Abstracts
OBJECTIVES: To compare subjective and objective pain intensity and associated characteristics in myofascial pain (MFP) patients with and without migraine. METHODS: The sample was comprised by 203 consecutive patients, mean age of 40.3 (89.2% of females), primarily diagnosed with MFP, who presented to the UCLA Orofacial Pain Clinic. Patients with secondary diagnosis of migraine (n=83) were included and comprised group 2. In order to compare group 1 (MFP) with group 2 (MFP + migraine) regarding objective pain (palpation scores) and subjective by means of visual analog scales (VAS) pain levels. Also, comparisons of mood problems, jaw function problems, sleep quality and disability levels using VAS were performed using the Mann-Whitney test. A significance level of 5% was adopted. RESULTS: Mann-Whitney test revealed that group 2 presented significantly higher pain levels on palpation of masticatory and cervical muscles in comparison to group 1 (p<0.05). Group 2 also presented higher levels of subjective pain, with statistical significance for "pain at the moment" and "highest pain" (p<0.05). Additionally, group 2 showed higher levels of mood problems, disability, jaw function impairment and sleep problems than group 1 with statistical significance for the later (p<0.05). CONCLUSIONS: Migraine comorbidity demonstrated a significant impact on pain intensity and life quality of patients with MFP. Clinicians should approach both conditions in order to achieve better treatment outcomes.
Temporomandibular disorders; Orofacial pain; Migraine
OBJETIVO: comparar a severidade da dor subjetiva e objetiva, além de outras características associadas entre pacientes com dor miofascial com e sem o diagnóstico adicional de enxaqueca. MÉTODOS: foram selecionados 203 pacientes, com idade média de 40,3 anos (89,2% do sexo feminino), que se apresentaram à Clínica de Dor Orofacial da Universidade da Califórnia, Los Angeles, EUA - todos com diagnóstico primário de dor miofascial. Pacientes com diagnóstico secundário de enxaqueca foram incluídos (n=83) e formaram o grupo 2. O teste de Mann-Whitney foi utilizado para comparar o grupo 1 (dor miofascial) com o 2 (dor miofascial + enxaqueca) quanto à intensidade de dor à palpação e subjetiva, através de Escalas Analógicas Visuais (EAV). Também com o auxílio de EAV, foram comparados estado de humor, problemas com a função, qualidade do sono e incapacidade. Em todos os testes foi adotado um nível de significância de 5%. RESULTADOS: o grupo 2 apresentou níveis de dor à palpação muscular estatisticamente maiores que o grupo 1 (p<0,05). Ao se analisar a intensidade de dor subjetiva obtida através da EAV, o grupo 2 apresentou níveis maiores de dor subjetiva (EAV) em todas as medições, com significância estatística para "dor no momento" e "dor máxima" (p<0,05). Da mesma maneira, o grupo 2 mostrou níveis maiores, obtidos através da EAV, de problemas com humor, incapacidade, problemas com a função mandibular e problemas com sono/descanso, sendo que apenas o último apresentou significância estatística (p<0,05). CONCLUSÕES: a comorbidade enxaqueca exerce forte impacto na severidade da dor e na qualidade de vida de pacientes que apresentam diagnóstico primário de dor miofascial.
Disfunção temporomandibular; Dor orofacial; Enxaqueca
ORIGINAL ARTICLE
Muscle pain intensity of patients with myofascial pain with different additional diagnoses
Rafael dos Santos SilvaI; Paulo Cesar Rodrigues ContiII; Somsak MitrirattanakulIII; Robert MerrillIV
IAdjunct Professor, Dentistry Department, Maringá State University
IIAssociate Professor, Prosthodontics Department, Bauru School of Dentistry, University of São Paulo
IIIAssociate Professor, Occlusion Department, Mahidol School of Dentistry, Bangkok, Thailand
IVAdjunct Professor, Orofacial Pain Department, UCLA School of Dentistry, Los Angeles, USA
Contact address
ABSTRACT
OBJECTIVES: To compare subjective and objective pain intensity and associated characteristics in myofascial pain (MFP) patients with and without migraine.
