Abstract
Starch derivatives of taro (Colocasia esculenta L. Schott) and rice were characterized as wall materials of orange oil (d-limonene) by spray drying. Native starches were initially hydrolyzed with HCl and then esterified. Succinylated starches were modified using a conventional method in a slurry and were extruded; whereas, the phosphorylated starches were modified using the extrusion process. Viscosity and solubility of starches reduced after acid hydrolysis, derivatization, and extrusion. The particle size of the wall materials ranged between 20.05 and 31.81 µm. The encapsulation efficiency of the phosphorylated taro, rice, and waxy corn starches was 96.9, 96.8 and 97.1% respectively, and 98.6, 98.1, and 98.8% for succynilated taro, rice, and waxy corn starches, respectively. Starch derivatives of taro and rice could potentially be used as wall materials of orange oil d-limonene.
taro; rice; starch derivatives; wall materials
ORIGINAL PAPERS
Characterization of new sources of derivative starches as wall materials of essential oil by spray drying
Iñigo Verdalet-GuzmánI; Laura Martínez-OrtizI; Fernando Martínez-BustosII,* * Corresponding author
IInstituto de Ciencias Básicas, Universidad Veracruzana - UV, Av. Dr. Rafael Sánchez Altamirano, s/n, Col. Industrial-Animas, C.P. 91190, Xalapa, VER, México
IICentro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional - CINVESTAV, Unidad Querétaro, Libramiento Norponiente #2000, Fracc. Real de Juriquilla, C.P. 76230, Santiago de Querétaro, QRO, México, e-mail: fmartinez@qro.cinvestav.mx
ABSTRACT
Starch derivatives of taro (Colocasia esculenta L. Schott) and rice were characterized as wall materials of orange oil (d-limonene) by spray drying. Native starches were initially hydrolyzed with HCl and then esterified. Succinylated starches were modified using a conventional method in a slurry and were extruded; whereas, the phosphorylated starches were modified using the extrusion process. Viscosity and solubility of starches reduced after acid hydrolysis, derivatization, and extrusion. The particle size of the wall materials ranged between 20.05 and 31.81 µm. The encapsulation efficiency of the phosphorylated taro, rice, and waxy corn starches was 96.9, 96.8 and 97.1% respectively, and 98.6, 98.1, and 98.8% for succynilated taro, rice, and waxy corn starches, respectively. Starch derivatives of taro and rice could potentially be used as wall materials of orange oil d-limonene.
Keywords: taro; rice; starch derivatives; wall materials.
1 Introduction
Food industry has focused on discovering and evaluating new wall materials for flavor encapsulation by spray drying considering their functionality as encapsulating agents, cost, authorized grade, and accessibility. Carbohydrates, mainly sugars such as glucose and sucrose, along with polysaccharides such as starch, maltodextrins, pectin, alginate, and chitosan are successfully used as wall materials for encapsulation of flavor ingredients (KENYON, 1995). However, carbohydrates cannot be used in wall systems without the presence of a surface-active constituent because they usually have no emulsifying properties (BANGS; REINECCIUS, 1988). Hydrolyzed starch products are hydrophilic compounds, which have little affinity for hydrophobic flavors (SHAIKH; BHOSALE; SINGHAL, 2006). Their hydrophilic nature can be modified by linking hydrophobic side chains (DRUSCH; SCHWARZ, 2006). Extrusion processes have unique characteristics compared to other thermal processes. Extrusion processes break covalent bonds in polymers, create intense structural disruption, mix and facilitate reactions in the absence of effluents that in other processes are limited or are made available to a lesser extent (ASP; BJÖRK, 1989). Extrusion may use reduced amounts of reagents; moreover, further hydrolysis of desirable starch for microencapsulation purposes can occur. Native rice starches consist of tiny granules (5 mm) with narrow size distribution (PUCHONGKAVARIN; VARAVINIT; BERGTHALLER, 2005). Taro starch granules have irregular and polygonal shapes with an average size of 3.0 µm (JANE et al., 1992); these granules exhibit the capability to form spherical aggregates. Small starch granules used as wall materials can be combined into porous spheres when dried by aspersion, and they have a wide range of useful applications. Thus, the aim of this study was to analyze the potential of taro and rice starch derivatives to be used as wall material of orange oil by spray drying.
2 Materials and methods
2.1 Raw materials
Fresh taro tubers were donated by producers from Papaloapan, Veracruz, México. Commercial "Valle Verde" rice flour was purchased from a local market. Waxy maize starch was acquired from National Starch, México. Sodium tripolyphosphate (Sigma-Aldrich, Toluca de Lerdo, México) and η-octenyl succinic anhydride (Sigma-Aldrich, Toluca de Lerdo, México) were used to prepare the modified starches. Orange oil (d-limonene) was donated by Food Specialities, México.
2.2 Extraction of starch and starch hydrolysis
Taro starch was extracted from fresh tubers (PEREIRA PACHECO; MOLINA MEDINA, 1992). Rice starch was extracted from rice flour with NaOH. Before esterification, both starches were hydrolyzed using 3.4 g of pure HCl/100 g of starch (d.b.); hydrolysis was carried out in a water bath at 50 ºC for 6 hours (ZAMBRANO; CAMARGO, 2001).
