Abstract:
This study investigated whether antenatal exposure to antidepressants (ADs) increases the risks of autism spectrum disorders (ASD), attention deficit/hyperactivity disorders (ADHD), schizophrenia and other mental illnesses, and cognitive and developmental deficits in infants or preschool children. PubMed, EMBASE, BIREME/BVS databases were searched to identify studies examining associations of ADs in pregnancy with neurodevelopmental and psychiatric disorders. Twenty studies addressed ASD and/or ADHD risks while 30 focused on developmental and cognitive deficits in infants or preschool children. Most studies detected no association of antenatal AD with ASD after adjustment of risk ratios for maternal depression or psychiatric disorders. Some studies showed that maternal depression, regardless of whether it is treated or untreated, increased ASD risks. Seven out of 8 studies found no increase in ADHD risk associated with antenatal exposure to selective serotonin reuptake inhibitors, the most commonly used AD. No consistent evidence was found linking AD in pregnancy to neurocognitive developmental deficits in infants or preschool children. A residual confounding by indication (depression severity) remained in almost all studies. This systematic review found no consistent evidence suggesting that ADs in pregnancy increase risks of ASD, ADHD, and neurocognitive development deficits. Some studies, however, found evidence that maternal depression increases ASD risks.
Keywords:
Antidepressant Drugs; Depression; Autism Spectrum Disorders; Attention Deficit Disorder with Hyperactivity; Pregnancy
Resumo:
O estudo teve como objetivo investigar se a exposição intrauterina a antidepressivos (ADs) aumenta o risco de transtornos do espectro autista (TEA), transtorno de déficit de atenção e hiperatividade (TDAH), esquizofrenia e outros transtornos mentais e déficits cognitivos e de desenvolvimento em lactentes e pré-escolares. Foram realizadas buscas nas bases PubMed, EMBASE e BIREME/BVS para identificar estudos sobre associações entre o uso de ADs durante a gestação e transtornos de neurodesenvolvimento e psiquiátricos. Vinte estudos trataram de riscos de TEA e/ou TDAH, enquanto 30 focaram em déficits cognitivos e de desenvolvimento em lactentes ou pré-escolares. A maioria dos estudos não detectou associação entre AD na gestação e TEA, depois de ajustar as razões de risco para depressão ou outros transtornos psiquiátricos maternos. Alguns estudos mostraram que a depressão materna, quer tratada ou não, aumenta o risco de TEA. Sete entre oito estudos não detectaram aumento de risco de TDAH associado à exposição intrauterina a inibidores seletivos da recaptação da serotonina, o AD mais comumente utilizado. Não foram encontradas evidências consistentes entre o uso de AD na gestação e déficits de desenvolvimento neurocognitivo em lactentes ou pré-escolares. Em quase todos os estudos, permaneceu um confundimento residual por indicação (gravidade da depressão). A revisão sistemática não encontrou evidências consistentes de que os ADs na gestação aumentassem o risco de TEA, TDAH ou déficits de desenvolvimento neurocognitivo. Entretanto, alguns estudos evidenciaram que a depressão materna aumenta o risco de TEA.
Palavras-chave:
Antidepressivos; Depressão; Transtorno do Espectro Autista; Transtorno do Déficit de Atenção com Hiperatividade; Gravidez
Resumen:
Este estudio investigó si la exposición prenatal a antidepresivos (ADs) incrementa los riesgos de trastornos del espectro autista (TEA), trastornos de déficit de atención/hiperactividad (TDAH), esquizofrenia, así como otras enfermedades mentales, cognitivas, y déficits en el desarrollo de niños de primaria o preescolares. Se consultaron las bases de datos PubMed, EMBASE, BIREME/BVS para identificar estudios de asociaciones de ADs durante el embarazo con trastornos de desarrollo neurológico y psiquiátricos. Veinte estudios estaban centrados en riesgos de TEA y/o TDAH, mientras que 30 se centraron en déficits de desarrollo y cognitivos en niños de primaria o preescolares. La mayor parte de los estudios no detectaron asociación de AD, durante la etapa prenatal, con TDA tras el ajuste de las ratios de riesgo para depresión materna o trastornos psiquiátricos. Algunos estudios mostraron que la depresión materna, independientemente de si es tratada o no, incrementó los riesgos de TEA. Siete de los 8 estudios no encontraron un incremento en el riesgo de TDAH, asociado con la exposición prenatal a inhibidores selectivos de la recaptación de serotonina, el antidepresivo más usado habitualmente durante el período prenatal. No se encontraron evidencias consistentes relacionando AD durante el embarazo y déficits en el desarrollo neurocognitivo de niños de primaria o preescolares. En casi todos los estudios hubo una desviación residual señalada como gravedad de la depresión. Esta revisión sistemática no halló evidencias consistentes, sugiriendo que el consumo de ADs durante el embarazo incremente el riesgo de TEA, TDAH, y déficits en el desarrollo neurocognitivo. Algunos estudios, no obstante, encontraron evidencias de que la depresión materna incrementa riesgos de TEA.
Palabras-clave:
Antidepresivos; Depresión; Trastorno del Espectro Autista; Trastorno por Déficit de Atención con Hiperactividad; Embarazo
Introduction
Depression is a common but serious disorder characterized by a constellation of symptoms, namely, insomnia or hypersomnia, anhedonia, feelings of worthlessness, deep sadness and excessive guilt, extreme fatigue, loss of energy, diminished ability to concentrate and to make decisions, loss or increase of appetite and/or weight, psychomotor retardation or agitation, and suicidal thoughts and attempts 11. Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev 2011; 31:1117-25..
In 2015, the World Health Organization (WHO) estimated that 322 million people, or 4.4% of world’s population, suffered from depression 22. World Health Organization. Depression and other common mental disorders. Global health estimates. Geneva: World Health Organization; 2017.. By 2030 depression will become the leading cause of global burden of disease as measured by the disability-adjusted life years (DALY) 22. World Health Organization. Depression and other common mental disorders. Global health estimates. Geneva: World Health Organization; 2017.. Depression is nearly 1.7-fold more common in females than in males and vulnerability to it increases in pregnancy and puerperium when many women experience their first depressive episode, or have a recurrent manifestation of a previously diagnosed disorder 33. Biaggi A, Conroy S, Pawlby S, Pariante CM. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord 2016; 191:62-77.. It affects up to 10%, or even more, of pregnant women and mothers who have recently given birth 44. Vigod SN, Wilson CA, Howard LM. Depression in pregnancy. BMJ 2016; 352:i1547.. Although depression is treatable, only approximately 12% of depressed pregnant women receive adequate treatment 55. Byatt N, Xiao RS, Dinh KH, Waring ME. Mental health care use in relation to depressive symptoms among pregnant women in the USA. Arch Womens Ment Health 2016; 19:187-91..
There is little consensus about the management of depression in pregnancy and reluctance to prescribe antidepressants (ADs) to pregnant women arises mostly from unresolved concerns regarding their risks to the unborn child 66. Campagne DM. Antidepressant use in pregnancy: are we closer to consensus? Arch Womens Ment Health 2019; 22:189-97.. Accumulated evidence from observational investigations and meta-analyses indicates that maternal use of selective serotonin reuptake inhibitors (SSRI) in the first trimester of gestation entails a slightly higher risk of congenital malformations, particularly of heart defects 77. Gao SY, Wu QJ, Sun C, Zhang TN, Shen ZQ, Liu CX, et al. Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: a systematic review and meta-analysis of cohort studies of more than 9 million births. BMC Med 2018; 16:205.. Some studies revealed associations of use of non-SSRIs with adverse pregnancy outcomes other than congenital anomalies, such as preterm delivery, low birth weight and perinatal complications 88. Källén B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med 2004; 158:312-6.,99. Gentile S. Tricyclic antidepressants in pregnancy and puerperium. Expert Opin Drug Saf 2014; 13:207-25.. A recent systematic review, however, found no consistent differences between AD-treated and untreated patients in the risk level for poor pregnancy outcomes, such as small-for-gestational-age babies, spontaneous abortions and preterm births 1010. Mitchell J, Goodman J. Comparative effects of antidepressant medications and untreated major depression on pregnancy outcomes: a systematic review. Arch Womens Ment Health 2018; 21:505-16..
Concerns about harmful effects of maternal depression and its pharmacological treatment on the unborn child go beyond the endpoints evaluated at term pregnancy and neonatal period. It is thought, for instance, that antenatal exposure to ADs might increase the risk of neurodevelopmental disorders such as autism spectrum (ASD) and attention-deficit/hyperactivity (ADHD) disorders, and/or cognitive deficits 1111. Rotem-Kohavi N, Oberlander TF. Variations in neurodevelopmental outcomes in children with prenatal SSRI antidepressant exposure. Birth Defects Res 2017; 109:909-23.. This notion is plausible because the fetal brain undergoes a process of rapid cell proliferation and migration, synaptogenesis and circuitry formation. Considerable experimental and clinical evidence apparently links dysfunctions of serotoninergic transmission to disruption of neural network shaping and subsequent appearance of neurodevelopmental disorders 1212. Rice D, Barone Jr. S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 2000; 108 Suppl 3:511-33.,1313. Lesch KP, Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 2012; 76:175-91.. It is of note that the most commonly prescribed antidepressants inhibit, either selectively or non-selectively, serotonin uptake from the synaptic cleft. Serotonin acts as a nervous tissue growth factor and, by doing this, it modulates neural plasticity and network formation in the developing brain 1313. Lesch KP, Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 2012; 76:175-91.. Serotonin-dependent shaping of neural circuitry provides an insight into a possible mode of action by which antenatal exposure to serotonin reuptake inhibitors might increase the risk of neurodevelopmental disorders. Along this line, Rotem-Kohavi et al. 1414. Rotem-Kohavi N, Williams LJ, Virji-Babul N, Bjornson BH, Brain U, Werker JF, et al. Alterations in resting-state networks following in utero selective serotonin reuptake inhibitor exposure in the neonatal brain. Biol Psychiatry Cogn Neurosci Neuroimaging 2019; 4:39-49. recently reported findings of a possible neural correlate of epidemiologic associations between antenatal exposure to SSRIs and neurodevelopmental disorders. Using a resting-state functional magnetic resonance imaging, the authors noted that, compared with healthy control infants and infants whose depressed mothers did not receive antidepressants, newborns prenatally exposed to SSRIs exhibited a hyperconnectivity in auditory resting-state networks.
