = 0%), and studies published in the peer-reviewed literature (OR = 1.56; 95% CI, 1.19-2.04; I2 = 0%).
Exposure to residential insecticides during childhood was also positively associated with childhood leukemia (OR = 1.61; 95% CI, 1.33-1.95; I2 = 0%) when combining results from seven original studies (Figure 3B, Table 3). With restriction to studies of higher total methodological quality, the magnitude of the association was reduced and was no longer significant (OR = 1.36; 95% CI, 0.84-2.21; I2 = 30%). Our findings were similar when evaluating population-based studies (OR = 1.48; 95% CI, 1.03-2.11; I2 = 40%), studies of ALL (OR = 1.35; 95% CI, 0.76-2.38; I2 = 51%), and studies with results for outdoor insecticide use only (OR = 1.43; 95% CI, 0.71-2.86; I2 = 78%). Sensitivity analysis using an alternate exposure metric for Leiss and Savitz37 (using home extermination for insects as opposed to pest strips for insects in the home) decreased the magnitude of the association and also increased heterogeneity (OR = 1.29; 95% CI, 0.84-1.97; I2 = 62%). ORs were elevated, however, with recent year of publication (OR = 1.70; 95% CI, 1.28-2.27; I2 = 0%) and among studies published in the peer-reviewed literature (OR = 1.73; 95% CI, 1.41-2.12; I2 = 0%).
Finally, we observed no association between exposure to residential herbicides during childhood and childhood leukemia when combining results for four studies overall (OR = 0.96; 95% CI, 0.59-1.58; I2 = 72%) (Figure 3C, Table 4). We also observed substantial heterogeneity. Sensitivity analysis using alternate exposure indices for Ma et al.41 (using year 2 of childhood as opposed to year 1) and for Davis35 (using garden herbicide use between 7 months of age and age at diagnosis as opposed to yard herbicides from 0 to 6 months of age) resulted in a summary OR for childhood leukemia of 1.38 (95% CI, 1.10-1.72; I2 = 0%).
Discussion
In this meta-analysis, we examined previous observational epidemiologic studies of the association between residential pesticide exposure during critical exposure time windows (preconception, pregnancy, childhood) and childhood leukemia. Overall, exposure to residential pesticides during pregnancy was positively associated with childhood leukemia. We observed the strongest association for insecticides, with little evidence of heterogeneity. Results for childhood exposures were less clear. Although we observed overall positive and significant associations for exposure to unspecified pesticides and insecticides during childhood, for insecticides the association attenuated among studies with higher total methodological quality. Few studies examined childhood exposure to herbicides, and we observed no overall positive association. Although we also examined preconceptional residential pesticide exposure, only two studies had clearly defined exposure time windows on which to base an assessment of the effect of this type of exposure on childhood leukemia. We obtained some differences in results in subgroup analysis according to study quality, study design, and publication status or when using alternate exposure indices for some of the associations we observed.
Previous reviews have concluded that there is likely to be a positive association between pesticide exposure and childhood leukemia15,44. Results from a companion article revealed positive associations between childhood leukemia and prenatal maternal occupational exposure to pesticides20. Occupational pesticide exposures are of greater magnitude compared with those from other sources45-47. Summary ORs for the relation between prenatal maternal occupational exposure to pesticides and childhood leukemia were larger compared with those here, with an overall summary OR of 2.08 (95% CI, 1.51-2.88), reported for any pesticide exposure, and 2.72 (95% CI, 1.47-5.04), reported for insecticide exposure, lending further credibility to the hypothesis.
Among the potential limitations of the present analysis is the possibility for publication bias. Although such bias can be difficult to assess, we found several small studies that were either unpublished (PhD dissertations)30,35,36 or written in languages other than English28,29. The magnitude of the association observed between unspecified pesticides and childhood leukemia tended to strengthen, and the heterogeneity reduce, on restriction to studies published in the peer-reviewed literature only.