2.3 Dextrose Equivalent (DE)
DE was determined by the phenol-sulfuric acid method (DUBOIS et al., 1956).
2.4 Phosphorylated and succynilated starches preparation
Starch phosphorylation was achieved following the Chang and Lii (1992) method using a single screw extruder designed and manufactured by CINVESTAV-IPN. The extrusion process was done at 70, 125 and 180 ºC, feeding, transition, and high pressure zones respectively. Screw speed was 25 rpm with a 70 g/min feed rate, 3:1 compression ratio, and 2.9 mm diameter die nozzle. The extruded products were dried for 24 hours in a convection oven at 45 ºC and milled in a Braun coffee mill. They were then passed through a 100 mesh sieve (149 µm) and stored in polyethylene bags at 4 ºC for further analysis. Starch succinylation was performed with η-octenyl succinic anhydride, according to the Jeon, Viswanathan and Gross (1999) method. The dried powder was stored in polyethylene bags at 4 ºC. After esterification, η-OSA starches were further extruded in a single screw extruder, under the same conditions used for starch phosphorylation. Waxy maize starch, used as control, was also modified under the same conditions used for taro and rice starches.
2.5 Analysis of modified starches
Substitution degree of phosphorylate and succinylate starches
Degree of phosphorus substitution (DS) was calculated following the method of Chang and Lii (1992).
The substitution degree of succinate was obtained following the method of alkenyl-succinates (JEON; VISWANATHAN; GROSS, 1999). Degree of substitution was calculated according to Song et al. (2006).
2.6 Water absorption index (WAI) and Water solubility index (WSI) of starches
WAI and WSI were registered following the method of Anderson et al. (1969). Three repetitions were performed for each analysis, from which the mean value was reported.
2.7 Pasting properties of starches
The pasting properties of starches were measured in a 3C Rapid Visco Analyzer (RVA, Newport Scientific PTY LTD, Sydney Australia). The analysis was based on the AACC standard program for RVA analysis with some modifications (AMERICAN..., 1999).
2.8 Microencapsulation
Microcapsules were prepared by Spray Drying (SD-Basic Spray-Dryer, LabPlant, Huddersfield, UK). Emulsions of 30 g (d.b.) were prepared dispersing 20g of wall material in 100 mL of deionized water at room temperature.
Emulsions were homogenized at 14,000 rpm × g for 4 minutes (Ultra Turrax T-25-SI, IKA Works, USA). Drying conditions were: inlet air temperature at 180±1 ºC, outlet air temperature of 100±5 ºC, 0.5 mm nozzle diameter, liquid flow rate of 7.5 mL/min, and fixed equipment air flow (approximately 70 m3/h).
2.9 Emulsions particle size
The particle size analysis of the emulsions of modified starches with d-limonene was carried out by laser diffraction (model Beckman Coulter LS).
2.10 Apparent viscosity of the emulsions
Rheological measurements were performed with 8 ml of the emulsion (4:1 oil:starch) at 25 ºC controlled temperature using a digital rotational Brookfield Viscometer (Model RVT, Brookfield Engineering Laboratories, Stoughton, MA) (SOPADE; FILIBUS, 1995).
2.11 Microcapsules characterization
Morphology and particle size of the microcapsules were measured using scanning electronic microscopy (ESEM EDDAX, GSE detector), at an acceleration voltage of 20 kV. The encapsulated samples were fixed in stubs containing a double-faced adhesive metallic tape.
2.12 Yield and efficiency of encapsulation
These parameters were calculated from determinations of non-encapsulated surface d-limonene content and the total oil of the microcapsules. The non-encapsulated surface d-limonene from the powdered samples was extracted using the Soxhlet extraction method, following Beristain and Vernon-Carter (1994) method. The total oil in the microcapsules was determined using a Clevenger apparatus.
2.13 Water activity (a w )
Water activity (aw) was measured using an Aqua Lab equipment (ASSOCIATION..., 1990).
2.14 Moisture content of the microcapsules
The moisture content of the microcapsules was determined gravimetrically by oven-drying at 100 ºC over a 24 hours period.
2.15 Statistical analysis
All experiments were performed in triplicate and assessed by analysis of variance (STATSOFT, 2001) The data were calculated using the STATISTICA (Analysis of variance) and the Tukey's test (p<0.05), Software package version 6.0 (STATSOFT, 2001).
3 Results and discussion
3.1 Characterization of modified starches
Dextrose equivalent (DE). DE values after hydrolysis of starches with HCl (3.5%) for 6 hours were 4.5, 4.8, and 5.8 for taro, rice, and waxy maize, respectively.
Degree of substitution (DS)
The highest values of phosphorus content and DS of phosphorylated starches were found for rice, and the lowest one was found for taro starch (Table 1). These values corroborate the data found by Moorthy (2002), who reported phosphorus content between 0.006 and 0.013 in native taro starch. Murúa-Pagola, Beristain-Guevara and Martinez-Bustos (2009); Chang and Lii (1992) reported higher phosphorous values for waxy and normal corn starches than those here reported. For all the η-octenylsuccinylated (η-OSA) starches, the DS values were 0.07. Song et al. (2006) found a similar DS value for Early Indica rice starch using OSA concentrations higher than 15%. These values were lower than those found in the present research.