Several cohort and case-control studies addressed a potential association between antidepressant drug use by pregnant women and neurodevelopmental disorders such as ASD and ADHD in their children 1515. Sujan AC, Öberg AS, Quinn PD, D'Onofrio BM. Annual research review: maternal antidepressant use during pregnancy and offspring neurodevelopmental problems - a critical review and recommendations for future research. J Child Psychol Psychiatry 2019; 60:356-76.. These studies arrived at conflicting conclusions about whether or not antenatal exposure to antidepressants is associated with ASD or ADHD. There is far less information about risks of neurodevelopmental disorders other than ASD and ADHD, such as mental illnesses of later onset and long-term cognitive deficits.
Eight reviews of observational studies showed a positive association between exposure to ADs (SSRIs) in utero and ASD 1616. Rais TB, Rais A. Association between antidepressants use during pregnancy and autistic spectrum disorders: a meta-analysis. Innov Clin Neurosci 2014; 11:18-22.,1717. Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, Wong IC. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev 2015; 49:82-9.,1818. Kobayashi T, Matsuyama T, Takeuchi M, Ito S. Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: a systematic review and meta-analysis. Reprod Toxicol 2016; 65:170-8.,1919. Healy D, Le Noury J, Mangin D. Links between serotonin reuptake inhibition during pregnancy and neurodevelopmental delay/spectrum disorders: a systematic review of epidemiological and physiological evidence. Int J Risk Saf Med 2016; 28:125-41.,2020. Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: a systematic review and meta-analysis. Reprod Toxicol 2016; 66:31-43.,2121. Andalib S, Emamhadi MR, Yousefzadeh-Chabok S, Shakouri SK, Høilund-Carlsen PF, Vafaee MS, et al. Maternal SSRI exposure increases the risk of autistic offspring: a meta-analysis and systematic review. Eur Psychiatry 2017; 45:161-6.,2222. Mezzacappa A, Lasica PA, Gianfagna F, Cazas O, Hardy P, Falissard B, et al. Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: a systematic review and meta-analysis. JAMA Pediatr 2017; 171:555-63.,2323. Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: a meta-analysis of cohort studies. Br J Clin Pharmacol 2017; 83:2798-806., whereas two studies found no evidence of association or inconsistent findings 2424. Zhou XH, Li YJ, Ou JJ, Li YM. Association between maternal antidepressant use during pregnancy and autism spectrum disorder: an updated meta-analysis. Mol Autism 2018; 9:21.,2525. Morales DR, Slattery J, Evans S, Kurz X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Med 2018; 16:6.. Whether these associations were causal, however, remained unclear. Three reviews evaluated whether prenatal AD exposure was associated with ADHD and found no evidence of a causal link 2525. Morales DR, Slattery J, Evans S, Kurz X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Med 2018; 16:6.,2626. Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, et al. Prenatal antidepressant exposure and the risk of attention-deficit hyperactivity disorder in children: a systematic review and meta-analysis. Neurosci Biobehav Rev 2018; 86:1-11.,2727. Uguz F. Maternal antidepressant use during pregnancy and the risk of attention-deficit/hyperactivity disorder in children: a systematic review of the current literature. J Clin Psychopharmacol 2018; 38:254-9.. Only one recent review examined whether AD use in pregnancy was associated with neurodevelopmental outcomes other than ASD and ADHD 2828. Prady SL, Hanlon I, Fraser LK, Mikocka-Walus A. A systematic review of maternal antidepressant use in pregnancy and short- and long-term offspring's outcomes. Arch Womens Ment Health 2018; 21:127-40..
Autistic disorders are as a rule diagnosed around 3-4 years of age, and last throughout the individuals’ life 2929. Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health 2017; 38:81-102.. They are characterized by symptoms such as deficits of social interaction and communication, limited and repetitive patterns of behavior, poor eye contact, lack of response to his/her name and/or indifference to caregivers, difficulty to express his/her emotions or feelings, apparent indifference to the feelings of others, inability to start a conversation or keep one going, and others 2929. Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health 2017; 38:81-102.. Some children with ASD have learning disabilities whereas others show normal to high intelligence, yet having great difficulty to communicate or apply what they learned. Both genetic and environmental factors seem to contribute to ASD susceptibility and severity 2929. Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health 2017; 38:81-102.. According to current estimates, ASD affects approximately 1% of people, being more frequently diagnosed in boys than in girls. There is no effective therapy for ASD, nor are there effective means to prevent it 2929. Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health 2017; 38:81-102.. Identification of environmental risk factors for ASD is, therefore, a public health goal of utmost importance.
ADHD is characterized by difficulty in paying attention and or hyperactivity/impulsivity 3030. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet 2005; 366:237-48.. It is about 2-fold more frequent in boys than in girls, generally diagnosed in children under the age of 12 years, and lasts throughout the persons’ life. Most patients have combined attention deficit and hyperactivity symptoms, and their enhanced distractibility and impulsivity impairs school performance and social skills 3030. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet 2005; 366:237-48.,3131. Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry 2018; 5:175-86.. Although ADHD pathophysiology remains unclear, its symptoms show a good response to treatment with dopaminergic drugs. ADHD is one of the most prevalent childhood psychiatric disorders, affecting 5-7% children, when diagnosed by DSM-IV criteria, and 1-2% when diagnosed by WHO’s (ICD-10) criteria 3030. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet 2005; 366:237-48.,3131. Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry 2018; 5:175-86.. Overdiagnosis and overtreatment of ADHD, however, is a matter of debate.
The objective of this review was to answer a question of relevance for both public health and clinical practice: Is there epidemiologic evidence suggesting that prenatal exposure to ADs increases risks of ASD, ADHD, other psychiatric disorders of later onset and neurodevelopmental deficits in the exposed offspring? A well-founded response to this question would enable physicians and health authorities to make informed decisions about the use of antidepressant drugs to treat maternal depression in pregnancy. A corollary aim of the systematic review was to disclose research gaps requiring further epidemiology studies.
This study adds to existing reviews due to its broader research question, encompassing ASD, ADHD, psychiatric disorders of later onset and other neurodevelopmental outcomes, and because it reviewed a greater number of observational studies and critically appraised their methodological limitations.
Methods
This review followed the recommendations of the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 3232. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015; 350:g7647., and was registered with PROSPERO 2018 (CRD42018080950).
Search strategy and study selection
A systematic search on PubMed, EMBASE, BIREME/BVS (Virtual Health Library - Brazil) electronic databases identified epidemiologic studies addressing the association of ADs in pregnancy with neurodevelopmental disorders in the offspring. The search covered the period between the inception of the database and December 2nd, 2018 (PubMed and BIREME/BVS), or January 1st, 2019 (EMBASE). The BIREME/BVS database includes articles published in periodicals indexed in the Latin American and Caribbean Literature on Health Sciences (LILACS). We also conducted a manual screening of reference lists of articles, reviews, and other documents, to identify additional studies potentially eligible for reviewing. There was no restriction regarding the language of the article. The search strategy combined Medical Subject Heading (MeSH; https://www.ncbi.nlm.nih.gov/mesh) terms for the pharmacological classes of antidepressants (with their variations) with pregnancy (or gestation) and with MeSH terms for neurodevelopmental disorders and cognitive skills (and variations). To design the search strings, Boolean connectors “AND” and “OR” were used for combining the search terms. Term combinations and the full search strategy are shown in Figure 1 and Supplementary Material - Appendix 1 (http://cadernos.ensp.fiocruz.br/site/public_site/arquivo/suppl-app1-e00026619_3711.pdf).
Design of systematic review search strings (MeSH terms) with Boolean connectors “AND” and “OR”.
Inclusion criteria
Studies were eligible for inclusion if they investigated, in children born to mothers with any exposure to AD in pregnancy, outcomes such as diagnoses of ASD, ADHD, mental disorders (schizophrenic, affective or anxiety disorders), or emotional, internalizing and externalizing behaviors, speech and language, intelligence, neuromotor development, or any other form of cognitive functioning, assessed at least 3-4 weeks after birth, using scales or any other method. Internalizing behaviors (or disorders) are children’s negative behaviors characterized primarily by processes focusing inward (on the self) such as anxiety, fearfulness, social withdrawal, somatization and depression. Contrasting with internalizing behaviors, externalizing behaviors are directed outwards (the external world) such as hostility, antisocial behavior, and aggression.
Exclusion criteria
Articles were not eligible for reviewing if they met any of the exclusion criteria, namely, in vivo studies conducted in animals, in vitro/ex-vivo investigations, ecologic and non-analytic epidemiology studies, cross-sectional studies, case-reports, case-series, letters, editorials, reviews, meta-analyses, notes, comments, clinical guidelines, opinion papers, full-paper not available, and articles not available in English, Portuguese, Spanish, German or French.