METHODS: The sample was comprised by 203 consecutive patients, mean age of 40.3 years (89.2% of females), primarily diagnosed with MFP, who presented to the UCLA Orofacial Pain Clinic. Patients with secondary diagnosis of migraine (n=83) were included and comprised group 2. In order to compare group 1 (MFP) with group 2 (MFP + migraine) regarding objective (palpation scores) and subjective pain levels by means of visual analog scales (VAS). Also, comparisons of mood problems, jaw function problems, sleep quality and disability levels using VAS were performed using the Mann-Whitney test. A significance level of 5% was adopted.
RESULTS: Mann-Whitney test revealed that group 2 presented significantly higher pain levels on palpation of masticatory and cervical muscles in comparison to group 1 (p<0.05). Group 2 also presented higher levels of subjective pain, with statistical significance for "pain at the moment" and "highest pain" (p<0.05). Additionally, group 2 showed higher levels of mood problems, disability, jaw function impairment and sleep problems than group 1 with statistical significance for the later (p<0.05).
CONCLUSIONS: Migraine comorbidity demonstrated a significant impact on pain intensity and life quality of patients with MFP. Clinicians should approach both conditions in order to achieve better treatment outcomes.
Keywords: Temporomandibular disorders. Orofacial pain. Migraine.
INTRODUCTION
Headaches, facial pain and Temporomandibular Disorders (TMD) comprise a serious contemporary problem, especially when presented simultaneously. Common complaint among TMD patients, headaches, including migraines, present prevalence between 48 and 77%.7,9,13,15,19
However, the actual impact of various types of headache in TMD patients is still subject to much debate and controversy and necessarily involves an understanding of the pathophysiology of each condition. The current literature reports improvement of headache after treatment of the signs and symptoms of TMD, a fact that strengthens the possible interplay between the two entities.10,16,19
Although the triggering mechanism of migraine is still not completely understood, it is well known that its pathophysiology does not primarily involve the masticatory muscle activity. On the other hand, considering that the temporomandibular joint (TMJ) and masticatory muscles receive trigeminal sensory innervation which, in turn, is also responsible for conducting nociceptive input from cranial blood vessels involved in the genesis of migraine, it is obvious the understanding of a possible overlap of nociceptive impulses in cases of comorbidity. Thus, the presence of TMD symptoms seems to cause an excitatory impact on migraine, and vice versa, especially in patients with severe and/or persistent pain, naturally more susceptible to the phenomenon of central sensitization.18,19
Based on the above, the present study aims to compare patients with myofascial pain (MFP) of the masticatory muscles with and without the additional diagnosis of migraine regarding pain intensity and associated characteristics.
METHODS
Initially, 424 consecutive patients who presented with complaint of pain in the facial region to the Orofacial Pain Clinic of the UCLA School of Dentistry, Los Angeles, were examined. Of these, 203 were included in the final sample, 181 women (89.2%) and 22 men (10.8%), with a mean age of 40.3 years (± 15.44).
In order to be included in the final sample, the patient should receive a primary diagnosis of myofascial pain, presenting one or more trigger points in the masticatory and/or cervical muscles. Considering the difficulty in obtaining single diagnoses of myofascial pain due to the multifactorial nature of TMD, patients with secondary diagnoses of osteoarthritis, capsulitis, tension-type headache (TTH) and migraine were also included in the final sample.
Diagnostic criteria of myofascial pain, capsulitis and osteoarthritis were based on the recommendations of the American Academy of Orofacial Pain,21 while the TTH and migraine were diagnosed according to the criteria established by the International Headache Society.29
Patients with the diagnosis of neuropathic pain were excluded. The presence of other diagnostic of primary headaches (cluster or chronic paroxysmal headaches), as well as any secondary headaches, were also exclusion criteria for patients in this study. Finally, patients with systemic conditions such as rheumatoid arthritis or fibromyalgia, among others, as well as those with mental or neurological issues were also excluded.