Jeon, Viswanathan and Gross (1999) found a DS of 0.11 in modified OSA waxy corn starch, having performed the esterification in similar conditions to those described in the present research, 60% starch slurry (w/w relative to water), and a concentration higher than 15% OSA (w/w relative to starch).
Bhosale and Singhal (2006) reported a linear increase in the DS of the OSA-modified amaranth and waxy corn starches with an increase in concentration of OSA. The amount of 3% OSA provided maximum DS to both modified OSA-amaranth and OSA-waxy corn starch under similar reaction conditions.
Pasting properties of starches
Table 2 shows the maximum viscosity during heating cycle, the water absorption index, and the water solubility index of native and hydrolyzed starches. Hydrolyzed starches considerably lowered its maximum viscosity compared to that of native starches. Taro starch was the most affected by hydrolysis, followed by waxy corn starch and finally rice starch. The maximum phosphorylated starches viscosity values were 18.94, 18.72, and 18.47 for taro, rice, and waxy corn, respectively, whereas, succynilated taro, rice and waxy corn starches showed values of 18.76, 15.35, and 11.37 respectively. This is attributable to the fact it occurs during the hydrolysis and extrusion process, in which the structure of starch is depolymerized by shearing, high pressure, and temperature (CHANG; LII, 1992). Un-depolymerized starches are rarely used for spray drying because of their high viscosity. Extruded starches usually show lower viscosity profile compared with native starches. Hydrolyzed starches had lower WAI values than those of its native starches. Acid hydrolysis resulted in low molecular weight products that showed reduced water absorption.
Waxy corn starch showed the highest difference in WSI as an effect of the acid. The WAI values of phosphorylated starches were 1.7, 2.3, and 1.5 for taro, rice, and waxy corn, respectively. Succynilated taro, rice, and waxy corn starches had values of 0.86, 1.3, and 1.0, respectively. The addition of hydrophobic groups to the starch molecules and cross linking formation decreased the WAI. Murúa-Pagola, Beristain-Guevara and Martinez-Bustos (2009) reported WAI values from 2.22 to 4.09 for phosphorylated, acetylated, and succynilated waxy maize starches. Changes in the WAI as a result of the studied variables could be due to the effects of acid. In addition, the extrusion of starch granules would open their structures which would then result in high water retention.
3.2 Characterization of emulsions
Apparent viscosity of the emulsions
The microstructure of spray dried microcapsules can be affected by the composition and properties of wall materials, its drying parameters, and the emulsion viscosity, which in consequence result in resistance to flow and prolonged storage conditions (BHOSALE; SINGHAL, 2006).
Viscosity of the modified taro starches was higher than those of the other two derivative starches. The highest viscosity was reported for succynilated taro starch, and the lowest values for succinylated rice starch. Nevertheless, fine emulsions from the powders were also obtained in all the modified starches (Table 3). This could be explained by the differences in the stability of emulsions during spray drying process. Bhandari et al. (1992) reported a direct relationship between the viscosity of liquid emulsions prior to spray drying and the moisture content of the powder. In addition, particle structure and porosity are additional parameters that influence their water-holding properties during drying and the evaporative rate, and inlet and outlet air temperature differentials (REINECCIUS, 2004).
Particle size of the emulsions
Table 3 shows the particle size of emulsions prepared with starch derivatives. Particle size of the modified starches was equal or lower than 9.0 µm, which allowed a successful step of the emulsions through the nozzle of the spray dryer. Succynilated taro and rice starches showed the highest particle size values of the evaluated emulsions, whereas, phosphorylated taro and rice starches, and succinylated waxy corn were the lowest. Sheu and Rosenberg (1995) suggested that retention of volatiles during microencapsulation by spray drying could be enhanced by reducing the mean emulsion size of the dispersed core material during emulsification. According to Jafari et al. (2008), the higher surface oil in the particles produced from emulsions with larger droplets can be attributed to droplets breakdown during atomization. Moreover, lower oil content resulted in higher emulsion viscosity, which reduces oil droplets diffusion inside atomized droplets and makes oil migration to the particle surface difficult. Soottitantawat et al. (2004) reported the influence of the mean emulsion droplet size on d-limonene retention during spray drying; ethyl butyrate and ethyl propionate were used as model flavors. Gum Arabic, soybean water-soluble polysaccharides, or modified starch blended with maltodextrin were used as wall materials, and these authors found that the increasing emulsion droplet decreased the retention of flavors. Risch and Reineccius (1998) have also shown in a similar research that a smaller emulsion size yields a higher retention of orange oil. Soottitantawat et al. (2005) reported the influence of emulsion size on the retention during spray drying of soluble and insoluble flavor.
The smaller emulsion size showed higher retention than the large emulsion size, especially for the insoluble flavor. The distribution curve containing larger emulsion droplets shifted toward a smaller size after atomization, indicating that the larger emulsion droplets would be changed in size during atomization and result in decreasing flavor retention. Paramita, Furuta and Yoshii (2012) used modified starch (wall material) by spray drying to produce oil-rich powders of oil mixtures of medium-chain triglycerides (MCT) and d-limonene in mixing ratios from 10 to 100 wt%. The viscosity of the infeed liquid and the particle size of the powder exponentially decreased with increasing oil load, while the emulsion droplet size in the infeed liquid increased. In addition, retention of d-limonene during spray drying also decreased markedly with increasing oil load, irrespective of the different oil loads. For diverse combinations of carbohydrate carrier solids, the increasing emulsion diameter resulted in a decreasing retention of d-limonene. This implies that a fine emulsion is stable during atomization and spray drying, and indicates that, for the proper wall materials, the emulsion droplet size is a significant factor for flavor retention (AGHBASHLO et al., 2012). Risch and Reineccius (1988) also reported in a similar research that a smaller emulsion size yields a higher retention of orange oil. As for the effect of the emulsion size on the shelf life of encapsulated orange oil flavor and encapsulated fatty acid, a longer shelf life of larger feed emulsion size was showed.