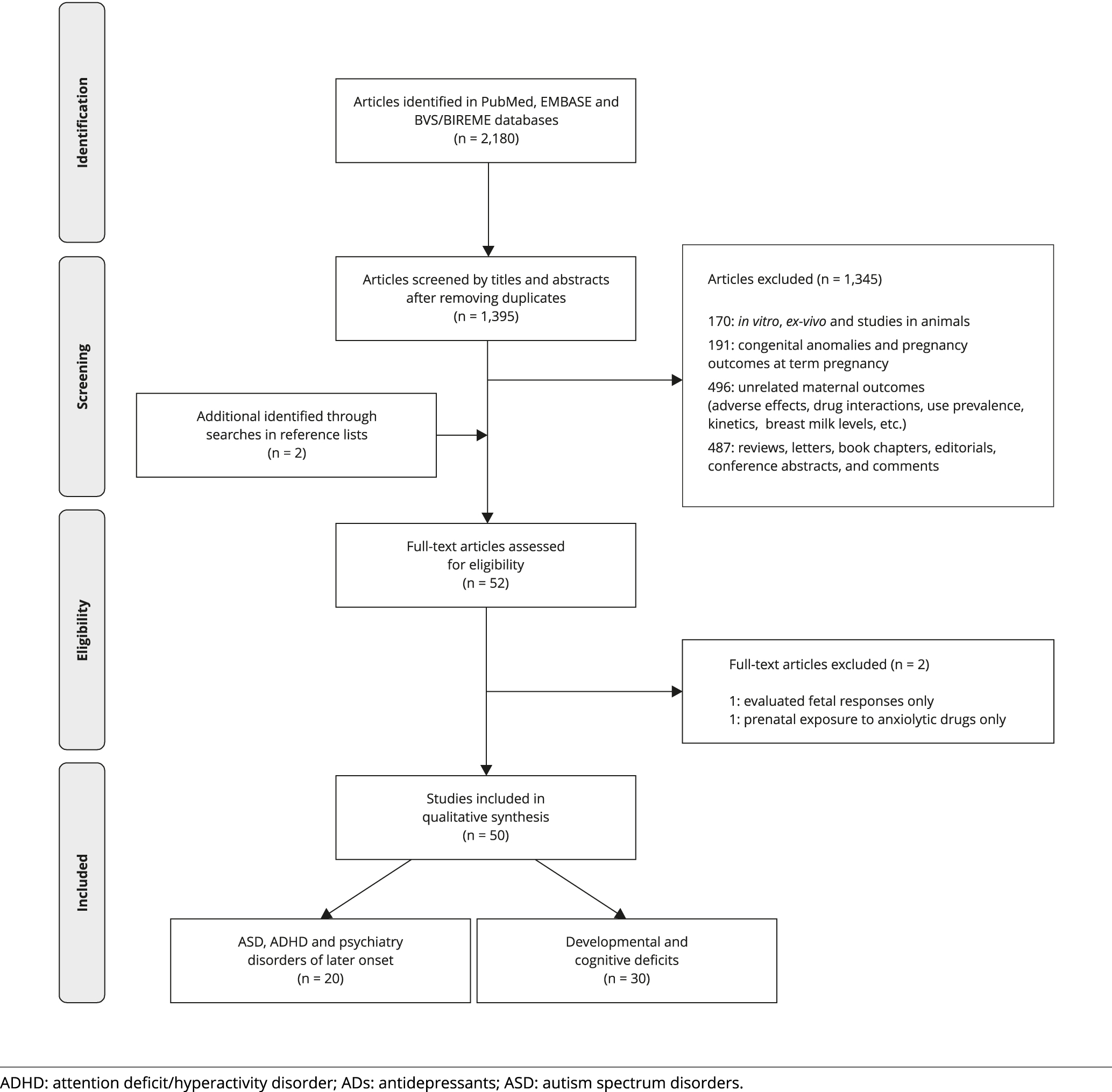
As shown in Figure 2, selection of retrieved studies (after excluding duplicates) for reviewing was a two-step screening process: at first, publications were screened by titles and abstracts and, if deemed potentially eligible, at a subsequent phase full articles were retrieved and read. Two reviewers independently screened retrieved studies for eligibility and, if they did not reach a consensus after extensive discussion, a third reviewer was asked to resolve it.
Data extraction
Based on the STROBE statement checklist of items that should be included in reports from observational studies 3333. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335:806-8., two reviewers extracted a predetermined set of data from each study selected for review. Extracted data included first author’s name, publication year, geographic location, study period, sample size, drug exposure (type of antidepressant and pharmacological class), period of exposure during pregnancy, strengths and limitations of the study, findings, outcome with risk estimates and 95% confidence intervals (95%CI), adjusted confounders, and study conclusions. A third reviewer examined the compiled data and study summaries, and differences between reviewers were resolved with discussion.
Assessment of the methodological quality
The assessment of study quality was based on the Newcastle-Ottawa Scale (NOS) for observational (case-control and cohort) epidemiology studies 3434. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 11/Dec/2018).
http://www.ohri.ca/programs/clinical_epi...
. NOS consists of eight items grouped into three domains for selection, comparability and outcome and two reviewers independently assessed each of the studies assigning a score (maximum score = 9) to the study quality. Any discrepancy between reviewers was resolved by a third reviewer.
Data synthesis
For each outcome evaluated, we present the characteristics and findings of all included studies. A qualitative (narrative) synthesis of the evidence for ASD, ADHD and other neurodevelopmental outcomes responded the research question. It took into account not only the results of the reviewed studies but also their methodological limitations and residual confounding. We considered undertaking a quantitative synthesis for ASD and ADHD. Nonetheless, we did not perform a meta-analysis because of the heterogeneity in design and characteristics of the included studies and the fact that several studies (national-based cohorts) used the same national data source (registry) with overlapping of data collection times.
Results
As shown in the PRISMA flow diagram of study selection process (Figure 2), of the 50 studies included in the review, 20 addressed risks of ASD and ADHD while 30 investigated risks of developmental and cognitive impairments in infants, toddlers and preschool children. One Finnish study investigated the impact of gestational exposure to SSRIs on offspring psychiatric disorders including not only ASD and ADHD, but also anxiety and depression in early adolescents 3535. Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry 2016; 55:359-66..
Autism spectrum disorders
Five out of 16 studies evaluating whether prenatal ADs increased risks of ASD found no increase in risk 3535. Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry 2016; 55:359-66.,3636. Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med 2013; 369:2406-15.,3737. Castro VM, Kong SW, Clements CC, Brady R, Kaimal AJ, Doyle AE, et al. Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry 2016; 6:e708.,3838. Brown HK, Ray JG, Wilton AS, Lunsky Y, Gomes T, Vigod SN. Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children. JAMA 2017; 317:1544-52.,3939. Viktorin A, Uher R, Reichenberg A, Levine SZ, Sandin S. Autism risk following antidepressant medication during pregnancy. Psychol Med 2017; 47:2787-96.. Three studies detected a weak association of AD in pregnancy with ASD that were no longer significant when risk estimates were adjusted for confounding factors such as maternal depression or history of psychiatric disorders 4040. Sørensen MJ, Grønborg TK, Christensen J, Parner ET, Vestergaard M, Schendel D, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 2013; 5:449-59.,4141. Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727-34.,4242. Sujan AC, Rickert ME, Öberg AS, Quinn PD, Hernández-Díaz S, Almqvist C, et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA 2017; 317:1553-62.. Hagberg et al. 4343. Hagberg KW, Robijn AL, Jick S. Maternal depression and antidepressant use during pregnancy and the risk of autism spectrum disorder in offspring. Clin Epidemiol 2018; 10:1599-612. found a significant association of ASD with antenatal AD, when the unexposed offspring (no AD, no history of depression) was compared with a group exposed to both AD and maternal depression in pregnancy. No increase in risk of ASD was evident, however, when unexposed offspring was compared with offspring of pregnant women who received AD for disorders other than depression 4343. Hagberg KW, Robijn AL, Jick S. Maternal depression and antidepressant use during pregnancy and the risk of autism spectrum disorder in offspring. Clin Epidemiol 2018; 10:1599-612.. Six cohort and case-control (nested in population-based cohorts) investigations, on the other hand, found a modest association of antenatal AD with ASD 4444. Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104-12.,4545. Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, Newschaffer CJ. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J Autism Dev Disord 2014; 44:2558-67.,4646. Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr 2016; 170:117-24., ASD in boys 4747. Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics 2014; 133:e1241-8., and ASD without intellectual disability 4848. Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ 2013; 346:f2059.,4949. Rai D, Lee BK, Dalman C, Newschaffer C, Lewis G, Magnusson C. Antidepressants during pregnancy and autism in offspring: population based cohort study. BMJ 2017; 358:j2811.. One of the reviewed studies evaluated whether antenatal exposure to SSRI was associated with higher scores for autistic traits in children 5050. El Marroun H, White TJ, van der Knaap NJ, Homberg JR, Fernández G, Schoemaker NK, et al. Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. Br J Psychiatry 2014; 205:95-102.. El Marroun et al. 5050. El Marroun H, White TJ, van der Knaap NJ, Homberg JR, Fernández G, Schoemaker NK, et al. Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. Br J Psychiatry 2014; 205:95-102. found a modest association between maternal (self-reported) use of SSRIs in pregnancy and higher scores for autistic traits in infants assessed by child behavior checklists and a social responsiveness scale. Since depressed women are likely to overestimate problems with their children, and a residual confounding by indication of SSRIs (severity of depression) remained, associations of SSRI use in pregnancy with higher scores for autistic traits in infants may be non-causal.
Attention deficit/hyperactivity disorders
Four out of 8 studies that examined possible associations between AD in pregnancy and ADHD found no association between exposure and outcome 3535. Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry 2016; 55:359-66.,3737. Castro VM, Kong SW, Clements CC, Brady R, Kaimal AJ, Doyle AE, et al. Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry 2016; 6:e708.,4242. Sujan AC, Rickert ME, Öberg AS, Quinn PD, Hernández-Díaz S, Almqvist C, et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA 2017; 317:1553-62.,5151. Laugesen K, Olsen MS, Telén Andersen AB, Frøslev T, Sørensen HT. In utero exposure to antidepressant drugs and risk of attention deficit hyperactivity disorder: a nationwide Danish cohort study. BMJ Open 2013; 3:e003507.. Three studies revealed no association of ADHD with maternal use of SSRIs, yet they found that this disorder was - weakly to moderately - associated with prenatal exposure to non-SSRIs, tricyclic ADs (TCA) and atypical antidepressants.