The 203 patients of the sample were divided into two groups. The first group comprised patients with the exclusive diagnosis of myofascial pain, while the second one included patients with myofascial pain and the additional diagnosis of migraine. The evaluation was performed by four examiners, all residents from the Orofacial Pain Program of the UCLA School of Dentistry, previously submitted to calibration and training by an experienced professional.
The objective muscle pain intensity was recorded from conventional digital muscle palpation. The muscles included in the survey were: temporalis (anterior, middle and posterior), masseter (superficial and deep), sternocleidomastoid (SCM), trapezius and splenius capitis.
Five pairs of facial muscles and three pairs of cervical muscles were included, totalizing 16 palpation sites. The exam was performed according to the recommendations of the American Academy of Orofacial Pain,21 with palpation pressure according to the findings of Silva et al.25
The palpation score was then recorded in each point, as follows:
» 0 = no pain;
» 1 = mild pain or discomfort;
» 2 = moderate pain;
» 3 = severe pain, with eyelid reflex or other severe pain signal;
» 4 = severe pain with referral.
The final score which represented the objective pain intensity (palpation) experienced by each patient was obtained using the arithmetic mean of all sites of muscle palpation.
The intensity of subjective pain, mood state, disability level because of the pain, jaw function problems and quality of sleep/rest were obtained by Visual Analog Scales (VAS).4,23,28 This type of scale has proven to be effective in comparison to others, as noted Conti et al4 in 2001. As for the subjective pain, four parameters of pain were recorded, through four different VAS. They measured pain levels "at the moment" and "highest", "lowest" and "average" pain experienced in the last month.
In order to detect differences between groups 1 (MFP) and 2 (MFP + migraine) regarding subjective and objective pain intensity, mood, disability, jaw function problems and sleep quality, Mann-Whitney test was performed with a significance level of 5%.
The present study was submitted for assessment and approved by the Ethics Committee of the University of California, Los Angeles, USA.
RESULTS
Mann-Whitney test showed that group 2 had higher pain levels upon muscle palpation than group 1 (p<0.05), as illustrated in Table 1.
VAS analysis has shown that group 2 presented higher pain levels for all variables, as shown in Figure 1. Statistically significant differences between groups were found with regard to "highest pain" and "pain at the moment" (p<0.05) (Table 2).
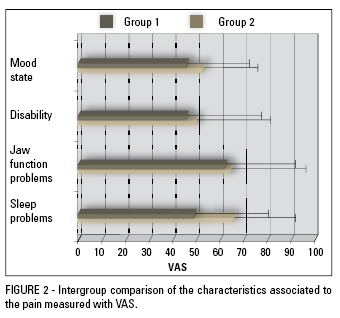
Comparisons between groups regarding characteristics associated to the pain such as mood, disability, problems with jaw function and problems with sleep/rest were also performed. For all variables, group 2 showed higher values (Fig 2), however only the variable "problems with sleep/rest" presented statistically significant difference (p<0.05) (Table 3).
DISCUSSION
The possible overlap between headaches, especially migraine and TTH, and TMD has been the subject of numerous studies in the literature. On one side, different pathophysiology theories contradict this hypothesis. On the other, studies of prevalence in populations with different types of headache, migraine, and asymptomatic TMD have detected and confirmed the overlap of symptoms of both conditions.9,10,14,30 When studying a population of patients with chronic headache (migraine and TTH), Glaros et al9 found more muscle pain and capsulitis compared to asymptomatic control subjects, confirming the possible association between the two entities. In another study, Mitrirattanakul and Merrill19 found significantly higher prevalence of headache in the TMD group (72.7%) compared to the asymptomatic control group (31.9%).
