Abstracts
The peak frequency of Hodgkin's disease convergesmatches with women of reproductive fertility age. Currently, this disease is the fourth more diagnosed neoplasia during pregnancy. In addition, there is no consensus in the literature on how to treat pregnant women because of the risks of chemotherapy for mothers and for fetuses. We report three cases of pregnant women with Hodgkin's disease. A review of the literature was made aiming to suggest a protocol to treat these patients.
Hodgkin disease/drug therapy; Pregnancy complications, neoplastic/drug therapy; Case reports
O pico de incidência do linfoma de Hodgkin coincide com a idade fértil feminina, sendo atualmente a quarta neoplasia mais diagnosticada na gravidez. Entretanto, não existe consenso na literatura sobre como tratar essas pacientes, devido aos riscos da quimioterapia tanto para a gestante quando para o feto. Relatamos três casos de gestantes acometidas por linfoma de Hodgkin e realizamos a revisão de literatura com o objetivo sugerir um protocolo de tratamento para essas pacientes.
Doença de Hodgkin/quimioterapia; Complicações neoplásicas na gravidez/quimioterapia; Relatos de casos
INTRODUCTION
Cancer is the second cause of death in women of reproductive age, and may complicate approximately 1 of each 1,000 pregnancies (11. Yahalom J. Management of Hodgkin lymphoma during pregnancy. Up to Date (Internet). April 2008. Available from: www.uptodate.com
www.uptodate.com...
). In this context, lymphomas are the forth more diagnosed neoplasia in pregnancy. The most common one is Hodgkin's disease (HD) for its high incidence in women of reproductive age (22. Lishner M, Zemlickis D, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Maternal and fetal outcome following Hodgkins disease in pregnancy. Br J Cancer. 1992;65(1):114-7.).
However, the association between HD and pregnancy is uncommon yet corresponding to 3.2% of all patients with HD (22. Lishner M, Zemlickis D, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Maternal and fetal outcome following Hodgkins disease in pregnancy. Br J Cancer. 1992;65(1):114-7.). Some reports estimate its frequency in approximately 1:6000 gestations (22. Lishner M, Zemlickis D, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Maternal and fetal outcome following Hodgkins disease in pregnancy. Br J Cancer. 1992;65(1):114-7.,33. Pereg D, Koren G, Lishner M. The treatment of Hodgkin's and non-Hodgkin's lymphoma in pregnancy. Haematologica. 2007;92(9):1230-7.). Decisions on treatment in this phase must take into account the clinical presentation, the drug interactions with pregnancy and the effects on fetuses and newborns.
We aim to report Hospital Albert Einstein's experience in the treatment of pregnant women with HD, and to establish a protocol for the treatment based on a review of the literature.
CASE REPORTS
Case 1
ACSW, second pregnancy, 35 years old. Gestational age in the first visit was 29 weeks gestational age (GA) and 5 days. The patient reported rubella at 12 weeks and appearance of cervical ganglion at 23 weeks GA. She also presented comorbid conditions of hypothyroidism and antiphospholipid syndrome (APS), and was using heparin of low molecular weight.
The patient was submitted to nuclear magnetic resonance that showed lymphnodemegaly in the low internal jugular chain, in the right posterior cervical and in left supraclavicular chains, measuring up to 2.5 cm of diameter. It was also observed an extrinsic pressure on the left internal jugular vein and a lymph node conglomerate on the para-aortic and on the right inferior paratracheal chains.
A lymph node biopsy showed syncytial variant of nodular sclerosing Hodgkin's disease (NSHD) and a Cotswold staging IIA. A conservative treatment was proposed with corticotherapy starting at 32 weeks GA up to childbirth because the patient did not have oncologic emergency and she had decided not to expose the fetus to chemotherapy. The baby was delivered at 34 weeks GA as suggested by the obstetrician. The newborn presented at birth discrete hypoxia with 6/8 Apgar scores and icterus neonatorum. Birth weight was 2.7 kg and height was 40 cm. The newborn had a good and uneventful evolution at the nursery and was discharged on the seventh day.
The patient was submitted to PET/CT 10 days after delivery. The test did not change the initial staging. Chemotherapy was introduced according to the ABVD protocol (doxorubicin, bleomycin, vinblastine and dacarbazine) protocol for six cycles. Nowadays, the patient is in total remission.