Figueroa 5252. Figueroa R. Use of antidepressants during pregnancy and risk of attention-deficit/hyperactivity disorder in the offspring. J Dev Behav Pediatr 2010; 31:641-8. found that maternal use of SSRIs and ADs other than bupropion in pregnancy did not increase the risk of ADHD in children aged 5 years or younger. Antenatal bupropion, on the other hand, was moderately associated with ADHD especially when exposure occurred in the 2nd trimester of gestation. Bupropion inhibits both norepinephrine (NE) and dopamine (DA) reuptakes and, additionally, releases these neurotransmitters into the synaptic cleft. It also acts as a nicotinic receptor antagonist 5353. Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev 2006; 12:178-207.. This atypical antidepressant is used in combination with another AD, when patients are resistant to SSRI, and as an adjunct therapy for smoking cessation, weight loss and ADHD 5454. Zisook S, Rush AJ, Haight BR, Clines DC, Rockett CB. Use of bupropion in combination with serotonin reuptake inhibitors. Biol Psychiatry 2006; 59:203-10.,5555. Dunner DL. Combining antidepressants. Shanghai Arch Psychiatry 2014; 26:363-4.,5656. Patel K, Allen S, Haque MN, Angelescu I, Baumeister D, Tracy DK. Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Ther Adv Psychopharmacol 2016; 6:99-144.. Boukhris et al. 5757. Boukhris T, Sheehy O, Bérard A. Antidepressant use in pregnancy and the risk of attention deficit with or without hyperactivity disorder in children. Paediatr Perinat Epidemiol 2017; 31:363-73. found that TCA in the 2nd/3rd trimesters of pregnancy was weakly associated with ADHD, even after adjustment for maternal history of depression. A territory population-based study by Man et al. 5858. Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ 2017; 357:j2350. found that non-SSRI use in pregnancy was associated with a modest increase in the risk of ADHD in the offspring, yet a subsequent sibling analysis showed no association.
Finally, a case-control study by Clements et al. 4141. Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727-34. found a modest association of ADHD with prenatal exposure to SSRIs & non-SSRIs, especially during the 1st trimester of gestation. This association was significant even after adjustment of risk estimates for maternal history of depression.
Quality assessment
The quality of cohort and case-control studies selected for review ranged from fair to very good (NOS scores 6 to 9), and 16 and 8 of them investigated risks of ASD and/or ADHD, respectively, in children born to AD-treated pregnant women (Tables 1 and 2). The reasons for lower assessments (fair quality) were lower scores in Selection (2) and Comparability (1), and Outcome (1) and Comparability (1) domains for one case-control 4444. Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104-12. and one cohort 5050. El Marroun H, White TJ, van der Knaap NJ, Homberg JR, Fernández G, Schoemaker NK, et al. Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. Br J Psychiatry 2014; 205:95-102. study, respectively (Supplementary Material - Appendix 2: http://cadernos.ensp.fiocruz.br/site/public_site/arquivo/suppl-app2-e00026619_2540.pdf).
Mental disorders of later onset
Malm et al. 3535. Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry 2016; 55:359-66. found that prenatal exposure to SSRIs modestly increased risks of depression in the offspring (SSRI exposed vs. non-exposed, after adjustment for maternal psychiatric disorder; HR = 1.78; 95%CI: 1.12-2.82). In this Finnish national register-based cohort study, cumulative incidence of diagnoses of depression was determined up to adolescence (age of 14) 3535. Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry 2016; 55:359-66.. Notwithstanding the attempt to adjust risk estimates for records of maternal history of psychiatric illnesses, the severity of maternal depressive disorder remained as a residual confounding. It is of note that association of SSRIs with depression among the offspring was also detected (adjusted HR = 1.84; 95%CI: 1.14-2.97) for preconception exposures (i.e., SSRIs discontinued in pregnancy). In this study, the authors found no association of SSRI in pregnant women with anxiety in children prenatally exposed 3535. Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry 2016; 55:359-66.. No other study examined a possible association between antenatal exposure to ADs and psychiatric disorders of later onset (adolescents and young adults) such as schizophrenia 5959. Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull 2011; 37:504-13., and affective and anxiety disorders 6060. Jones PB. Adult mental health disorders and their age at onset. Br J Psychiatry Suppl 2013; 54:s5-10..
Developmental and cognitive deficits
Thirty out of 50 studies selected for review focused on the development and/or cognitive deficits in infants, toddlers and preschool children that might have arisen from prenatal exposures to ADs (Table 3). A majority of these 30 studies found no association between prenatal ADs (mostly SSRIs) and deficits of IQ and/or cognitive 6161. Nulman I, Rovet J, Stewart DE, Wolpin J, Gardner HA, Theis JG, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med 1997; 336:258-62.,6262. Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002; 159:1889-95.,6363. Nulman I, Koren G, Rovet J, Barrera M, Pulver A, Streiner D, et al. Neurodevelopment of children following prenatal exposure to venlafaxine, selective serotonin reuptake inhibitors, or untreated maternal depression. Am J Psychiatry 2012; 169:1165-74.,6464. Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 2003; 142:402-8.,6565. Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr 2013; 102:1054-9.,6666. Johnson KC, Smith AK, Stowe ZN, Newport DJ, Brennan PA. Preschool outcomes following prenatal serotonin reuptake inhibitor exposure: differences in language and behavior, but not cognitive function. J Clin Psychiatry 2016; 77:e176-82.,6767. Hermansen TK, Røysamb E, Augusti EM, Melinder A. Behavior and inhibitory control in children with prenatal exposure to antidepressants and medically untreated depression. Psychopharmacology (Berl) 2016; 233:1523-35.,6868. El Marroun H, White TJ, Fernandez G, Jaddoe VW, Verhulst FC, Stricker BH, et al. Prenatal exposure to selective serotonin reuptake inhibitors and non-verbal cognitive functioning in childhood. J Psychopharmacol 2017; 31:346-55.,6969. Viktorin A, Uher R, Kolevzon A, Reichenberg A, Levine SZ, Sandin S. Association of antidepressant medication use during pregnancy with intellectual disability in offspring. JAMA Psychiatry 2017; 74:1031-8., language 7070. Handal M, Skurtveit S, Roth C, Hernandez-Diaz S, Selmer R. Prenatal exposure to folic acid and antidepressants and language development: a population-based cohort study. J Clin Psychopharmacol 2016; 36:333-9., and behavior development 6565. Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr 2013; 102:1054-9.,7171. Reebye PN, Morison SJ, Panikkar H, Misri S, Grunau RE. Affect expression in prenatally psychotropic exposed and nonexposed mother-infant dyads. Infant Ment Health J 2002; 23:403-16.,7272. Casper RC, Gilles AA, Fleisher BE, Baran J, Enns G, Lazzeroni LC. Length of prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants: effects on neonatal adaptation and psychomotor development. Psychopharmacology (Berl) 2011; 217:211-9.,7373. Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol 2013; 30:297-301.,7474. Santucci AK, Singer LT, Wisniewski SR, Luther JF, Eng HF, Dills JL, et al. Impact of prenatal exposure to serotonin reuptake inhibitors or maternal major depressive disorder on infant developmental outcomes. J Clin Psychiatry 2014; 75:1088-95.. Some studies, however, indicated that the maternal depressive disorder, regardless of whether it is treated with ADs or not during pregnancy, may slightly increase the risks of deficits of IQ and language development 6262. Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002; 159:1889-95., language competence 7575. Skurtveit S, Selmer R, Roth C, Hernandez-Diaz S, Handal M. Prenatal exposure to antidepressants and language competence at age three: results from a large population-based pregnancy cohort in Norway. BJOG 2014; 121:1621-31., and/or behavior development 7676. Pedersen LH, Henriksen TB, Bech BH, Licht RW, Kjaer D, Olsen J. Prenatal antidepressant exposure and behavioral problems in early childhood: a cohort study. Acta Psychiatr Scand 2013; 127:126-35.,7777. Grzeskowiak LE, Morrison JL, Henriksen TB, Bech BH, Obel C, Olsen J, et al. Prenatal antidepressant exposure and child behavioural outcomes at 7 years of age: a study within the Danish National Birth Cohort. BJOG 2016; 123:1919-28..
Six studies evaluated the impact of prenatal ADs (mostly SSRI) on levels of internalizing and/or externalizing behaviors in preschool children. One study found that both prenatal SSRI and depression increased risks of externalizing behaviors while SSRI increased risks of internalizing behaviors 6767. Hermansen TK, Røysamb E, Augusti EM, Melinder A. Behavior and inhibitory control in children with prenatal exposure to antidepressants and medically untreated depression. Psychopharmacology (Berl) 2016; 233:1523-35.. Another investigation indicated that prenatal SRI predicts internalizing but not externalizing behaviors in early childhood 7878. Hanley GE, Brain U, Oberlander TF. Prenatal exposure to serotonin reuptake inhibitor antidepressants and childhood behavior. Pediatr Res 2015; 78:174-80.. Oberlander et al. 7979. Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 2007; 161:22-9. found that antenatal SSRI and depression are not associated with the child’s externalizing behavior while current maternal mood predicts it. Two additional studies found no association between prenatal SSRIs or ADs with internalizing 8080. Misri S, Reebye P, Kendrick K, Carter D, Ryan D, Grunau RE, et al. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry 2006; 163:1026-32. and externalizing behaviors 8181. Lupattelli A, Wood M, Ystrom E, Skurtveit S, Handal M, Nordeng H. Effect of time-dependent selective serotonin reuptake inhibitor antidepressants during pregnancy on behavioral, emotional, and social development in preschool-aged children. J Am Acad Child Adolesc Psychiatry 2018; 57:200-8.. Overall, the foregoing findings showed no consistent association of antenatal SSRIs and depression with children’s internalizing or externalizing behaviors.