Despite displaying different mechanisms, headache symptoms are often found in TMD patients, with prevalence ranging from 48 to 77%.5,9,10,19,30 The pain reported by TMD patients is usually located in the masticatory muscles, preauricular area and/or temporomandibular joint (TMJ). According to Glaros et al,9 headache symptoms described by patients with TMD are similar to those reported by patients diagnosed with migraine or TTH.
In general, females are three times more prevalent than males when it comes to the pain subject.13,19,22,26,27 Vazquez-Delgado et al,30 in 2004, in a study with a population with chronic daily headache and another with MFP, found a prevalence of 81% of women, value of around the 81.3% found by Mitrirattanakule and Merrill19 and the 81.2% reported by Kim and Kim,13 all similar to the number found in this study. Of the 203 patients who comprised the sample, 89.2% were female.
But how can conditions with different pathophysiology in relation to TMD, such as migraine and TTH, cause significant impact on myofascial pain, measured by palpation of the masticatory and cervical muscles?
Theories that explain the phenomenon of headaches, especially migraines, are based on cortical spreading depression phenomena, descending modulatory system dysfunction (serotonergic and noradrenergic), and sensitization of second order neurons.13 The phenomena of central sensitization leads to peripheral allodynia and secondary hyperalgesia, felt on the scalp of patients with migraine, or as masticatory and cervical muscle tenderness, both during and after crisis.11,19 Some studies have shown increases in muscle pain on palpation during the attack of migraine. Indeed, the nociception caused by muscle pain, commonly found in patients with MFP, as in the patients included in the present study, can induce or increase the phenomena of central sensitization in patients with headache, resulting in the induction or exacerbation of headache in a region previously sensitized (subnucleus caudalis in the trigeminal nucleous).19
This overlap of stimuli would occur because the structures involved in TMD share the sensory innervation of the trigeminal nerve with the cranial blood vessels, important in the genesis of migraine. Possible noxious stimuli would leave by different routes towards the subnucleous caudalis, where the synapse with a second order neuron takes place. At this point, first order neurons occasionally share the same second order neuron in a phenomena known as the convergence theory.17,26
This fact may explain the findings described in this study, in which the group 2 presented mean pain on palpation significantly higher compared to group 1 (p<0.05). The comparison of subjective pain (VAS) also showed group 2 with higher levels of pain. However, this difference was significant only for "pain at the moment" and "highest pain" (p<0.05). The same results were found by Mitrirattanakul and Merrill,19 in 2006, who showed levels of pain and disability significantly higher in patients with musculoskeletal disorders associated with primary headaches, compared to individuals with exclusive musculoskeletal disorders. Some studies report the presence of muscle tenderness on palpation during the migraine attack and sometimes persisting after the attack,14,20,28 depending on the severity and/or frequency of the migraine episode. That fact may contribute to the interpretation of the findings of this study.
If the muscle tenderness is just a result of central sensitization caused by the migraine, it should be resolved with specific abortive migraine medications. There are reports of the veracity of this fact in the literature. Dao et al,6 in 1995, analyzing a sample of migraine patients, showed improvement in the symptoms of muscle pain after the administration of dihydroergotamine-45 or a triptan. However, in populations with orofacial pain, especially with MFP, there are reports of the contrary.7 In such cases, the frequency and intensity of the headaches tend to improve after reduction of nociceptive impulses from the masticatory muscles and the TMJ through physical therapy approaches9,19 or after myofascial trigger points injections.7
Considering that muscle pain caused by the MFP does not improve after administration of specific abortive migraine medications (triptans, for example)19, one can infer that not only the nociceptive activity of cranial blood vessel can induce neurogenic inflammation, an event that culminates with the migraine attack. Other noxious stimuli coming from other branches of the trigeminal nerve, i.e. the masticatory muscles or the TMJ, can exacerbate or even initiate the headache. According to Davidoff,7 muscle pain from trigger points can be severe enough to trigger a migraine attack. Furthermore, the author found that palpation of the trigger point during the migraine attack increases the headache significantly.