Case 2
BFS, primigravida, 20 years old. The patient comes to medical service at 31 weeks GA. She reported fever and cervical adenomegaly for one year, and was treated for infection. Although we were not able to proof if the disease had started a year ago. A biopsy was performed and it showed syncytial variant of NSHD. The initial Costwold staging was II B confirmed by magnetic resonance. The patient also presented symptomatic compression of the superior vena cava. Because of the B symptoms and compression of the superior vena cava met the criteria of an oncologic emergency, we decided to start the first ABVD cycle. The baby was delivered at 35 weeks GA and presented 8/9 Apgar scores, birth weight was 2.8kg and height was 45 cm. There were no complications and the baby was discharged on the fourth day. The patient underwent six uneventful complete cycles of ABVD and is kept in complete remission.
Case 3
PKGS, second pregnancy, 29 years old. The patient looked for the hematologist at 36 weeks GA for a lymphnodemegaly recently observed. She did not present B symptoms and underwent a magnetic resonance that showed cervical and mediastinal adenomegalies. We decided to perform a ganglionar biopsy which was compatible with the diagnosis of syncytial variant of NSHD. She did not undergo any treatment until delivery that happened at 38 weeks GA and gave birth through pelvic pathway to a full-term infant, who received 9/10 Apgar scores, had birth weight of 3.2kg and height of 48 cm.
After the delivery, the patient was submitted to PETCT which was compatible with clinic staging IIA and a bulky mediastinal mass, which is considered as a tumor when the mass is greater than 7 cm in mediastinal area. Then, she was submitted to six cycles of ABVD without any events.
A summary of the cases is showed in chart 1.
DISCUSSION
HD is commonly associated to pregnancy because the peak its frequency occurs during women reproductive age (22. Lishner M, Zemlickis D, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Maternal and fetal outcome following Hodgkins disease in pregnancy. Br J Cancer. 1992;65(1):114-7.). In pregnant women the most observed clinical finding is cervical lymphnodemegaly that did not differ from non pregnant women. The biopsy diagnosis does not present difficulties, however, some aspects must be observed in pregnant women.
In pregnant women the characteristics of HD are the same when compared to non pregnant women. The majority of data on this subject come from case series.
In a study with 48 cases, the patients’ mean age was 26 years. Twelve were diagnosed before getting pregnant, 10 during pregnancy and 27 until the 9th month after pregnancy termination. When compared to non pregnant women with HD clinical stage (CS), 46% were CS II, 17% were CS III and 12% CS IV (22. Lishner M, Zemlickis D, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Maternal and fetal outcome following Hodgkins disease in pregnancy. Br J Cancer. 1992;65(1):114-7.).
Usually, for HD staging computerized tomographies of chest, abdomen and pelvis or PET-CT are requested. The computerized tomography of abdomen and pelvis might expose the fetus to radiation of at least 0.02 Gy which is considered potentially teratogenic(44. Cohen-Kerem R, Nulman I, Abramow-Newerly M, Medina D, Maze R, Brent RL, et al. Diagnostic radiation in pregnancy: perception versus true risks. J Obstet Gynaecol Can. 2006;28(1):43-8.). However, there are imaging tests as magnetic resonance and ultrasonography which could provide information on lymph node megalies without measurable radiation risk to the fetus, although ultrasonography is a less sensible and dependable test, besides the lack of data on the unrestrictive use of magnetic resonance in pregnant women(55. Kawabata I, Takahashi Y, Iwagaki S, Tamaya T. MRI during pregnancy. J Perinat Med. 2003;31(6):449-58.).
The PET-CT in oncology uses 18 F-FDG, a substance that can cross placental barrier and offer a potential radiation risk to the fetus. PET-CT is also contraindicated during breastfeeding. Mothers must interrupt breastfeeding for 24 hours after underwenting this exam (66. Benveniste H, Fowler JS, Rooney WD, Moller DH, Backus WW, Warner DA, Carter P, King P, Scharf B, Alexoff DA, Ma Y, Vaska P, Schlyer D, Volkow ND; PET study; MRI study. Maternal-fetal in vivo imaging: a combined PET and MRI study. J Nucl Med. 2003;44(9):1522-30.).