Discussion
Residual confounding by indication and severity of depression
A residual confounding by indication was a shortcoming with major impact on the interpretation of most research findings. ADs are indicated mainly for the treatment of depressive disorders and so the possibility exists that associations resulted from the maternal depression for which the drug was prescribed and not from the pharmacological intervention. In other words, reported associations between children’s neurodevelopmental disorders (ASD and ADHD) and maternal use of ADs in pregnancy may be non-causal. A few studies did not control confounding by indication at all and simply compared the offspring of AD-treated depressed mothers with the offspring of untreated and non-depressed pregnant women. These studies generally found a higher risk of ASD/ADHD among children prenatally exposed to ADs. In most studies, however, investigators made an attempt to control this confounding in the statistical analysis by adjusting risk ratios for effects of dichotomous presence or absence of maternal depression or history of psychiatric disorders, or yet, by also making a maternal sibling sub-cohort analysis.
It is fair to think that depression entailing prescription for AD is probably more severe than the disorder of patients not receiving pharmacological intervention. The lack of adjustment for severity of depression may have led to spurious associations of AD exposure in pregnancy with any analyzed outcome, if there is a causal link between the outcome (ASD, ADHD or other neurodevelopmental disorder) and the mother’s major depressive illness.
Depression and risks of ASD, ADHD and neurodevelopmental impairment
The notion that maternal depression might be an independent risk factor for adverse pregnancy outcomes and offspring neurodevelopmental disorders is not only plausible but it is also consistent with findings from observational studies. Systematic reviews, with or without meta-analysis, found that untreated depression, anxiety and/or perceived stress during pregnancy were associated with small increases in the risk of adverse pregnancy outcomes such as preterm births, low birth weights and small-for-gestational-age infants 8282. Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 2010; 67:1012-24.,8383. Staneva A, Bogossian F, Pritchard M, Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: a systematic review. Women Birth 2015; 28:179-93.. Untreated depression also causes effects on the developing fetus such as hyperactivity, irregular fetal heart rate and altered EEG patterns 8484. Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience 2017; 342:154-66.. The impact of maternal depression on child development and mental health has been far less explored. Nonetheless, some researches provided indirect evidence for such association of unmedicated maternal depression with ASD. A strong evidence along this line was provided by studies showing that preconception-only maternal exposure to ADs increased risks of ASD in the offspring 3636. Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med 2013; 369:2406-15.,3737. Castro VM, Kong SW, Clements CC, Brady R, Kaimal AJ, Doyle AE, et al. Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry 2016; 6:e708.,4141. Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727-34.,4545. Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, Newschaffer CJ. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J Autism Dev Disord 2014; 44:2558-67.. AD therapy before pregnancy indicates that these women suffered from a depressive disorder severe enough to require a pharmacological intervention. It is difficult to foresee, on the other hand, how a preconception-only exposure to ADs could have any detrimental effect on the further development of the conceptus. Moreover, associations of ASD with AD use in pregnancy detected by several studies proved to be nonsignificant after adjustment for maternal psychiatric disorders. Recently, Hagberg et al. 4343. Hagberg KW, Robijn AL, Jick S. Maternal depression and antidepressant use during pregnancy and the risk of autism spectrum disorder in offspring. Clin Epidemiol 2018; 10:1599-612. found that untreated depression, but not AD use in pregnancy for disorders other than depression, increased risks of ASD in the offspring. Using the duration of the last episode of depression as a proxy for disorder severity, the authors noted that risk of ASD increased with increasing severity of maternal depression. Collectively, these findings are consistent with the interpretation that a maternal history of depression might be an independent risk factor for ASD.
Seven studies found that maternal depression in pregnancy and/or after birth, regardless of whether it is treated or untreated, might impair child development 6262. Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002; 159:1889-95.,6767. Hermansen TK, Røysamb E, Augusti EM, Melinder A. Behavior and inhibitory control in children with prenatal exposure to antidepressants and medically untreated depression. Psychopharmacology (Berl) 2016; 233:1523-35.,7575. Skurtveit S, Selmer R, Roth C, Hernandez-Diaz S, Handal M. Prenatal exposure to antidepressants and language competence at age three: results from a large population-based pregnancy cohort in Norway. BJOG 2014; 121:1621-31.,7676. Pedersen LH, Henriksen TB, Bech BH, Licht RW, Kjaer D, Olsen J. Prenatal antidepressant exposure and behavioral problems in early childhood: a cohort study. Acta Psychiatr Scand 2013; 127:126-35.,7777. Grzeskowiak LE, Morrison JL, Henriksen TB, Bech BH, Obel C, Olsen J, et al. Prenatal antidepressant exposure and child behavioural outcomes at 7 years of age: a study within the Danish National Birth Cohort. BJOG 2016; 123:1919-28.,7979. Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 2007; 161:22-9.,8080. Misri S, Reebye P, Kendrick K, Carter D, Ryan D, Grunau RE, et al. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry 2006; 163:1026-32.. These findings showed that, as noted for ASD and ADHD, the non-adjustment of risk ratios for severity of depression may have led to spurious associations of antenatal AD with deficits in the cognitive and behavioral development of infants and preschool children.
Genetics and mother-child interaction
Both inheritable genetic traits and depression-caused abnormal mother-child interaction might provide plausible explanations for associations between maternal depression and enhanced risks of neurodevelopmental and psychiatric disorders in the offspring.
Affective disorders have well-established genetic components and family studies indicated 2- to 3-fold increases in lifetime risk of major depression among first-degree relatives 8585. Flint J, Kendler KS. The genetics of major depression. Neuron 2014; 81:1214.,8686. Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018; 50:668-81.. A recent genome-wide association meta-analysis identified 44 genetic risk loci for major depressive disorders and strongly suggested the existence of biological processes common to major depression and schizophrenia. The genome analysis indicated that some biological processes might be common to major depression and other psychiatric disorders such as ASD and ADHD 8686. Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018; 50:668-81.. In the study by Malm et al. 3535. Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry 2016; 55:359-66., for instance, inherited risk factors for major depression were lurking variables that might have influenced the apparent association between SSRI use in pregnancy and depressive disorders in the adolescent offspring. Although the association was significant after adjustment for maternal psychiatric illness, the authors did not adjust risk ratios for severity of maternal depression. SSRI use in pregnancy could have been a surrogate or marker for more severe maternal depressive disorders and, as mentioned, major depression has a genetic and inheritable component.
Furthermore, various studies provided evidence that depression can adversely affect mother-child bonding as well as the child’s development and mental health 8787. Kingston D, Tough S, Whitfield H. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry Hum Dev 2012; 43:683-714..
Sibling analysis designs are useful to investigate associations thought likely to suffer confounding arising from genetics and environmental postnatal factors, including the detrimental effects of maternal depressive symptoms on mother-child interaction. In principle, such strategy to control potential confounding in the design of the study seems to be better than merely adjusting risk ratios in the statistical analysis using dichotomous data on the presence/absence of maternal history of depression. Nonetheless, a possible disadvantage of sibling analysis in a sub-cohort of population-based cohorts is that it drastically reduces the sample size and thus the statistical power of the analysis. In all sibling analyses conducted by reviewed studies, no association of ADs in pregnancy with ASD was found 3838. Brown HK, Ray JG, Wilton AS, Lunsky Y, Gomes T, Vigod SN. Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children. JAMA 2017; 317:1544-52.,4040. Sørensen MJ, Grønborg TK, Christensen J, Parner ET, Vestergaard M, Schendel D, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 2013; 5:449-59.,4242. Sujan AC, Rickert ME, Öberg AS, Quinn PD, Hernández-Díaz S, Almqvist C, et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA 2017; 317:1553-62.,4343. Hagberg KW, Robijn AL, Jick S. Maternal depression and antidepressant use during pregnancy and the risk of autism spectrum disorder in offspring. Clin Epidemiol 2018; 10:1599-612., nor was it detected with ADHD 4242. Sujan AC, Rickert ME, Öberg AS, Quinn PD, Hernández-Díaz S, Almqvist C, et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA 2017; 317:1553-62.,5151. Laugesen K, Olsen MS, Telén Andersen AB, Frøslev T, Sørensen HT. In utero exposure to antidepressant drugs and risk of attention deficit hyperactivity disorder: a nationwide Danish cohort study. BMJ Open 2013; 3:e003507.,5858. Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ 2017; 357:j2350..
Non-differential misclassification of exposure
A notable shortcoming common to all reviewed studies is a potential non-differential misclassification of exposure status due to lack of confirmation of the actual drug intake in pregnancy. Exposure assessment (binary exposure) took into account records of drug prescription and dispensing, but no study confirmed patients’ adherence to AD treatment. The exposure status relevant for the outcome of interest is not merely a matter of using or not ADs during pregnancy. The effect of exposure on the outcome is likely to depend on the magnitude (dose), timing and adherence to prescribed pharmacotherapy. Nonadherence to prescribed medication and, particularly, poor AD adherence is a challenging issue in psychiatric practice. It is estimated that up to approximately 50% of psychiatric patients, including those with major depression prematurely discontinue drug therapy 8888. Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci 2012; 9:41-6.,8989. Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry 2013; 26:446-52.. Nonadherence with medication is also a common problem among pregnant women whose drug-taking behavior can be negatively influenced by concerns about harmful effects of the pharmacological therapy on the unborn child, among other factors 9090. Matsui D. Adherence with drug therapy in pregnancy. Obstet Gynecol Int 2012; 2012:796590.. The influence of concomitant pregnancy and depressive illness on the high rates of nonadherence with medication noted in either condition alone is unclear. At any rate, non-differential misclassification of exposure is likely to bias towards the null estimates of detrimental effects of prenatal AD exposure on offspring’s health.
National scenarios
Another potential drawback of the reviewed cohort or case-control studies is the fact that nearly all of them used data from nationwide (or territory-based) health registries (e.g., Scandinavian countries, Finland, Hong Kong), or large databases from health insurance companies (e.g., United States, United Kingdom). These studies investigated associations between exposure and outcomes in people living in a few highly developed countries and findings might be somewhat different for distinct exposure scenarios and populations from Asia, Africa and Latin America.