3.3 Characterization of the microcapsules
Particle size of the microcapsules
The size of microcapsules is dependent on physical factors such as the rate of shear, the phase viscosity, and the concentration of stabilizers, as well as, the design of the stirrer and vessel (HONG; PARK, 1999). The materials showed a similar bimodal particle distribution (data not shown). Table 3 shows that the particle size of the materials varied from 20.05 to 31.81 µm in accordance with the results reported in the literature for capsules produced by spray drying (THIES, 1995). Drusch and Schwarz (2006) reported the microencapsulation of a fish oil rich in long-chain polyunsaturated fatty acids with two different types of η-octenylsuccinate-derivative starch. The particle size expressed as 50th percentile of the particle distribution ranged from 22 to 29 µm. Particle size was significantly affected by the composition of the emulsion and drying conditions. Data indicate that microcapsules size decreased with a decrease in oil and starch content, whereas an increase in the drying air inlet and outlet temperature led to an increase in particle size (MINEMOTO et al., 2002). Emulsified linoleic acid encapsulated with gum Arabic was smaller in droplet size and more stable than those prepared with maltodextrins, and it was also more resistant to oxidation than that in a maltodextrin-based microcapsule. Higher inlet air temperature leads to the production of larger particles and causes increasing swelling (TONON; FREITAS; HUBINGER, 2011).
Water activity (a w ) of the microcapsules
Water activity (aw) is an important index for spray-dried powder because it can significantly affect the food safety stability and, simultaneously with temperature, can control the physico-chemical properties, along with shelf life of the fabricated microcapsules. There were no significant changes in the water activities of the spray-dried microcapsules for all the investigated derivate starches (Table 3). The highest values were found for modified taro and rice starches, and the lowest values were found for modified waxy corn starch. Pitalua et al. (2010) reported that in dry atmospheres, the water is strongly attracted to the polar sites on the surface of the microcapsules and is, therefore, not available for any type of reaction. These authors reported that low and intermediate values of degradation were found with beetroot juice using gum Arabic as wall material, with water activity below 0.521 (aw < 0.521). On other hand, when water activity values are high (aw > 0.748), the adsorption phenomenon produces conformational swelling and changes in the structure of the dry powder (SERRIS; BILIADERIS, 2001). Drusch and Schwarz (2006) found that the aw in microcapsules of fish oil with two different types of η-octenylsuccinate-derivative starch ranged from 0.097 to 0.273. This parameter indicates that spray-drying resulted in a stable product in terms of microbiological and physicochemical alterations during storage. An aw value of less than 0.300 is generally considered to ensure product stability. Moisture content depends on the wall material; it is known that when wall material reaches moisture content < 7%, the diffusion coefficient of water is reduced, and this decreases its movement through the dry matrix (BHANDARI et al., 1992). The moisture content of the microcapsules ranged from 2.25 to 2.37 g water/100g solids d.b. (Table 3). Carrillo-Navas et al. (2011) reported moisture content values from 4.82 to 5.51% in microcapsules of gum Arabic-mesquite and gum-maltodextrin. Beristain, Azuara and Vernon-Carter (2002) reported 4.91% moisture content in microcapsules of orange peel with mesquite gum solutions as wall materials using spray drying.
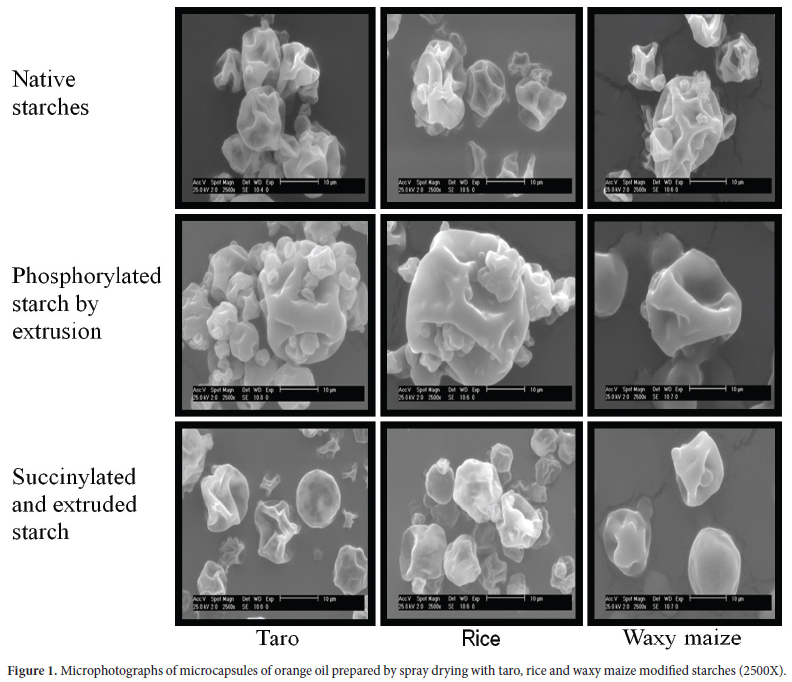
External morphology of the microcapsules
Microstructures of spray-dried microcapsules have been shown to be affected by wall composition and properties, core-to-wall ratio, drying parameters, and storage conditions (SANKARIKUTTY et al., 1988). Microcapsules of the modified starches showed similar external morphologies with a smooth surface without granulate in the form of irregular spheres, although free of pores and cracks (Figure 1). Encapsulated orange oil with several commercial food proteins and gum Arabic showed that the structural morphology of the microcapsules exhibited slightly rough surfaces with cavities or dented surfaces; probably due to rapid shrinkage of the liquid droplets during the early stages of the drying process. The inner structure of the open capsules was found without any deliberate fracturing. There were slight agglomerations among individual spray dried particles (KIM; MORR, 1996). This type of capsules has been reported by Soottitantawat et al. (2005) in encapsulated d-limonene with mixtures of commercial waxy corn and maltodextrins.