Original studies may be subject to limitations related to exposure assessment and reporting. Typically, the quality of environmental epidemiology studies is influenced by the quality of exposure measurement48. The studies in the present meta-analysis measured residential pesticide exposure entirely by parental report, and only in some instances were detailed data collected on specific types of pesticides or frequency of use. Although based on small numbers of exposed subjects, some limited evidence supports a positive exposure-response relationship between childhood leukemia and both pregnancy and childhood household pesticide or insecticide exposure8,34,39,41. Although there may be differential misclassification of exposure among cases, it has also been suggested that nondifferential misclassification may be of greater concern49. Although none of the studies we included here appear to have attempted to validate self-reported residential pesticide exposure information, Meinert et al.38,40 examined the risk of both childhood leukemia and solid tumors in the same study. They found a positive association between pesticides and childhood leukemia, but not solid tumors, possibly suggesting that the extent of recall bias by parents may be limited. The concordance of pesticide exposure among farmers, as measured via either self-report or biomonitoring, was poor50,51. Recently, Ward et al.52 examined exposure to persistent organochlorine pesticides in residential carpet dust samples in the Northern California Childhood Leukemia Study. They observed no positive associations with childhood leukemia for chlordane, DDT (dichlorodiphenyltrichloroethane) or its metabolite DDE (dichlorodiphenyldichloroethylene), methoxychlor, or pentachlorophenol concentrations.
Studies differed in the precise exposure time windows captured and reported. Some studies reported results only for all time windows combined, which may obscure the potential association linked to specific exposure time windows; however, because high correlations have been found between pesticide exposures in different exposure time windows8,34, the extent to which such obfuscation might occur is difficult to determine. Sensitivity analyses that included studies reporting results in wide or ill-defined exposure time windows tended to increase the degree of heterogeneity we observed, as quantified by the I2 statistic. In models comparing pesticide exposures occurring during pregnancy, in childhood, and in both pregnancy and childhood, Menegaux et al.31 observed the strongest associations with childhood leukemia when exposures were experienced during both exposure time windows, as opposed to during one exposure time window only.
Exposure to different types of pesticides may also be correlated, and few studies have attempted to disentangle the independent effects of specific pesticides. Menegaux et al.31 incorporated different insecticide exposures simultaneously and found that the positive associations remained. Lowengart et al.33 reported that the positive associations observed for parental exposure to either household pesticides or garden pesticides/herbicides during pregnancy remained after mutual adjustment. Davis35 reported little change in pesticide ORs after adjustment for other pesticide use. However, Rudant et al.43 reported that associations with paternal pesticide use were confounded by maternal use. Residential pesticides also represent only one potential pathway through which parental or childhood pesticide exposure may occur, with food, occupation, and the transport of agricultural pesticides representing other potentially important exposure pathways52-56. Among studies of residential pesticides that also collected data on maternal occupational pesticide exposures, the prevalence of occupational pesticide exposure was low, and there is little information on the potential interrelationships of occupational and residential pesticide exposures for childhood leukemia. However, Rudant et al.43 reported that excluding children with occupationally exposed parents did not change results, and Buckley et al.34 reported that the positive associations observed for residential pesticide exposure remained in multivariate models containing parental occupational pesticide exposure. Residential pesticides may be an important exposure source even in agricultural areas57.
In terms of other potential confounding variables, as noted above, most studies examined or adjusted for at least a range of sociodemographic and maternal characteristics. Leiss and Savitz37 also considered magnetic field exposure. Menegaux et al.31 examined early common infections, child care attendance, and residence near a gas station/garage as potential confounders, with no change in results. Rudant et al.43 also reported that early infections and daycare attendance did not change results for residential pesticides. Lowengart et al.33 reported that the positive associations observed for both residential pesticide exposure and paternal occupational exposure to chlorinated solvents remained after mutual adjustment. Among the studies that assessed the potential influence of maternal or childhood X-ray exposures, Lowengart et al.33 and Fajardo-Gutierrez et al.28 reported no change in findings; however, Dell30 reported that the positive association observed between pregnancy exposure to yard pesticides and childhood leukemia disappeared after adjusting for maternal X-ray exposure and use of antibiotics during pregnancy.