Publication bias and searching strategy
In principle, publication biases may misdirect the qualitative and/or quantitative synthesis of any systematic review. It is unlikely, however, that any good quality observational study on the topic addressed by this systematic review would have remained unpublished. The possibility exists, on the other hand, that the adopted searching strategy (search strings and databases) was not effective to identify all relevant studies. To verify whether the search strategy failed to identify articles of interest, we compared the set of studies included in this review with those analyzed by previous systematic reviews. Nine reviews conducted between 2014 and 2018 examined a possible association of ADs in pregnancy with ASD 1616. Rais TB, Rais A. Association between antidepressants use during pregnancy and autistic spectrum disorders: a meta-analysis. Innov Clin Neurosci 2014; 11:18-22.,1717. Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, Wong IC. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev 2015; 49:82-9.,1818. Kobayashi T, Matsuyama T, Takeuchi M, Ito S. Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: a systematic review and meta-analysis. Reprod Toxicol 2016; 65:170-8.,1919. Healy D, Le Noury J, Mangin D. Links between serotonin reuptake inhibition during pregnancy and neurodevelopmental delay/spectrum disorders: a systematic review of epidemiological and physiological evidence. Int J Risk Saf Med 2016; 28:125-41.,2020. Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: a systematic review and meta-analysis. Reprod Toxicol 2016; 66:31-43.,2121. Andalib S, Emamhadi MR, Yousefzadeh-Chabok S, Shakouri SK, Høilund-Carlsen PF, Vafaee MS, et al. Maternal SSRI exposure increases the risk of autistic offspring: a meta-analysis and systematic review. Eur Psychiatry 2017; 45:161-6.,2222. Mezzacappa A, Lasica PA, Gianfagna F, Cazas O, Hardy P, Falissard B, et al. Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: a systematic review and meta-analysis. JAMA Pediatr 2017; 171:555-63.,2323. Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: a meta-analysis of cohort studies. Br J Clin Pharmacol 2017; 83:2798-806.,2424. Zhou XH, Li YJ, Ou JJ, Li YM. Association between maternal antidepressant use during pregnancy and autism spectrum disorder: an updated meta-analysis. Mol Autism 2018; 9:21.. Our review about ASD risks covered not only all studies included in the previous reviews but also analyzed two more studies than the most recently published review. Two reviews, both published in 2018, addressed the risks of ADHD 2626. Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, et al. Prenatal antidepressant exposure and the risk of attention-deficit hyperactivity disorder in children: a systematic review and meta-analysis. Neurosci Biobehav Rev 2018; 86:1-11.,2727. Uguz F. Maternal antidepressant use during pregnancy and the risk of attention-deficit/hyperactivity disorder in children: a systematic review of the current literature. J Clin Psychopharmacol 2018; 38:254-9.. Again, this study covered not only all studies included in published reviews but one more than those examined by one of the two previous reviews. Two reviews published in 2011 9191. Gentile S, Galbally M. Prenatal exposure to antidepressant medications and neurodevelopmental outcomes: a systematic review. J Affect Disord 2011; 128:1-9. and 2018 2828. Prady SL, Hanlon I, Fraser LK, Mikocka-Walus A. A systematic review of maternal antidepressant use in pregnancy and short- and long-term offspring's outcomes. Arch Womens Ment Health 2018; 21:127-40., including 5 and 7 studies, respectively, evaluated risks of neurodevelopmental disorders arising from prenatal exposures to ADs. Except for one study 9292. Suri R, Hellemann G, Stowe ZN, Cohen LS, Aquino A, Altshuler LL. A prospective, naturalistic, blinded study of early neurobehavioral outcomes for infants following prenatal antidepressant exposure. J Clin Psychiatry 2011; 72:1002-7., this review analyzed all studies included in previous reviews and 23 additional articles. Suri et al. 9292. Suri R, Hellemann G, Stowe ZN, Cohen LS, Aquino A, Altshuler LL. A prospective, naturalistic, blinded study of early neurobehavioral outcomes for infants following prenatal antidepressant exposure. J Clin Psychiatry 2011; 72:1002-7. found no association of AD in pregnancy with neurobehavioral outcomes and their study was included in the review by Prady et al. 2828. Prady SL, Hanlon I, Fraser LK, Mikocka-Walus A. A systematic review of maternal antidepressant use in pregnancy and short- and long-term offspring's outcomes. Arch Womens Ment Health 2018; 21:127-40..
The foregoing comparison with previous reviews suggests that this updated review, encompassing the three outcomes of interest, did not fail to retrieve and include any relevant study about potential associations between prenatal exposure to AD and risks of ASD, ADHD and neurodevelopmental disorders.
SSRIs and neurodevelopmental outcomes
Studies of associations of ADs in pregnancy with impaired neurocognitive and behavioral development during infancy and early childhood were heterogeneous regarding the design, instruments used to assess neurocognitive development outcomes and child age at assessment. Overall, their findings indicated that antenatal exposure to SSRI did not impair further child development. Many of these studies used scales to assess child development such as the Bayley Scales of Infant Development (BSID) for infants with ages ranging from one to 42 months. BSID has high reliability and validity but, unless the scores are very low, its predictive value for long-term intellectual and motor disabilities is questionable 9393. Anderson PJ, Burnett A. Assessing developmental delay in early childhood - concerns with the Bayley-III scales. Clin Neuropsychol 2017; 31:371-81.,9494. Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res 2014; 75:670-4.,9595. Harris SR, Langkamp DL. Predictive value of the Bayley mental scale in the early detection of cognitive delays in high-risk infants. J Perinatol 1994; 14:275-9.. The clinical relevance of significant yet small differences in BDSI scores detected by some studies is unclear.
Since observational studies have limitations for controlling confounding and making causal inferences, a prospective, randomized, placebo-controlled, double-blinded clinical trial is under way in Sweden to clarify whether a SSRI (sertraline) and/or depression in pregnancy might in fact affect child neurocognitive development. The primary objective of this trial is to assess cognitive development at 2 years of age using the BSID-III scale. The recruitment of pregnant women for this study (MAGDALENA study protocol) will be finished in 5 to 6 years’ time 9696. Heinonen E, Szymanska-von Schultz B, Kaldo V, Nasiell J, Andersson E, Bergmark M, et al. MAGDALENA: study protocol of a randomised, placebo-controlled trial on cognitive development at 2 years of age in children exposed to SSRI in utero. BMJ Open 2018; 8:e023281..
Concluding remarks
This study found no consistent evidence of association between antenatal exposure to ADs and increased risks of ASD, ADHD, psychiatric illnesses, and cognitive and or developmental deficits in preschool children.
The conclusion that prenatal exposure to SSRIs and/or serotonin-norepinephrine reuptake inhibitors (SNRIs) does not predict risks of ASD is particularly robust. It is of note that results from some studies strongly suggested that maternal depression, regardless of whether it is treated or untreated during pregnancy, increases risks of ASD in the offspring. A recent population-based cohort study (mother-newborn pairs from Manitoba, Canada, born 1996-2009 with follow-up through 2014) found no association (HR = 0.92; 95%CI: 0.42-2.03) between antenatal exposure to SSRI or SNRI and ASD 9797. Singal D, Chateau D, Dahl M, Katz L, Ruth C, Wall-Wieler E, et al. In utero SSRI and SNRI exposure and the risk of neurodevelopmental outcomes in children: a population-based retrospective cohort study utilizing linked administrative data. Pharmacoepidemiol Drug Saf 2018; 27 Suppl 2:240-1.. This study was not included because only a conference abstract was available when the review was completed.
As commented before, eight reviews of observational studies found a positive association of ASD with use of SSRIs in pregnancy 1616. Rais TB, Rais A. Association between antidepressants use during pregnancy and autistic spectrum disorders: a meta-analysis. Innov Clin Neurosci 2014; 11:18-22.,1717. Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, Wong IC. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev 2015; 49:82-9.,1818. Kobayashi T, Matsuyama T, Takeuchi M, Ito S. Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: a systematic review and meta-analysis. Reprod Toxicol 2016; 65:170-8.,1919. Healy D, Le Noury J, Mangin D. Links between serotonin reuptake inhibition during pregnancy and neurodevelopmental delay/spectrum disorders: a systematic review of epidemiological and physiological evidence. Int J Risk Saf Med 2016; 28:125-41.,2020. Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: a systematic review and meta-analysis. Reprod Toxicol 2016; 66:31-43.,2121. Andalib S, Emamhadi MR, Yousefzadeh-Chabok S, Shakouri SK, Høilund-Carlsen PF, Vafaee MS, et al. Maternal SSRI exposure increases the risk of autistic offspring: a meta-analysis and systematic review. Eur Psychiatry 2017; 45:161-6.,2222. Mezzacappa A, Lasica PA, Gianfagna F, Cazas O, Hardy P, Falissard B, et al. Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: a systematic review and meta-analysis. JAMA Pediatr 2017; 171:555-63.,2323. Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: a meta-analysis of cohort studies. Br J Clin Pharmacol 2017; 83:2798-806.. At least four of these studies highlighted that maternal psychiatric condition was a major confounding 1818. Kobayashi T, Matsuyama T, Takeuchi M, Ito S. Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: a systematic review and meta-analysis. Reprod Toxicol 2016; 65:170-8.,2020. Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: a systematic review and meta-analysis. Reprod Toxicol 2016; 66:31-43.,2222. Mezzacappa A, Lasica PA, Gianfagna F, Cazas O, Hardy P, Falissard B, et al. Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: a systematic review and meta-analysis. JAMA Pediatr 2017; 171:555-63.,2323. Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: a meta-analysis of cohort studies. Br J Clin Pharmacol 2017; 83:2798-806. or that causality remained to be confirmed 1717. Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, Wong IC. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev 2015; 49:82-9.. Two additional reviews found no association 2424. Zhou XH, Li YJ, Ou JJ, Li YM. Association between maternal antidepressant use during pregnancy and autism spectrum disorder: an updated meta-analysis. Mol Autism 2018; 9:21., or pointed out that a residual confounding by indication and inconsistent findings precluded a conclusion about risks of ASD 2525. Morales DR, Slattery J, Evans S, Kurz X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Med 2018; 16:6.. Along the same line, three reviews concluded that a residual confounding by indication cannot be ruled out as an explanation for observed associations of prenatal AD exposure with ADHD 2525. Morales DR, Slattery J, Evans S, Kurz X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Med 2018; 16:6.,2626. Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, et al. Prenatal antidepressant exposure and the risk of attention-deficit hyperactivity disorder in children: a systematic review and meta-analysis. Neurosci Biobehav Rev 2018; 86:1-11.,2727. Uguz F. Maternal antidepressant use during pregnancy and the risk of attention-deficit/hyperactivity disorder in children: a systematic review of the current literature. J Clin Psychopharmacol 2018; 38:254-9.. Only one review 2828. Prady SL, Hanlon I, Fraser LK, Mikocka-Walus A. A systematic review of maternal antidepressant use in pregnancy and short- and long-term offspring's outcomes. Arch Womens Ment Health 2018; 21:127-40. evaluated studies that compared behavioral and neurodevelopmental outcomes for children whose mothers took ADs during pregnancy with those whose mothers suffered from mental disorders but did not take medication. This study 2828. Prady SL, Hanlon I, Fraser LK, Mikocka-Walus A. A systematic review of maternal antidepressant use in pregnancy and short- and long-term offspring's outcomes. Arch Womens Ment Health 2018; 21:127-40. found very limited evidence indicating that gestational use of ADs might impair behavioral and neurodevelopmental outcomes in the offspring.