3.4 Encapsulation characteristics of orange oil during spray drying
Retention of orange peel oil during spray drying
In general, modified starches had good flavor retention (Table 4). Succynylated waxy maize starch showed the best results in terms of total oil retention (86.06%). The efficiency of inner oil retention ranged from 96.8% to 98.8% for all wall materials evaluated. These values are similar to those reported for microencapsulated lime essential oil with gum Arabic and maltodextrin (99%) (BRINGAS-LANTIGUA; VALDÉS; PINO, 2012), and mandarin microencapsulated essential oil (KIM; MORR, 1996), and are highly related to microencapsulated orange essential oil (BRINGAS-LANTIGUA et al., 2011), which range between 72.7%, 85.7% and 82-100% for microencapsulated limonene (SOOTTITANTAWAT et al., 2005; MURÚA-PAGOLA; BERISTAIN-GUEVARA; MARTINEZ-BUSTOS, 2009).
Boutboul et al. (2002) reported that the aroma retention was increased with the specific area of starch materials. For microencapsulation by spray-drying, low viscosity of the n-OSA starch is desirable, with and high total solids concentration of the feed emulsion and prevention of excessive air inclusion in the microcapsules during spray drying.
On other hand, the molecular weight must not be too low since the film-formation property of starches is a prerequisite for coating and entrapping active substances by forming microcapsules. Qi and Xu (1999) reported that starch hydrolysis products have a hydrophilic nature, thus having little affinity for hydrophobic flavor oils. All evaluated modified starches showed inner oil retention higher than 96%. Murúa-Pagola, Beristain-Guevara and Martinez-Bustos (2009) reported values of encapsulated d-limonene of 93.0, 91.9, and 95.6 for phosphorylated, succinylated, and N-Lok (control) waxy starches, respectively. Varavinit, Chaokasem and Shobsngob (2001) reported maximum values of total retained lemon oil of 62.53 and 55.00% for sago starch and taro stearate starch, respectively; 70.12 and 55.57% for pre-gelatinized hydrolyzed sago starch and taro stearate starch, respectively; and 59.15% for Arabic gum. These values were lower than those found in the present research.
In phosphorylated taro starch, total oil retention was of 53.16 and 81.00% for succinylated taro starch, respectively. Phosphorylated and succinylated rice starches had 50.63 and 65.81 % of total oil retention. Starch phosphates showed suitable properties as emulsion stabilizers. Starch phosphates could probably improve oil retention in blends with emulsifying starches. Surface oil contents of microencapsulated products ranged from 1.02 to 1.60 % of total solids. Surface oil was higher in phosphorylated taro and rice starches than in other modified starches. Succinylated waxy corn starch had the lowest values of surface oil. The high displayed stability may be attributed to good surface-active properties (ROMÁN-GUERRERO et al., 2009) of starch derivatives which possess hydrophobic groups that are accessible in short timescale, which enable them to adhere and spread out at the interface, thereby protecting newly formed droplets from aggregating or coalescing through steric stabilization and electrostatic stabilization mechanisms (DICKINSON, 2003). According to Risch and Reineccius (1988), this parameter is strongly associated with the emulsion droplet size, and low surface oil content is very important for providing storage stability to flavorings subject to oxidative deterioration. It is desirable that a high inlet air temperature is used to allow rapid formation of a semipermeable membrane on the droplet surface, but yet not so high as to cause heat damage to the dry product or excessive bubble growth and surface disruption, which increases losses during drying or causes particles to become sticky and cake (OZMEN; LANGRISH, 2003). Huynh et al. (2008) reported that encapsulation of lemon myrtle oil, in which the outlet air temperature of the spray dryer had little effect on oil retention, surface oil significantly increased with increasing outlet air temperature. However, Bringas-Lantigua et al. (2011) reported that an inlet air temperature of 220 ºC and an outlet air temperature of 85 ºC were optimal for the spray drying of lime essential oil. Drusch and Schwarz (2006) reported that moderate spray-drying conditions (air inlet temperature of 160 ºC and outlet air temperature of 60 ºC) were advantageous since ballooning of particles and lipid oxidation during spray drying were limited compared to drying at high spray-drying temperatures. In the present study, inlet air temperature of 180±1 ºC and outlet air temperature of 100±5 ºC resulted in high efficiency of d-limonene retention in all the wall materials evaluated. Flavor retention of d-limonene by spray drying with various kinds of matrices (gum Arabic, maltodextrin, and modified starch) systems was higher than 80% (SOOTTITANTAWAT et al., 2005). According to Soottitantawat et al. (2003), the highest retention of an encapsulated moderately soluble flavor can be obtained at the optimal value of the mean emulsion droplet size.