For childhood leukemia, the pregnancy exposure time window may be of particular importance10. Most childhood leukemia cases occur in the first few years of life3. Most childhood leukemia cases have gross chromosomal abnormalities, including translocations; however, little is known regarding their underlying cause58. A study of routinely collected blood samples in neonates revealed leukemia clones with specific chromosomal translocations in children who later developed ALL59. Preleukemic clones may persist throughout childhood and may require postnatal exposures for leukemia progression60. In a small study of infants born in an agricultural region in the Philippines, the prevalence of a common AML translocation [t(8;21)] in cord blood samples was about 2-fold higher among those with detectable meconium levels of the methylcarbamate insecticide propoxur61,62. The prenatal origin of AML may be less frequent than that of ALL63.
Conclusions
This systematic review and meta-analysis reveals positive associations between exposure to residential pesticides in pregnancy and childhood and childhood leukemia, with the strongest associations observed for insecticides. Further work is needed to confirm previous findings based on self-report, to better describe potential exposure-response relationships, to assess specific pesticides and toxicologically related subgroups of pesticides in more detail, and to assess the potential role of preconceptional paternal exposures. Large prospective studies of children with biomonitoring data and discovery of biomarkers of past exposure (especially for rapidly excreted pesticides) would aid in this regard64. Additional studies are needed in order to better understand potential mechanisms of action and gene-pesticide interactions. In terms of precautionary public health implications, cosmetic pesticide bylaws implemented in various Canadian jurisdictions typically do not address the use of pesticides indoors or for essential purposes, such as to intervene in a health hazard or infestation to property. Further consideration of the need to reduce prenatal and childhood exposure to residential pesticides may be warranted.
Acknowledgements
We thank D. Salzwedel for assistance in developing the literature search strategy. This review was funded in part by a financial contribution from the Public Health Agency of Canada through the National Collaborating Centre for Environmental Health. M Turner holds a Canada graduate scholarship from the Canadian Institutes of Health Research. The views expressed herein do not necessarily represent the views of the Public Health Agency of Canada or the National Collaborating Centre for Environmental Health. D Krewski is chief executive officer and chief risk scientist for Risk Sciences International (http://www.risksciencesint.com). The other authors declare they have no competing financial interests.
Received 11 May 2009
Accepted 29 July 2009
This article was originally published by Environ Health Perspect 118:33-41 (2010).doi:10.1289/ehp.0900966 available via http://dx.doi.org/ [Online 29 July 2009] and is part of the scientific collaboration between Cien Saude Colet and EHP. Supplemental material is available online (doi:10.1289/ehp.0900966.S1 via http://dx.doi.org/).
Appendix 1
Appendix 1 - Clique para ampliar Appendix 1
- 1. American Cancer Society. Cancer facts and figures, 2009. Atlanta, GA: American Cancer Society; 2009.
- 2. Canadian Cancer Society/National Cancer Institute of Canada. Canadian cancer statistics 2009. Toronto: Canadian Cancer Society; 2009.
- 3. Agha M, BiMonte B, Greenberg M, Greenberg C, Barr R, McLaughlin J. Incidence trends and projections for childhood cancer in Ontario. Int J Cancer 2006; 118:2809-2815.
- 4. MacArthur A, Spinelli J, Rogers P, Goddard K, Abanto Z, McBride M. Mortality among 5-year survivors of cancer diagnosed during childhood or adolescence in British Columbia, Canada. Pediatr Blood Cancer 2007; 48:460-467.
- 5. Speechley K, Barrera M, Shaw A, Morrison H, Maunsell E. Health-related quality of life among child and adolescent survivors of childhood cancer. J Clin Oncol 2006; 24:2536-2543.
- 6. Greaves M. Childhood leukaemia. BMJ 2002; 324:283-287.
- 7. Rossig C, Juergens H. Aetiology of childhood acute leukaemias: current status of knowledge. Radiat Prot Dosimetry 2008; 132:114-118.