Overall, findings from this study and those from other systematic reviews addressing risks of poor pregnancy outcomes and developmental disorders do not support concerns on the risks of SSRIs/SNRIs for the unborn child.
References
-
1Richards D. Prevalence and clinical course of depression: a review. Clin Psychol Rev 2011; 31:1117-25.
-
2World Health Organization. Depression and other common mental disorders. Global health estimates. Geneva: World Health Organization; 2017.
-
3Biaggi A, Conroy S, Pawlby S, Pariante CM. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord 2016; 191:62-77.
-
4Vigod SN, Wilson CA, Howard LM. Depression in pregnancy. BMJ 2016; 352:i1547.
-
5Byatt N, Xiao RS, Dinh KH, Waring ME. Mental health care use in relation to depressive symptoms among pregnant women in the USA. Arch Womens Ment Health 2016; 19:187-91.
-
6Campagne DM. Antidepressant use in pregnancy: are we closer to consensus? Arch Womens Ment Health 2019; 22:189-97.
-
7Gao SY, Wu QJ, Sun C, Zhang TN, Shen ZQ, Liu CX, et al. Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: a systematic review and meta-analysis of cohort studies of more than 9 million births. BMC Med 2018; 16:205.
-
8Källén B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med 2004; 158:312-6.
-
9Gentile S. Tricyclic antidepressants in pregnancy and puerperium. Expert Opin Drug Saf 2014; 13:207-25.
-
10Mitchell J, Goodman J. Comparative effects of antidepressant medications and untreated major depression on pregnancy outcomes: a systematic review. Arch Womens Ment Health 2018; 21:505-16.
-
11Rotem-Kohavi N, Oberlander TF. Variations in neurodevelopmental outcomes in children with prenatal SSRI antidepressant exposure. Birth Defects Res 2017; 109:909-23.
-
12Rice D, Barone Jr. S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 2000; 108 Suppl 3:511-33.
-
13Lesch KP, Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 2012; 76:175-91.
-
14Rotem-Kohavi N, Williams LJ, Virji-Babul N, Bjornson BH, Brain U, Werker JF, et al. Alterations in resting-state networks following in utero selective serotonin reuptake inhibitor exposure in the neonatal brain. Biol Psychiatry Cogn Neurosci Neuroimaging 2019; 4:39-49.
-
15Sujan AC, Öberg AS, Quinn PD, D'Onofrio BM. Annual research review: maternal antidepressant use during pregnancy and offspring neurodevelopmental problems - a critical review and recommendations for future research. J Child Psychol Psychiatry 2019; 60:356-76.
-
16Rais TB, Rais A. Association between antidepressants use during pregnancy and autistic spectrum disorders: a meta-analysis. Innov Clin Neurosci 2014; 11:18-22.
-
17Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, Wong IC. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev 2015; 49:82-9.
-
18Kobayashi T, Matsuyama T, Takeuchi M, Ito S. Autism spectrum disorder and prenatal exposure to selective serotonin reuptake inhibitors: a systematic review and meta-analysis. Reprod Toxicol 2016; 65:170-8.
-
19Healy D, Le Noury J, Mangin D. Links between serotonin reuptake inhibition during pregnancy and neurodevelopmental delay/spectrum disorders: a systematic review of epidemiological and physiological evidence. Int J Risk Saf Med 2016; 28:125-41.
-
20Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: a systematic review and meta-analysis. Reprod Toxicol 2016; 66:31-43.
-
21Andalib S, Emamhadi MR, Yousefzadeh-Chabok S, Shakouri SK, Høilund-Carlsen PF, Vafaee MS, et al. Maternal SSRI exposure increases the risk of autistic offspring: a meta-analysis and systematic review. Eur Psychiatry 2017; 45:161-6.
-
22Mezzacappa A, Lasica PA, Gianfagna F, Cazas O, Hardy P, Falissard B, et al. Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: a systematic review and meta-analysis. JAMA Pediatr 2017; 171:555-63.
-
23Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Maternal SSRI discontinuation, use, psychiatric disorder and the risk of autism in children: a meta-analysis of cohort studies. Br J Clin Pharmacol 2017; 83:2798-806.
-
24Zhou XH, Li YJ, Ou JJ, Li YM. Association between maternal antidepressant use during pregnancy and autism spectrum disorder: an updated meta-analysis. Mol Autism 2018; 9:21.
-
25Morales DR, Slattery J, Evans S, Kurz X. Antidepressant use during pregnancy and risk of autism spectrum disorder and attention deficit hyperactivity disorder: systematic review of observational studies and methodological considerations. BMC Med 2018; 16:6.
-
26Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, et al. Prenatal antidepressant exposure and the risk of attention-deficit hyperactivity disorder in children: a systematic review and meta-analysis. Neurosci Biobehav Rev 2018; 86:1-11.
-
27Uguz F. Maternal antidepressant use during pregnancy and the risk of attention-deficit/hyperactivity disorder in children: a systematic review of the current literature. J Clin Psychopharmacol 2018; 38:254-9.
-
28Prady SL, Hanlon I, Fraser LK, Mikocka-Walus A. A systematic review of maternal antidepressant use in pregnancy and short- and long-term offspring's outcomes. Arch Womens Ment Health 2018; 21:127-40.
-
29Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health 2017; 38:81-102.
-
30Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet 2005; 366:237-48.
-
31Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry 2018; 5:175-86.
-
32Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015; 350:g7647.
-
33von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335:806-8.
-
34Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 11/Dec/2018).
» http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp -
35Malm H, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomäki S, McKeague IW, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry 2016; 55:359-66.
-
36Hviid A, Melbye M, Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N Engl J Med 2013; 369:2406-15.
-
37Castro VM, Kong SW, Clements CC, Brady R, Kaimal AJ, Doyle AE, et al. Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Transl Psychiatry 2016; 6:e708.
-
38Brown HK, Ray JG, Wilton AS, Lunsky Y, Gomes T, Vigod SN. Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children. JAMA 2017; 317:1544-52.
-
39Viktorin A, Uher R, Reichenberg A, Levine SZ, Sandin S. Autism risk following antidepressant medication during pregnancy. Psychol Med 2017; 47:2787-96.
-
40Sørensen MJ, Grønborg TK, Christensen J, Parner ET, Vestergaard M, Schendel D, et al. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol 2013; 5:449-59.
-
41Clements CC, Castro VM, Blumenthal SR, Rosenfield HR, Murphy SN, Fava M, et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry 2015; 20:727-34.
-
42Sujan AC, Rickert ME, Öberg AS, Quinn PD, Hernández-Díaz S, Almqvist C, et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA 2017; 317:1553-62.
-
43Hagberg KW, Robijn AL, Jick S. Maternal depression and antidepressant use during pregnancy and the risk of autism spectrum disorder in offspring. Clin Epidemiol 2018; 10:1599-612.
-
44Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104-12.
-
45Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, Newschaffer CJ. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J Autism Dev Disord 2014; 44:2558-67.
-
46Boukhris T, Sheehy O, Mottron L, Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr 2016; 170:117-24.
-
47Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics 2014; 133:e1241-8.
-
48Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ 2013; 346:f2059.
-
49Rai D, Lee BK, Dalman C, Newschaffer C, Lewis G, Magnusson C. Antidepressants during pregnancy and autism in offspring: population based cohort study. BMJ 2017; 358:j2811.
-
50El Marroun H, White TJ, van der Knaap NJ, Homberg JR, Fernández G, Schoemaker NK, et al. Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. Br J Psychiatry 2014; 205:95-102.
-
51Laugesen K, Olsen MS, Telén Andersen AB, Frøslev T, Sørensen HT. In utero exposure to antidepressant drugs and risk of attention deficit hyperactivity disorder: a nationwide Danish cohort study. BMJ Open 2013; 3:e003507.
-
52Figueroa R. Use of antidepressants during pregnancy and risk of attention-deficit/hyperactivity disorder in the offspring. J Dev Behav Pediatr 2010; 31:641-8.
-
53Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev 2006; 12:178-207.
-
54Zisook S, Rush AJ, Haight BR, Clines DC, Rockett CB. Use of bupropion in combination with serotonin reuptake inhibitors. Biol Psychiatry 2006; 59:203-10.