From the pathology point of view the presentation during pregnancy is also the same. In a study with 17 women diagnosed in about 22 weeks GA the more frequent histological type was nodular sclerosing (NS) (77. Jacobs C, Donaldson SS, Rosenberg SA, Kaplan HS. Management of the pregnant patient with Hodgkin's disease. Ann Intern Med. 1981;95(6):669-75.), founded in 13 patients. In our case series, all observed cases were NS, subtype of syncytial variant. Despite the lack of designed studies with pregnant women with HD, some information could be found in published case series. There is a description of 24 cases of HD in gestation, with 2 spontaneous abortions and 5 cases of fetal malformation due to exposition to MOPP scheme or to cyclophosphamide and radiotherapy in the first trimester. In that study 15 pregnant women received ABDV independently of the trimester of gestation and had normal children who were healthy at posterior evaluations. The MOPP or COPP scheme in the second and third trimester of gestation was not associated to malformation (88. Ebert U, Löffler H, Kirch W. Cytotoxic therapy and pregnancy. Pharmacol Ther. 1997;74(2):207-20.).
The ABVD scheme was also assessed in two case series with 13 pregnant women who underwent chemotherapy in the first, second and third trimesters. These series also did not show malformation in the newborns. However, one patient who did decide to use dacarbazine had progression of the disease one year later (99. Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5(5):283-91.). The safety and efficacy of ABVD was also reported in pregnant women with HD and with HIV, presenting excellent outcome for mothers and children (1010. Avilés A, Neri N. Hematological malignancies and pregnancy: a final report of 84 children who received chemotherapy in utero. Clin Lymphoma. 2001;2(3):173-7.).
Pregnancy does not seem to influence the route of the disease. The case series from 1962 evaluated 112 gestations of 84 women and did not find differences in survival curve, mean of 90 months, among patients who interrupted pregnancy and those who did not interrupt (1111. Barry RM, Diamond HD, Carver LF. Influence of pregnancy on the course of Hodgkins disease. Am J Obstet Gynecol. 1962;84:445-54.). Another series showed that 12 from 14 mothers submitted to chemotherapy during pregnancy are fine or in lymphoma remission (1212. Avilés A, Díaz-Maqueo JC, Talavera A, Guzmán R, García EL. Growth and development of children of mothers treated with chemotherapy during pregnancy: current status of 43 children. Am J Hematol. 1991;36(4):243-8.).
Radiation might be efficient in initial stage of the disease, however, it can be performed in the second and third trimesters only. The maximum dose that the fetus can receive must be limited to 0.10 Gy. A case series with 16 pregnant women (two CS IA and 14 CS IIA) treated at MD Anderson Cancer Center showed a 10 year survival of 83% and all children had normal birth. Eight patients had to undergone chemotherapy or radiotherapy after delivery (22. Lishner M, Zemlickis D, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Maternal and fetal outcome following Hodgkins disease in pregnancy. Br J Cancer. 1992;65(1):114-7.).
A Danish cohort study, published in 2008, compared 292 newborn from mothers with HD to another 14,402 healthy mothers. It concluded that there were no significant associations of malformation or low birth weight in their children, although there was a frequency 26 times greater of prematurity among newborns from mothers with HD. This fact could be considered responsible for the decision of pregnant women, or their physicians, to interrupt the pregnancy earlier in order to start chemotherapy (1313. Langagergaard V, Horvath-Puho E, Nørgaard M, Nørgård B, Sørensen HT. Hodgkin's disease and birth outcome: a Danish nationwide cohort study. Br J Cancer. 2008;98(1):183-8.).
An extensive literature review was made in 2008 by the American Society of Hematology Education Book(1414. Bachanova, V, Connors, JM. How is Hodgkin in pregnancy best treated? ASH Evidence-based review, 2008. In: Hematology: American Society of Hematology Education Program Book. Philadelphia: American Society of Hematology; 2008. p. 33-4.). It assessed published papers at Medline database from 1950 to April, 2008. After exclusion of articles which did not addressed the research criteria, a total of eight case reports, nine case series and two case-controls were selected. The case reports highlighted the outcome of pregnant women who underwent chemotherapy related to the evolution of the disease and newborn well-being. From all case reports only one reported malformation. In this case, the newborn had syndactyly and the mother was treated with oral cyclophosphamide on the three trimesters.