- 8. Alderton LE, Spector LG, Blair CK, Roesler M, Olshan AF, Robison LL, Ross JA. Child and maternal household chemical exposure and the risk of acute leukemia in children with Down's syndrome: a report from the Children's Oncology Group. Am J Epidemiol 2006; 164:212-221.
- 9. Ross JA, Spector LG, Robison LL, Olshan AF. Epidemiology of leukemia in children with Down's syndrome. Pediatr Blood Cancer 2005; 44:8-12.
- 10. Belson M, Kingsley B, Holmes A. Risk factors for acute leukemia in children: a review. Environ Health Perspect 2007; 115:138-145.
- 11. Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer 2006; 6:193-203.
- 12. Infante-Rivard C, El-Zein M. Parental alcohol consumption and childhood cancers: a review. J Toxicol Environ Health B 2007; 10:101-129.
- 13. Lee KM, Ward MH, Han S, Ahn HS, Kang HJ, Choi HS, Shin HY, Koo HH, Seo JJ, Choi JE, Ahn YO, Kang D. Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res 2009; 33:250-258.
- 14. Schuz J, Ahlbom A. Exposure to electromagnetic fields and the risk of childhood leukaemia: a review. Radiat Prot Dosimetry 2008; 132:202-211.
- 15. Infante-Rivard C, Weichenthal S. Pesticides and childhood cancer: an update of Zahm and Ward's 1998 review. J Toxicol Environ Health B 2007; 10:81-99.
- 16. Arya N. Pesticides and human health: why public health officials should support a ban on non-essential residential use. Can J Public Health 2005; 96:89-92.
-
17Ontario Ministry of the Environment. Implementing the Cosmetic Pesticides Ban Proposed. New Regulation. 2008. [accessed 11 May 2009]. Available: http://www.ene.gov.on.ca/en/land/pesticides/Factsheet-pesticides.pdf
- 18. Lu C, Barr DB, Pearson MA, Walker LA, Bravo R. The attribution of urban and suburban children's exposure to synthetic pyrethroid insecticides: a longitudinal assessment. J Expo Sci Environ Epidemiol 2009; 19:69-78.
- 19. Lu C, Knutson DE, Fisker-Andersen J, Fenske RA. Biological monitoring survey of organophosphorus pesticide exposure among preschool children in the Seattle metropolitan area. Environ Health Perspect 2001; 108:299-303.
- 20. Wigle DT, Turner MC, Krewski D. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect 2009; 117:1505-1513.
- 21. Ovid. 2009. [accessed 31 March 2009]. Available: http://gateway.ovid.com
» link - 22. National Library of Medicine. TOXNET (Toxicology Data Network). 2009. [accessed 31 March 2009]. Available: http://toxnet.nlm.nih.gov
-
23Proquest. ProQuest Digital Dissertations and Theses. 2009. [accessed 31 March 2009]. Available: http://proquest.umi.com/login.23
- 24. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52:377-384.
- 25. Higgins J, Thompson S, Deeks J. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557-560.
- 26. Ioannidis J, Trikalinos T. The appropriateness of asymmetry tests for publication bias in meta-analyses a large survey. CMAJ 2007; 176:1091-1096.
- 27. Schwartzbaum JA, George SL, Pratt CB, Davis B. An exploratory study of environmental and medical factors potentially related to childhood cancer. Med Pediatr Oncol 1991; 19:115-121.
- 28. Fajardo-Gutierrez A, Garduno-Espinosa J, Yamamoto-Kimura L, Hernandez-Hernandez DM, Mejia-Arangure M, Gomez-Delgado A, Farfan-Canto JM, Ortiz-Fernandez A, Martinez-Garcia MDC. [Risk factors associated with the development of leukemia in children] [in Spanish]. Bol Med Hosp Infant Mex 1993; 50:248-257.
- 29. Kishi R, Katakura Y, Yuasa J, Miyake H. [Association of parents' occupational exposure to cancer in children: a case-control study of acute lymphoblastic leukemia] [in Japanese]. Sangyo Igaku 1993; 35:515-529.