-
55Dunner DL. Combining antidepressants. Shanghai Arch Psychiatry 2014; 26:363-4.
-
56Patel K, Allen S, Haque MN, Angelescu I, Baumeister D, Tracy DK. Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Ther Adv Psychopharmacol 2016; 6:99-144.
-
57Boukhris T, Sheehy O, Bérard A. Antidepressant use in pregnancy and the risk of attention deficit with or without hyperactivity disorder in children. Paediatr Perinat Epidemiol 2017; 31:363-73.
-
58Man KKC, Chan EW, Ip P, Coghill D, Simonoff E, Chan PKL, et al. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ 2017; 357:j2350.
-
59Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull 2011; 37:504-13.
-
60Jones PB. Adult mental health disorders and their age at onset. Br J Psychiatry Suppl 2013; 54:s5-10.
-
61Nulman I, Rovet J, Stewart DE, Wolpin J, Gardner HA, Theis JG, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med 1997; 336:258-62.
-
62Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002; 159:1889-95.
-
63Nulman I, Koren G, Rovet J, Barrera M, Pulver A, Streiner D, et al. Neurodevelopment of children following prenatal exposure to venlafaxine, selective serotonin reuptake inhibitors, or untreated maternal depression. Am J Psychiatry 2012; 169:1165-74.
-
64Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 2003; 142:402-8.
-
65Austin MP, Karatas JC, Mishra P, Christl B, Kennedy D, Oei J. Infant neurodevelopment following in utero exposure to antidepressant medication. Acta Paediatr 2013; 102:1054-9.
-
66Johnson KC, Smith AK, Stowe ZN, Newport DJ, Brennan PA. Preschool outcomes following prenatal serotonin reuptake inhibitor exposure: differences in language and behavior, but not cognitive function. J Clin Psychiatry 2016; 77:e176-82.
-
67Hermansen TK, Røysamb E, Augusti EM, Melinder A. Behavior and inhibitory control in children with prenatal exposure to antidepressants and medically untreated depression. Psychopharmacology (Berl) 2016; 233:1523-35.
-
68El Marroun H, White TJ, Fernandez G, Jaddoe VW, Verhulst FC, Stricker BH, et al. Prenatal exposure to selective serotonin reuptake inhibitors and non-verbal cognitive functioning in childhood. J Psychopharmacol 2017; 31:346-55.
-
69Viktorin A, Uher R, Kolevzon A, Reichenberg A, Levine SZ, Sandin S. Association of antidepressant medication use during pregnancy with intellectual disability in offspring. JAMA Psychiatry 2017; 74:1031-8.
-
70Handal M, Skurtveit S, Roth C, Hernandez-Diaz S, Selmer R. Prenatal exposure to folic acid and antidepressants and language development: a population-based cohort study. J Clin Psychopharmacol 2016; 36:333-9.
-
71Reebye PN, Morison SJ, Panikkar H, Misri S, Grunau RE. Affect expression in prenatally psychotropic exposed and nonexposed mother-infant dyads. Infant Ment Health J 2002; 23:403-16.
-
72Casper RC, Gilles AA, Fleisher BE, Baran J, Enns G, Lazzeroni LC. Length of prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants: effects on neonatal adaptation and psychomotor development. Psychopharmacology (Berl) 2011; 217:211-9.
-
73Batton B, Batton E, Weigler K, Aylward G, Batton D. In utero antidepressant exposure and neurodevelopment in preterm infants. Am J Perinatol 2013; 30:297-301.
-
74Santucci AK, Singer LT, Wisniewski SR, Luther JF, Eng HF, Dills JL, et al. Impact of prenatal exposure to serotonin reuptake inhibitors or maternal major depressive disorder on infant developmental outcomes. J Clin Psychiatry 2014; 75:1088-95.
-
75Skurtveit S, Selmer R, Roth C, Hernandez-Diaz S, Handal M. Prenatal exposure to antidepressants and language competence at age three: results from a large population-based pregnancy cohort in Norway. BJOG 2014; 121:1621-31.
-
76Pedersen LH, Henriksen TB, Bech BH, Licht RW, Kjaer D, Olsen J. Prenatal antidepressant exposure and behavioral problems in early childhood: a cohort study. Acta Psychiatr Scand 2013; 127:126-35.
-
77Grzeskowiak LE, Morrison JL, Henriksen TB, Bech BH, Obel C, Olsen J, et al. Prenatal antidepressant exposure and child behavioural outcomes at 7 years of age: a study within the Danish National Birth Cohort. BJOG 2016; 123:1919-28.
-
78Hanley GE, Brain U, Oberlander TF. Prenatal exposure to serotonin reuptake inhibitor antidepressants and childhood behavior. Pediatr Res 2015; 78:174-80.
-
79Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 2007; 161:22-9.
-
80Misri S, Reebye P, Kendrick K, Carter D, Ryan D, Grunau RE, et al. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry 2006; 163:1026-32.
-
81Lupattelli A, Wood M, Ystrom E, Skurtveit S, Handal M, Nordeng H. Effect of time-dependent selective serotonin reuptake inhibitor antidepressants during pregnancy on behavioral, emotional, and social development in preschool-aged children. J Am Acad Child Adolesc Psychiatry 2018; 57:200-8.
-
82Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 2010; 67:1012-24.
-
83Staneva A, Bogossian F, Pritchard M, Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: a systematic review. Women Birth 2015; 28:179-93.
-
84Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring. A systematic review. Neuroscience 2017; 342:154-66.
-
85Flint J, Kendler KS. The genetics of major depression. Neuron 2014; 81:1214.
-
86Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018; 50:668-81.
-
87Kingston D, Tough S, Whitfield H. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry Hum Dev 2012; 43:683-714.
-
88Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci 2012; 9:41-6.
-
89Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry 2013; 26:446-52.
-
90Matsui D. Adherence with drug therapy in pregnancy. Obstet Gynecol Int 2012; 2012:796590.
-
91Gentile S, Galbally M. Prenatal exposure to antidepressant medications and neurodevelopmental outcomes: a systematic review. J Affect Disord 2011; 128:1-9.
-
92Suri R, Hellemann G, Stowe ZN, Cohen LS, Aquino A, Altshuler LL. A prospective, naturalistic, blinded study of early neurobehavioral outcomes for infants following prenatal antidepressant exposure. J Clin Psychiatry 2011; 72:1002-7.
-
93Anderson PJ, Burnett A. Assessing developmental delay in early childhood - concerns with the Bayley-III scales. Clin Neuropsychol 2017; 31:371-81.
-
94Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res 2014; 75:670-4.
-
95Harris SR, Langkamp DL. Predictive value of the Bayley mental scale in the early detection of cognitive delays in high-risk infants. J Perinatol 1994; 14:275-9.
-
96Heinonen E, Szymanska-von Schultz B, Kaldo V, Nasiell J, Andersson E, Bergmark M, et al. MAGDALENA: study protocol of a randomised, placebo-controlled trial on cognitive development at 2 years of age in children exposed to SSRI in utero. BMJ Open 2018; 8:e023281.
-
97Singal D, Chateau D, Dahl M, Katz L, Ruth C, Wall-Wieler E, et al. In utero SSRI and SNRI exposure and the risk of neurodevelopmental outcomes in children: a population-based retrospective cohort study utilizing linked administrative data. Pharmacoepidemiol Drug Saf 2018; 27 Suppl 2:240-1.
-
98Salisbury AL, Wisner KL, Pearlstein T, Battle CL, Stroud L, Lester BM. Newborn neurobehavioral patterns are differentially related to prenatal maternal major depressive disorder and serotonin reuptake inhibitor treatment. Depress Anxiety 2011; 28:1008-19.
-
99Weikum WM, Oberlander TF, Hensch TK, Werker JF. Prenatal exposure to antidepressants and depressed maternal mood alter trajectory of infant speech perception. Proc Natl Acad Sci U S A 2012; 109 Suppl 2:17221-7.
-
100Hanley GE, Brain U, Oberlander TF. Infant developmental outcomes following prenatal exposure to antidepressants, and maternal depressed mood and positive affect. Early Hum Dev 2013; 89:519-24.
-
101de Vries NK, van der Veere CN, Reijneveld SA, Bos AF. Early neurological outcome of young infants exposed to selective serotonin reuptake inhibitors during pregnancy: results from the observational SMOK study. PLoS One 2013; 8:e64654.
-
102Eriksen HL, Kesmodel US, Pedersen LH, Mortensen EL. No association between prenatal exposure to psychotropics and intelligence at age five. Acta Obstet Gynecol Scand 2015; 94:501-7.
-
103Brown AS, Gyllenberg D, Malm H, McKeague IW, Hinkka-Yli-Salomäki S, Artama M, et al. Association of selective serotonin reuptake inhibitor exposure during pregnancy with speech, scholastic, and motor disorders in offspring. JAMA Psychiatry 2016; 73:1163-70.
-
104Handal M, Skurtveit S, Furu K, Hernandez-Diaz S, Skovlund E, Nystad W, et al. Motor development in children prenatally exposed to selective serotonin reuptake inhibitors: a large population-based pregnancy cohort study. BJOG 2016; 123:1908-17.
-
105Salisbury AL, O'Grady KE, Battle CL, Wisner KL, Anderson GM, Stroud LR, et al. The roles of maternal depression, serotonin reuptake inhibitor treatment, and concomitant benzodiazepine use on infant neurobehavioral functioning over the first postnatal month. Am J Psychiatry 2016; 173:147-57.
-
106Hermansen TK, Yrttiaho S, Røysamb E, Melinder A. Perceptual interference processing in preschool children, with and without prenatal exposure to selective serotonin reuptake inhibitors. Psychopharmacology (Berl) 2017; 234:339-51.
Publication Dates
-
Publication in this collection
31 Jan 2020 -
Date of issue
2020
History
-
Received
18 Feb 2019 -
Reviewed
12 Nov 2019 -
Accepted
02 Dec 2019