Despite disability is one of the most important features of migraine, being part of the diagnostic criteria, significant differences between groups, in this study, were not found. This fact may be explained by the simplicity of the VAS in measuring a variable that involves various aspects of life. A more comprehensive analysis, using more specific and sophisticaded tools to measure disability should be performed to clearly elucidate this aspect.
Although reported as common in patients with pain24,30, mood state, in the present study, revealed no significant differences between the groups. The same result was presented by Glaros et al9 who found no differences between TMD and headache patients compared to a control group regarding that feature. However, a study published in 2003 by Ruiz de Velasco et al24 reported significant different mood states in patients with migraine, such as sadness, anger, emotional instability and difficulty concentrating. Thus, it appears that mood state is not consistently different among painful conditions in relation to asymptomatic individuals. Likewise, the mood does not seem to be directly affected by the intensity of pain since the group 2 showed significantly higher pain levels than group 1. The mood state results of this study should, however, be considered with caution because it was measured using a VAS and not through a specific test.
Problems with jaw function also lacked significant differences between groups (p> 0.05), despite the mean values revealed to be relatively high in the VAS (62.14 in group 1 and 64.62 in group 2). Apparently, problems with jaw function are symptoms exclusively related to TMD21,23 and therefore primary headaches, especially migraine, have no influence on the severity of these problems.
Patients with pain, especially chronic, commonly report sleep disturbances.30 Liljestrom et al,15 however, found no association between sleep quality and TMD in a sample of children. In the present study, significant differences were detected between the groups in relation to problems with sleep, measured by VAS, with group 2 reporting significantly more problems (66.08) than group 1 (49.21). Once again, migraine seems to exert significant impact on the general scenario of patients with MFP, in this case interfering in the sleep quality. Vazquez-Delgado et al30 found poorer sleep quality in patients with MFP than in patients with capsulitis, chronic daily headache, chronic migraine and chronic TTH. According to Dodick et al,8 the patient who complains of sleep problems associated with primary headaches usually has consistent symptoms of depression and anxiety, and a history of analgesics abuse and fibromyalgia, conditions that individually cause problems with sleep.
Based on the findings of this study, the importance of addressing each of the pathologies involved in patients with orofacial pain is reinforced, especially disorders of musculoskeletal origin and primary headaches. Therefore, it is likely that the unawareness of one or more of these disorders lead to equivocal results in terms of resolution or improvement of the pain.
Still, no definitive conclusions can be obtained from the findings of this study due to the diversity of variables involved, a fact that reinforces the need for additional investigation to confirm the trend shown in the present study.
ACKNOWLEDGMENTS
The authors wish to thank CAPES for the financial support.
REFERENCES
- 1. Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89(23):107-10.
- 2. Burstein R, Cutrer MF, Yarnitski D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(8):1703-9.
- 3. Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55(1):27-36.
- 4. Conti PC, Azevedo LR, Souza NV, Ferreira FV. Pain measurement in TMD patients: evaluation of precision and sensitivity of different scales. J Oral Rehabil. 2001;28(6):534-9.
- 5. Dando WE, Branch MA, Maye JP. Headache disability in orofacial pain patients. Headache. 2006;46(2):322-6.
- 6. Dao TT, Lund JP, Rémillard G, Lavigne GJ. Is myofascial pain of the temporal muscles relieved by oral sumatriptan? A cross-over pilot study. Pain. 1995;62(2):241-4.
- 7. Davidoff RA. Trigger points and myofascial pain: toward understanding how they affect headaches. Cephalalgia. 1998;18(7):436-48.
- 8. Dodick DW, Eross EJ, Parish JM, Silber M. Clinical, anatomical, and physiologic relationship between sleep and headache. Headache. 2003;43(3):282-92.
- 9. Glaros A, Urban D, Locke J. Headache and temporomandibular disorders: evidence for diagnostic and behavioural overlap. Cephalalgia. 2007;27(6):542-9.