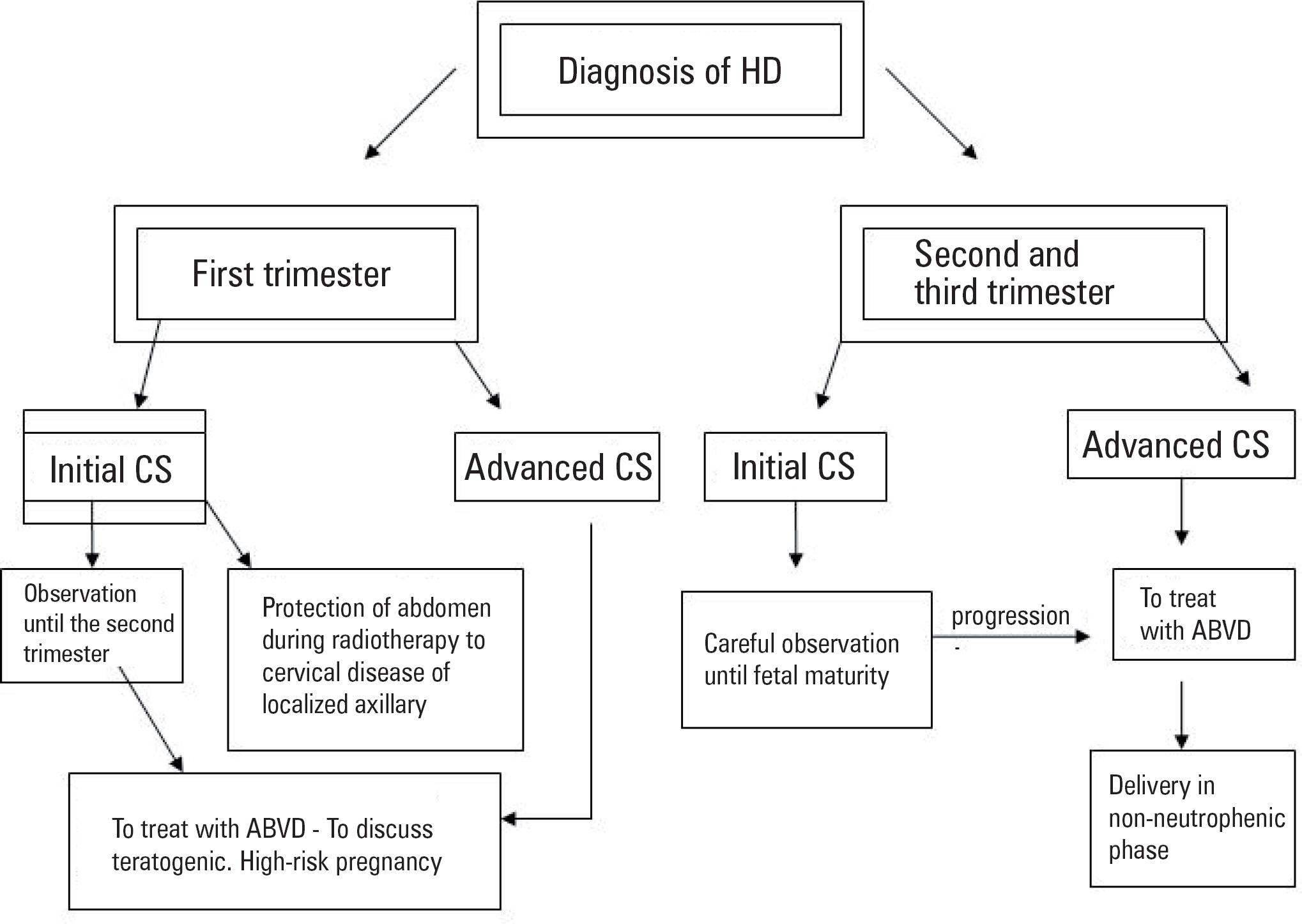
Despite the scarcity of evidences, we suggest a protocol (figure 1) that patients with low aggressive disease and who were diagnosed from the 20th weeks GA on might be kept under close observation associated or not to corticotherapy (77. Jacobs C, Donaldson SS, Rosenberg SA, Kaplan HS. Management of the pregnant patient with Hodgkin's disease. Ann Intern Med. 1981;95(6):669-75.). The CS IA or IAA of HD is considered a nonaggressive disease without high risk criteria (elevated lactic dehydrogenase and low albumin).
Radiotherapy is also an option to be considered in such cases of precocious CS with isolated axillary or cervical involvement. The maximum dose limited to the fetus must be taken into consideration(33. Pereg D, Koren G, Lishner M. The treatment of Hodgkin's and non-Hodgkin's lymphoma in pregnancy. Haematologica. 2007;92(9):1230-7.).
If the patients have aggressive disease with B symptoms or superior vena cava syndrome, chemotherapy should be started according to ABVD protocol and also to perform a follow-up with a specialized obstetrician in high risk pregnancy (11. Yahalom J. Management of Hodgkin lymphoma during pregnancy. Up to Date (Internet). April 2008. Available from: www.uptodate.com
www.uptodate.com...