- 30. Dell DM. Epidemiology of childhood leukemia: environmental and genetic determinants [PhD dissertation]. Pittsburgh, PA: University of Pittsburgh; 2004.
- 31. Menegaux F, Baruchel A, Bertrand Y, Lescoeur B, Leverger G, Nelken B, Sommelet D, Hémon D, Clavel J. Household exposure to pesticides and risk of childhood acute leukaemia. Occup Environ Med 2006; 63:131-134.
- 32. Pombo-de-Oliveira MS, Koifman S, Brazilian Collaborative Study Group of Infant Acute Leukemia. Infant acute leukemia and maternal exposures during pregnancy. Cancer Epidemiol Biomarkers Prev 2006; 15:2336-2341.
- 33. Lowengart RA, Peters JM, Cicioni C, Buckley J, Bernstein L, Preston-Martin S, Rappaport E. 1987. Childhood leukemia and parents' occupational and home exposures. J Natl Cancer Inst 1987; 79:39-46.
- 34. Buckley JD, Robison LL, Swotinsky R, Garabrant DH, LeBeau M, Manchester P, Nesbit ME, Odom L, Peters JM, Woods WG. Occupational exposures of parents of children with acute nonlymphocytic leukemia: a report from the Childrens Cancer Study Group. Cancer Res 1989; 49:4030-4037.
- 35. Davis JR. Childhood cancer and pesticide use in the home, garden, and yard [PhD dissertation]. Berkeley, CA: University of California, Berkeley; 1991.
- 36. Steinbuch M. The role of environmental exposures in the etiology of childhood acute myeloid leukemia [PhD dissertation]. Columbus, OH: Ohio State University; 1994.
- 37. Leiss JK, Savitz DA. Home pesticide use and childhood cancer: a case-control study. Am J Public Health 1995; 85:249-252.
- 38. Meinert R, Kaatsch P, Kaletsch U, Krummenauer F, Miesner A, Michaelis J. Childhood leukaemia and exposure to pesticides: results of a case-control study in northern Germany. Eur J Cancer 1996; 32A:1943-1948.
- 39. Infante-Rivard C, Labuda D, Krajinovic M, Sinnett D. Risk of childhood leukemia associated with exposure to pesticides and with gene polymorphisms. Epidemiology 1999; 10:481-487.
- 40. Meinert R, Schuz J, Kaletsch U, Kaatsch P, Michaelis J. Leukemia and non-Hodgkin's lymphoma in childhood and exposure to pesticides: results of a register-based case-control study in Germany. Am J Epidemiol 2000; 151:639-650.
- 41. Ma X, Buffler PA, Gunier RB, Dahl G, Smith MT, Reinier K, Reynolds P. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ Health Perspect 2002; 110:955-960.
- 42. Ma X. Environmental and genetic factors in the etiology of childhood leukemia [PhD dissertation]. Berkeley, CA: University of California, Berkeley; 2001.
- 43. Rudant J, Menegaux F, Leverger G, Baruchel A, Nelken B, Bertrand Y, Patte C, Pacquement H, Vérité C, Robert A, Michel G, Margueritte G, Gandemer V, Hémon D, Clavel J. Household exposure to pesticides and risk of childhood hematopoietic malignancies: the ESCALE study (SFCE). Environ Health Perspect 2007; 115:1787-1793.
- 44. Daniels JL, Olshan AF, Savitz DA. Pesticides and childhood cancers. Environ Health Perspect 1997; 105:1068-1077.
- 45. Bradman A, Eskenazi B, Barr DB, Bravo R, Castorina R, Chevrier J, Kogut K, Harnly ME, McKone TE. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect 2005; 113:1802-1807.
- 46. Mandel J, Alexander B, Baker B, Acquavella J, Chapman P, Honeycutt R. Biomonitoring for farm families in the farm family exposure study. Scand J Work Environ Health 2005; 31(Suppl.1):98-104.