Lee et al. (2008) found that the distribution of emulsion size in the powder showed increased fraction of large emulsion droplets and changed to a bimodal distribution. However, the modified starch HI-CAP 100 showed higher stability of encapsulated d-limonene than the modified potato starch. Kanakdande, Bhosale and Singhal (2007) reported that in general gum Arabic offered greater protection than maltodextrin and modified starch although the order of protection offered was volatiles > cuminaldehyde > p-cymene > γ-terpinene.
4 Conclusions
The extrusion process improved the hydrolysis of starch producing wall materials with enhanced characteristics, such as solubility and viscosity, to be used in spray-drying. Derivative starches showed good viscosity, and water absorption index and water solubility index..
In addition, particle size and viscosity of the emulsions were suitable to produce microcapsules by spray drying. Oil retention and surface oil contents were satisfactory for all wall materials evaluated. The proposed materials are a low cost technological innovation with good encapsulating capabilities.
Received 7/17/2013
Accepted 10/13/2013 (006162)
- AGHBASHLO, M. et al. The correlation of wall material composition with flow characteristics and encapsulation behavior of fish oil emulsion. Food Research International, v. 49, n. 1, p. 379-388, 2012. http://dx.doi.org/10.1016/j.foodres.2012.07.031
- AMERICAN ASSOCIATION OF CEREAL CHEMISTS - AACC. Methods of the AACC 3rd ed. St. Paul: Academic Press Inc., 1999.
- ANDERSON, R. A. et al. Gelatinization of corn grits by roll and extrusion-cooking. Cereal Science Today, v. 14, n. 4, p. 4-12, 1969.
- ASP, N.G.; BJÖRK, I. Nutritional properties of extruded foods. In: MERCIER, C.; LINKO, C. P.; HARPER, J. M. (Ed.). Extrusion cooking St. Paul: American Association of Cereal Chemists, 1989. p. 399-434.
- ASSOCIATION OF OFFICIAL ANALYTICAL CHEMISTS - AOAC. Official methods of analysis 15th ed. Arlington: AOAC International, 1990.
- BANGS, W. E.; REINECCIUS, G. A. Corn starch derivatives: possible wall materials for spray-dried flavor manufacture. In: RISCH, S. J.; REINECCIUS, G. A. (Ed.). Flavour Encapsulation Washington: American Chemical Society, 1988. p. 12-28. (ACS Symposium Series, 370).
- BERISTAIN, C. I.; AZUARA, E.; VERNON-CARTER, E. J. Effect of water activity on the stability to oxidation of spray-dried encapsulated orange peel oil using mesquite gum (Prosopis juliflora) as wall material. Journal of Food Science, v. 67, n. 1, p. 206-211, 2002. http://dx.doi.org/10.1111/j.1365-2621.2002.tb11385.x
- BERISTAIN, C. I.; VERNON-CARTER, E. J. Utilization of mesquite (Prosopis julijlora) gum as emulsion stabilizing agent for spray-dried encapsulated orange peel oil. Drying Technology, v. 12, n. 7, p. 1727-1733, 1994. http://dx.doi.org/10.1080/07373939408962195
- BHANDARI, B. R. et al. Flavor encapsulation by spray drying: application to citral and linalyl acetate. Journal of Food Science, v. 57, n. 1, p. 217-221, 1992. http://dx.doi.org/10.1111/j.1365-2621.1992.tb05459.x
- BHOSALE, R.; SINGHAL, R. Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohydrate polymers, v. 66, n. 4, p. 521-527, 2006. http://dx.doi.org/10.1016/j.carbpol.2006.04.007
- BOUTBOUL, A. et al. Influence of the nature and treatment of starch on aroma retention. Carbohydrate Polymers, v. 47, n. 1, p. 73-82, 2002. http://dx.doi.org/10.1016/S0144-8617(01)00160-6
- BRINGAS-LANTIGUA, M. et al. Influence of spray-dryer air temperatures on encapsulated mandarin oil. Drying Technology, v. 29, n. 5, p. 520-526, 2011. http://dx.doi.org/10.1080/07373937.2010.513780
- BRINGAS-LANTIGUA, M.; VALDÉS, D.; PINO, J. A. Influence of spray-dryer air temperatures on encapsulated lime essential oil. International Journal of Food Science and Technology, v. 47, n. 7, p. 1511-1517, 2012. http://dx.doi.org/10.1111/j.1365-2621.2012.02999.x
- CARRILLO-NAVAS, H. et al. Storage stability and physicochemical properties of passion fruit juice microcapsules by spray-drying. Revista Mexicana de Ingeniería Química, v. 10, n. 3, p. 421-430, 2011.
