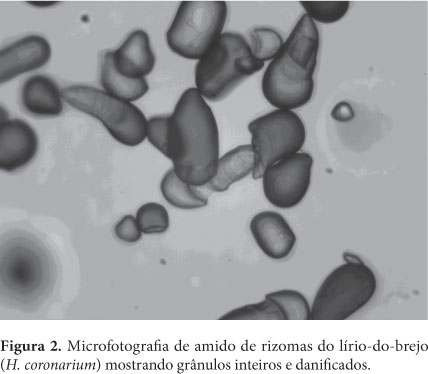
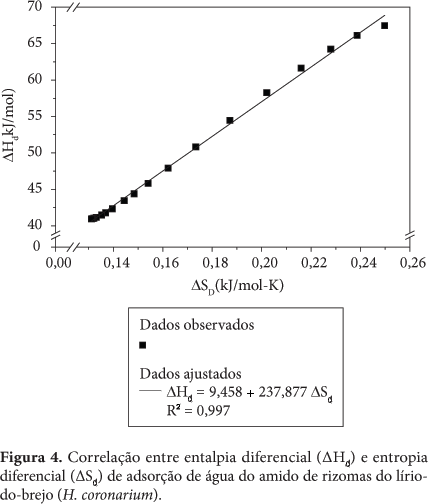
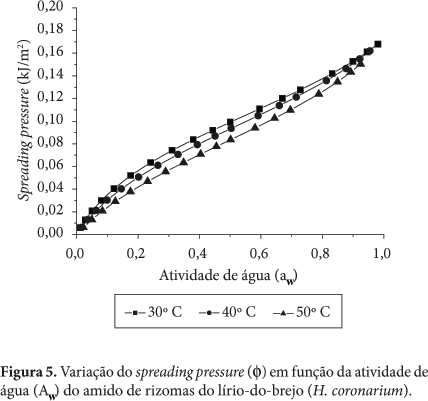
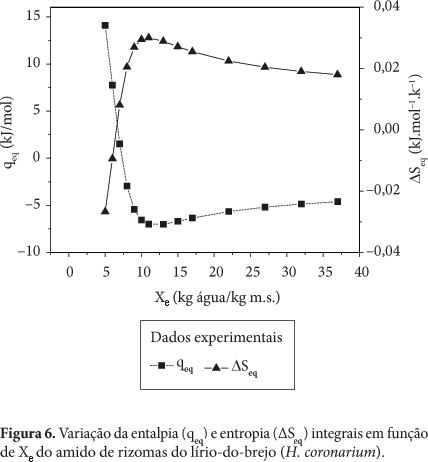
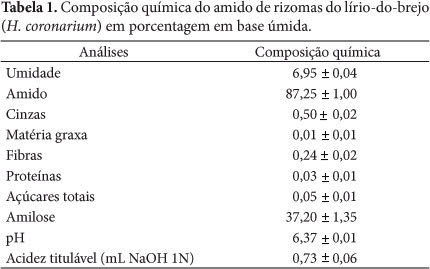
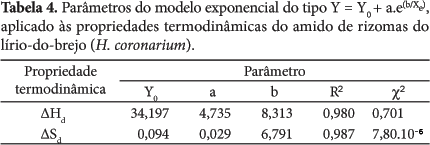
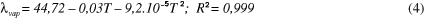Thermodynamic properties where determined (differential enthalpy, of differential entropy, integral enthalpy, and integral entropy) of the starch of rhizomes of swamp lily through water adsorption isotherms. The isotherms were determined in water activities in the range of 0.11 to 0.84, in temperatures ranging from 30 to 50 °C. The GAB equation which fits well to the experimental isotherms was used to estimate the properties of thermodynamic adsorption. The slight reversal of the isotherms indicates damaged starch. The enthalpy and entropy differential gap increased with the decrease in moisture and correlated to each other confirming the chemical linear compensation. An exponential model type Y = b.e(a/Xe) described adequately the dependence of these properties differential on the moisture content of balance. The integral enthalpy and entropy increased continuously with the moisture content of balance but presented negative values for the integral entropy. These thermodynamic properties of water adsorption showed that the starch extracted from the rhizomes of swamp lily has low hygroscopicity despite the occurrence of damaged starch granules.
Hedychium coronarium; water adsorption isotherm; isosteric heat; water activity; starch