- 10. Haley D, Schiffman E, Baker C, Belgrade M. The comparison of patients suffering from temporomandibular disorders and a general headache population. Headache. 1993;33(4):210-3.
- 11. Jensen R. Pathophysiological mechanisms of tension-type headache: a review of epidemiological and experimental studies. Cephalalgia. 1999;19(6):602-21.
- 12. Karli N, Zarifoglu M, Calisir N, Akgoz S. Comparison of pre-headache phases and trigger factors of migraine and episodic tension-type headache: do they share similar clinical pathophysiology? Cephalalgia. 2005;25(6):444-51.
- 13. Kim ST, Kim CY. Use of the ID Migraine questionnaire for migraine in TMJ and Orofacial Pain Clinic. Headache. 2006;46(2):253-8.
- 14. Laimi K, Vahlberg T, Salminen J, Metsähonkala L, Mikkelsson M, Anttila P, et al. Does neck pain determine the outcome of adolescent headache? Cephalalgia. 2007;27(3):244-53.
- 15. Liljestrom MR, Le Bell Y, Anttila P, Aromaa M, Jämsä T, Metsähonkala L, et al. Headache children with temporomandibular disorders have several types of pain and other symptoms. Cephalalgia. 2005;25(11):1054-60.
- 16. Magnusson T, Carlsson GE, Egermark-Eriksson I. An evaluation of the need and demand for treatment of craniomandibular disorders in a young Swedish population. J Craniomandib Disord. 1991;5(1):57-63.
- 17. Mense S. Considerations concerning the neurobiological basis of muscle pain. Can J Physiol Pharmacol. 1991;69(5):610-6.
- 18. Merrill RL. Central mechanisms of orofacial pain. Dent Clin North Am. 2007;51(1):45-59.
- 19. Mitrirattanakul S, Merrill RL. Headache impact in patients with orofacial pain. J Am Dent Assoc. 2006;137(9):1267-74.
- 20. Mongini F, Ciccone G, Deregibus A, Ferrero L, Mongini T. Muscle tenderness in different headache types and its relation to anxiety and depression. Pain. 2004;112(1-2):59-64.
- 21. Okeson JP. Orofacial pain: guidelines for assessment, diagnosis, and management. Chicago: Quintessence; 1996.
- 22. Okeson JP. Bell's orofacial's pains. The clinical management of orofacial pain. Carol Stream: Quintessence; 2005.
- 23. Okeson JP. Management of temporomandibular disorders and occlusion. St. Louis: CV Mosby; 1998.
- 24. Ruiz de Velasco I, González N, Etxeberria Y, Garcia-Monco JC. Quality of life in migraine patients: a qualitative study. Cephalalgia. 2003;23(9):892-900.
- 25. Silva RS, Conti PC, Lauris JR, da Silva RO, Pegoraro LF. Pressure pain threshold in the detection of masticatory myofascial pain: an algometer-based study. J Orofac Pain. 2005;19(4):318-24.
- 26. Simons DG, Travel JG, Simons LS, Cummings BD. Myofascial pain and dysfunction: the trigger point manual. Upper half of body. Baltimore: Williams & Wilkins; 1999.
- 27. Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. J Am Med Assoc. 1992;267(1):64-9.
- 28. Tfelt-Hansen P, Lous I, Olesen J. Prevalence and significance of muscle tenderness during common migraine attacks. Headache. 1981;21(2):49-54.
- 29. The International Classification of Headache Disorders: 2nd ed. Cephalalgia. 2004;24 Suppl 1:9-160.
- 30. Vazquez-Delgado E, Schmidt JE, Carlson CR, DeLeeuw R, Okeson JP. Psychological and sleep quality differences between chronic daily headache and temporomandibular disorders patients. Cephalalgia. 2004;24(6):446-54.
Endereço para correspondência:
Publication Dates
-
Publication in this collection
04 Nov 2011 -
Date of issue
Aug 2011
History
-
Received
12 Nov 2007 -
Accepted
17 Aug 2009