- CHANG, Y.-H.; LII, C.-Y. Preparation of starch phosphate by extrusion. Journal of Food Science, v. 57, n. 1, p. 203-205, 1992. http://dx.doi.org/10.1111/j.1365-2621.1992.tb05456.x
- DICKINSON, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocolloids, v. 17, n. 1, p. 25-39, 2003. http://dx.doi.org/10.1016/S0268-005X(01)00120-5
- DRUSCH, S.; SCHWARZ, K. Microencapsulation properties of two different types of n-octenylsuccinate derivative starch. European Food Research Technology, v. 222, p. 155-164, 2006. http://dx.doi.org/10.1007/s00217-005-0020-3
- DUBOIS, M. et al. Colorimetric method for determination of sugars and related substances. Analytical Chemistry, v. 28, n. 3, p. 350-356, 1956. http://dx.doi.org/10.1021/ac60111a017
- HONG, K.; PARK, S. Preparation of polyurea microcapsules with different composition ratios: structures and thermal properties. Materials Science and Engineering, v. 272, n. 2, p. 418-421, 1999. http://dx.doi.org/10.1016/S0921-5093(99)00509-2
- HUYNH, K. P. et al. Essential oil from lemongrass extracted by supercritical carbon dioxide and steam distillation. The Phillippine Agricultural Scientist, v. 91, n. 1, p. 36-41, 2008.
- JAFARI, S. M. et al. Encapsulation efficiency of food flavors and oils during spray drying. Drying Technology, v. 26, n. 7, p. 816-835, 2008. http://dx.doi.org/10.1080/07373930802135972
- JANE, J. et al. Physical and chemical studies of taro starches and flours. Cereal Chemistry, v. 69, p. 528-535, 1992.
- JEON, Y. S.; VISWANATHAN, A.; GROSS, R. A. Studies of starch esterification: Reactions with alkenyl-succinates in aqueous slurry systems. Starch/Stärke, v. 51, n. 2-3, p. 90-93, 1999. http://dx.doi.org/10.1002/(SICI)1521-379X(199903)51:2<90::AIDSTAR90>3.0.CO;2-M
- KANAKDANDE, D.; BHOSALE, R.; SINGHAL, R. S. Stability of cumin oleoresin microencapsulated in different combination of gum arabic, maltodextrin and modified starch. Carbohydrate Polymers, v. 67, n. 4, p. 536-541, 2007.
- KENYON, M. M. Modified starch, maltodextrin, and corn syrup solids as wall materials for food encapsulation. In: RISCH, S. J.; REINECCIUS, G. A. (Ed.). Encapsulation and controlled release of food ingredients Washington: American Chemical Society, 1995. p. 42-50. (ACS Symposium Series).
- KIM, Y. D.; MORR, C. V. Microencapsulation properties of gum Arabic and several food proteins: spray-dried orange oil emulsion particles. Journal of Agricultural and Food Chemistry, v. 44, n. 5, p. 1314-1320, 1996. http://dx.doi.org/10.1021/jf9503927
- LEE, S. C. et al. Characterization of new biosurfactant produced by Klebsiella sp. Y6-1 isolated from waste soybean oil. Bioresource Technology, v. 99, n. 7, p. 2288-2292, 2008. PMid:17596933. http://dx.doi.org/10.1016/j.biortech.2007.05.020
- MINEMOTO, Y. et al. Oxidation of linoleic acid encapsulated with gum arabic or maltodextrin by spray-drying. Journal of Microencapsulation, v. 19, n. 2, p. 181-189, 2002. PMid:11837973. http://dx.doi.org/10.1080/02652040110065468
- MOORTHY, S. N. Physicochemical and functional properties of tropical tubers starches: a review. Starch/Stärke, v. 54, n. 12, p. 559-592, 2002. http://dx.doi.org/10.1002/1521-379X(200212)54:12<559::AIDSTAR2222559>3.0.CO;2-F
- MURÚA-PAGOLA, B.; BERISTAIN-GUEVARA, C. I.; MARTINEZ-BUSTOS, F. Preparation of starch derivatives using reactive extrusion and evaluation of modified starches as shell materials for encapsulation of flavoring agents by spray drying. Journal of Food Engineering, v. 91, n. 3, p. 380-386, 2009. http://dx.doi.org/10.1016/j.jfoodeng.2008.09.035
- OZMEN, L.; LANGRISH, T. A. G. An experimental investigation of the wall deposition of milk powder in a pilot-scale spray dryer. Drying Technology, v. 21, n. 7, p. 1253-1272, 2003. http://dx.doi.org/10.1081/DRT-120023179
- PARAMITA, V.; FURUTA, T.; YOSHII, H. High-oil-load encapsulation of medium-chain triglycerides and d-limonene mixture in modified starch by spray drying. Journal of Food Science, v. 77, n. 2, p. 38-44, 2012. PMid:22251159. http://dx.doi.org/10.1111/j.1750-3841.2011.02534.x
- PEREIRA PACHECO, F. E.; MOLINA MEDINA, M. R. Starch extraction from Xanthosoma sagittifolium Tropical Science, v. 32, n. 2, p. 203-206, 1992.