- 47. Sala M, Sunyer J, Otero R, Santiago-Silva M, Ozalla D, Herrero C, To-Figueras J, Kogevinas M, Anto JM, Camps C, Grimalt J. Health effects of chronic high exposure to hexachlorobenzene in a general population sample. Arch Environ Health 1999; 54:102-109.
- 48. Hertz-Picciotto I. Environmental epidemiology. In: Rothman K, Greenland S, editors. Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins; 1998. p. 555-584.
- 49. Infante-Rivard C, Jacques L. Empirical study of parental recall bias. Am J Epidemiol 2000; 152:480-486.
- 50. Arbuckle T, Cole DC, Ritter L, Ripley B. Farm children's exposure to herbicides: comparison of biomonitoring and questionnaire data. Epidemiology 2004; 5:187-194.
- 51. Perry M, Marbella A, Layde P. Nonpersistent pesticide exposure self-report versus biomonitoring in farm pesticide applicators. Ann Epidemiol 2006; 16:701-707.
- 52. Ward MH, Colt JS, Metayer C, Gunier RB, Lubin J, Crouse V, Nishioka MG, Reynolds P, Buffler PA. Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environ Health Perspect 2009; 117:1007-1013.
- 53. Lu C, Toepel K, Irish R, Fenski R, Barr DB, Bravo R. Organic diets significantly lower children's dietary exposure to organophosphorus pesticides. Environ Health Perspect 2008; 114:260-263.
- 54. National Research Council. Pesticides in the diets of infants and children. Washington, DC: National Academy Press; 1993.
- 55. Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Harnly M, Hertz A. Agricultural pesticide use and childhood cancer in California. Epidemiology 2005; 16:93-100.
- 56. Ritz B, Rull RP. Assessment of environmental exposures from agricultural pesticides in childhood leukaemia studies: challenges and opportunities. Radiat Prot Dosimetry 2008; 132:148-155.
- 57. Quandt SA, Arcury TA, Rao P, Snively BM, Camann DE, Doran AM, Yau AY, Hoppin JA, Jackson DS. Agricultural and residential pesticides in wipe samples from farmworker family residences in North Carolina and Virginia. Environ Health Perspect 2004; 112:382-387.
- 58. Wiemels JL. Chromosomal translocations in childhood leukemia: natural history, mechanisms, and epidemiology. J Natl Cancer Inst Monogr 2008; 39:87-90.
- 59. Gale KB, Ford AM, Repp R, Borkhardt A, Keller C, Eden OB, Greaves MF. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proc Natl Acad Sci USA 1997; 94:13950-13954.
- 60. Maia A, Koechling J, Corbett R, Metzler M, Wiemels JL, Greaves M. Protracted postnatal natural histories in childhood leukemia. Genes Chromosomes Cancer 2004; 39:335-340.
- 61. Lafiura KM, Bielawski DM, Posecion NC Jr, Ostrea EM Jr, Matherly LH, Taub JW, Ge Y. Association between prenatal pesticide exposures and the generation of leukemia-associated T(8;21). Pediatr Blood Cancer 2007; 49:624-628.
- 62. Raimondi S, Chang M, Ravindranath Y, Behm F, Gresik MV, Steuber C, Steuber CP, Weinstein HJ, Carroll AJ. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a cooperative pediatric oncology group study-POG 8821. Blood 1999; 94:3707-3716.
- 63. Burjanivova T, Madzo J, Muzikova K, Meyer C, Schneider B, Votava F, Marschalek R, Stary J, Trka J, Zuna J. Prenatal origin of childhood AML occurs less frequently than in childhood ALL. BMC Cancer 2006; 6:100. [Review in Internet], [cited 2006 April 21]. Doi:10.1186/1471-2407-6-100.
- 64. Metayer C, Buffler PA. Residential exposures to pesticides and childhood leukaemia. Radiat Prot Dosimetry 2009; 132:212-219.
Appendix 1

Publication Dates
-
Publication in this collection
15 Apr 2011 -
Date of issue
Mar 2011
History
-
Received
11 May 2009 -
Accepted
29 July 2009