). In these cases, the delivery must occur at a neutropenia period.
If possible, patients diagnosed in the first trimester must wait until the beginning of the second trimester to start chemotherapy. When it is not possible, the risk of fetal malformation, even if lower, must be considered, and explained to the pregnant woman. Indications to a immediate start of chemotherapy in the first trimester are: compression of bulky mass, symptomatic subdiaphragmatic disease or advanced clinical HD stages (33. Pereg D, Koren G, Lishner M. The treatment of Hodgkin's and non-Hodgkin's lymphoma in pregnancy. Haematologica. 2007;92(9):1230-7.).
It is important to highlight that patients with previous diagnose of HD who had a relapse during pregnancy, might have a worse prognosis and the interruption of pregnancy must be considered (11. Yahalom J. Management of Hodgkin lymphoma during pregnancy. Up to Date (Internet). April 2008. Available from: www.uptodate.com
www.uptodate.com...
). The decision is personal, being the hematologist responsible to explain for the parents the risks and the benefits of the treatment.
Women who underwent treatment have similar fertility as women in general. However, those who were treated with MOPP and MVPP therapy have lower fertility. Patients who were submitted to radiotherapy must follow a hypothyroidism risk. It is recommended that women with HD must wait at least two or three years before getting pregnant again (11. Yahalom J. Management of Hodgkin lymphoma during pregnancy. Up to Date (Internet). April 2008. Available from: www.uptodate.com
www.uptodate.com...
).
REFERENCES
-
1Yahalom J. Management of Hodgkin lymphoma during pregnancy. Up to Date (Internet). April 2008. Available from: www.uptodate.com
» www.uptodate.com -
2Lishner M, Zemlickis D, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Maternal and fetal outcome following Hodgkins disease in pregnancy. Br J Cancer. 1992;65(1):114-7.
-
3Pereg D, Koren G, Lishner M. The treatment of Hodgkin's and non-Hodgkin's lymphoma in pregnancy. Haematologica. 2007;92(9):1230-7.
-
4Cohen-Kerem R, Nulman I, Abramow-Newerly M, Medina D, Maze R, Brent RL, et al. Diagnostic radiation in pregnancy: perception versus true risks. J Obstet Gynaecol Can. 2006;28(1):43-8.
-
5Kawabata I, Takahashi Y, Iwagaki S, Tamaya T. MRI during pregnancy. J Perinat Med. 2003;31(6):449-58.
-
6Benveniste H, Fowler JS, Rooney WD, Moller DH, Backus WW, Warner DA, Carter P, King P, Scharf B, Alexoff DA, Ma Y, Vaska P, Schlyer D, Volkow ND; PET study; MRI study. Maternal-fetal in vivo imaging: a combined PET and MRI study. J Nucl Med. 2003;44(9):1522-30.
-
7Jacobs C, Donaldson SS, Rosenberg SA, Kaplan HS. Management of the pregnant patient with Hodgkin's disease. Ann Intern Med. 1981;95(6):669-75.
-
8Ebert U, Löffler H, Kirch W. Cytotoxic therapy and pregnancy. Pharmacol Ther. 1997;74(2):207-20.
-
9Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5(5):283-91.
-
10Avilés A, Neri N. Hematological malignancies and pregnancy: a final report of 84 children who received chemotherapy in utero. Clin Lymphoma. 2001;2(3):173-7.
-
11Barry RM, Diamond HD, Carver LF. Influence of pregnancy on the course of Hodgkins disease. Am J Obstet Gynecol. 1962;84:445-54.
-
12Avilés A, Díaz-Maqueo JC, Talavera A, Guzmán R, García EL. Growth and development of children of mothers treated with chemotherapy during pregnancy: current status of 43 children. Am J Hematol. 1991;36(4):243-8.
-
13Langagergaard V, Horvath-Puho E, Nørgaard M, Nørgård B, Sørensen HT. Hodgkin's disease and birth outcome: a Danish nationwide cohort study. Br J Cancer. 2008;98(1):183-8.
-
14Bachanova, V, Connors, JM. How is Hodgkin in pregnancy best treated? ASH Evidence-based review, 2008. In: Hematology: American Society of Hematology Education Program Book. Philadelphia: American Society of Hematology; 2008. p. 33-4.
Publication Dates
-
Publication in this collection
Apr-Jun 2011
History
-
Received
21 Jan 2011 -
Accepted
15 Apr 2011