- PITALUA, E. et al. Antioxidative activity of microcapsules with beetroot juice using gum Arabic as wall material. Food and Bioproducts Processing, v. 88, n. 2-3, p. 253-258, 2010. http://dx.doi.org/10.1016/j.fbp.2010.01.002
- PUCHONGKAVARIN, H.; VARAVINIT, S.; BERGTHALLER, W. Comparative study of pilot scale rice starch production by an alkaline and an enzymatic process. Starch/Stärke, v. 57, n. 3-4, p. 134-144, 2005. http://dx.doi.org/10.1002/star.200400279
- QI, Z. H.; XU, A. Starch based ingredients for flow encapsulation. Cereal Food World, v. 44, p. 460-465, 1999.
- REINECCIUS, G. A. The spray drying of food flavors. Drying Technology, v. 22, n. 6, p. 1289-1324, 2004. http://dx.doi.org/10.1081/DRT-120038731
- RISCH, S. J.; REINECCIUS, G. A. Spray-dried orange oil: effect of emulsion size on flavor retention and shelf life. In: RISCH, S. J.; REINECCIUS, G. A. (Ed.). Flavor encapsulation Washington: American Chemical Society, 1988. p. 67-77. http://dx.doi.org/10.1021/bk-1988-0370.ch008
- ROMÁN-GUERRERO, A. et al. Application and evaluation of mesquite gum and its fractions as interfacial film formers and emulsifiers of orange peel-oil. Food Hydrocolloids, v. 23, n. 3, p. 708-713, 2009. http://dx.doi.org/10.1016/j.foodhyd.2008.06.005
- SANKARIKUTTY, B. et al. Studies on microencapsulation of cardamom oil by spray drying technique. Journal of Food Science Technology, v. 25, n. 6, p. 352-356, 1988.
- SERRIS, G. S.; BILIADERIS, C. G. Degradation kinetics of beetroot pigment encapsulated in polymeric matrices. Journal of the Science of Food and Agriculture, v. 81, n. 8, p. 691-700, 2001. http://dx.doi.org/10.1002/jsfa.864
- SHAIKH, J.; BHOSALE, R.; SINGHAL, R. Microencapsulation of black pepper oleoresin. Food Chemistry, v. 94, n. 1, p. 105-110, 2006. http://dx.doi.org/10.1016/j.foodchem.2004.10.056
- SHEU, T. Y.; ROSENBERG, M. Microencapsulation by spray drying ethyl caprylate in whey protein and carbohydrate wall systems. Journal of Food Science, v. 60, n. 1, p. 98-103, 1995. http://dx.doi.org/10.1111/j.1365-2621.1995.tb05615.x
- SONG, X. et al. Preparation and properties of octenyl succinic anhydride modified early indica rice starch. Starch/Stärke, v. 58, n. 2, p. 109-117, 2006. http://dx.doi.org/10.1002/star.200500444
- SOOTTITANTAWAT, A. et al. Microencapsulation by spray drying: influence of emulsion size on the retention of volatile compounds. Journal of Food Science, v. 68, n. 7, p. 2256-2262, 2003. http://dx.doi.org/10.1111/j.1365-2621.2003.tb05756.x
- SOOTTITANTAWAT, A. et al. Effect of water activity on the release characteristics and oxidative stability of d-limonene encapsulated by spray drying. Journal of Agriculture and Food Chemistry, v. 52, n. 5, p. 1269-1276, 2004. PMid:14995132. http://dx.doi.org/10.1021/jf035226a
- SOOTTITANTAWAT, A. et al. Influence of emulsion and powder size on the stability of encapsulated d-limonene by spray drying. Innovative Food Science and Emerging Technologies, v. 6, n. 1, p. 107-114, 2005. http://dx.doi.org/10.1016/j.ifset.2004.09.003
- SOPADE, P. A.; FILIBUS, T. E. The influence of solid and sugar contents on rheological characteristics of Akamu, a semi-liquid maize food. Journal of Food Engineering, v. 24, n. 2, p. 197-211, 1995. http://dx.doi.org/10.1016/0260-8774(94)P2643-J
- STATSOFT. Statistica Version 6.0. Tulsa: StatSoft Inc., 2001.
- THIES, C. Preparation of microcapsules by using centrifugal force, needles, nozzles and sprays. In: THIES, C. (Ed.). How to make microcapsules: lecture and laboratory manual. St. Louis: Thies Technology, 1995. chap. 3.
- TONON, R. V.; FREITAS, S. S.; HUBINGER, M. D. Spray drying of açai juice: effect of inlet temperature and type of carrier agent. Journal of Food Processing and Preservation, v. 35, n. 5, p. 691-700, 2011. http://dx.doi.org/10.1111/j.1745-4549.2011.00518.x
- VARAVINIT, S.; CHAOKASEM, N.; SHOBSNGOB, S. Studies of flavor encapsulation by agents produced from modified sago and tapioca starches. Starch/Stärke, v. 53, n. 6, p. 281-287, 2001. http://dx.doi.org/10.1002/1521-379X(200106)53:6<281::AIDSTAR281>3.0.CO;2-R
- ZAMBRANO, F.; CAMARGO, C. R. O. Optimisation of the conditions for the acid hydrolysis of cassava starch to obtain a fat replacer. Brazilian Journal of Food Technology, v. 4, p. 147-154, 2001.
Publication Dates
-
Publication in this collection
12 Feb 2014 -
Date of issue
Dec 2013
History
-
Received
17 July 2013 -
Accepted
13 Oct 2013