Abstracts
OBJECTIVE: To evaluate the quality of information about health and medication available on Brazilian websites. METHODS: A descriptive study with a quantitative approach regarding Brazilian websites, conducted from January to March 2011. The search sites were located using two search phrases: "medication information" and "health information." The choice of variables was based on the Internet information quality criteria of the World Health Organization and the International Code of Ethics for health and services sites on the Internet. The dependent variable was whether the site had information about health or medication. The independent variables were access, appearance, organization, honesty, transparency, responsibility and origin. For statistical analysis, the χ² and Fisher exact tests were applied, with a significance level of 5%. RESULTS: Of the 37 Brazilian sites analyzed, 24 (64.9%) contained health information and 13 (35.1%) contained medication information. Regarding appearance, organization and access criteria, most sites related to health and medication were easily accessible, easy to understand, used objective language, were updated and organized logically and provided accurate and scientifically grounded information. CONCLUSION: The honesty criterion differed significantly between sites, and the quality of information presented on health and medication websites showed significant differences, suggesting the need for a more systematic organization of these topics on the Internet.
Internet; Consumer health information; Information products and services
OBJETIVO: Avaliar a qualidade das informações sobre saúde e medicamentos disponíveis em sítios brasileiros. MÉTODOS: Estudo descritivo, de abordagem quantitativa em sítios brasileiros, realizado no período de janeiro a março de 2011. A localização dos sítios de busca ocorreu empregando duas palavras-chave: "informações sobre medicamentos" e "informações sobre saúde". A escolha das variáveis baseou-se nos critérios da qualidade da informação na internet da Organização Mundial da Saúde e do Código Internacional de Ética para sítios de saúde e serviços na internet. A variável dependente foi o sítio que apresentava informação sobre medicamento ou sobre saúde. As variáveis independentes foram acesso, aparência, organização, honestidade, transparência, responsabilidade e procedência. Para análise estatística, aplicaram-se os testes do χ² e exato de Fisher, com nível de significância de 5%. RESULTADOS: Dos 37 sítios brasileiros analisados, 24 (64,9%) eram de informações para saúde e 13 (35,1%) sobre medicamentos. Nos critérios de acesso, aparência e organização, a maioria dos sítios sobre saúde e sobre medicamentos era de fácil acesso, fácil entendimento, linguagem objetiva, atualizados e organizados de forma lógica e fornecia informação exata e cientificamente fundamentada. CONCLUSÃO: O critério honestidade apresentou diferença estatisticamente significante entre os sítios. A qualidade das informações geradas nos sítios sobre saúde e sobre medicamentos apresentou diferenças importantes, exigindo uma organização mais sistemática desses temas apresentados em seus ciberespaços.
Internet; Informação de saúde ao consumidor; Produtos e serviços de informação
ORIGINAL ARTICLE
Universidade de Fortaleza - UNIFOR, Fortaleza (CE), Brazil
Corresponding author
ABSTRACT
OBJECTIVE: To evaluate the quality of information about health and medication available on Brazilian websites.
METHODS: A descriptive study with a quantitative approach regarding Brazilian websites, conducted from January to March 2011. The search sites were located using two search phrases: "medication information" and "health information." The choice of variables was based on the Internet information quality criteria of the World Health Organization and the International Code of Ethics for health and services sites on the Internet. The dependent variable was whether the site had information about health or medication. The independent variables were access, appearance, organization, honesty, transparency, responsibility and origin. For statistical analysis, the χ2 and Fisher exact tests were applied, with a significance level of 5%.
RESULTS: Of the 37 Brazilian sites analyzed, 24 (64.9%) contained health information and 13 (35.1%) contained medication information. Regarding appearance, organization and access criteria, most sites related to health and medication were easily accessible, easy to understand, used objective language, were updated and organized logically and provided accurate and scientifically grounded information.
CONCLUSION: The honesty criterion differed significantly between sites, and the quality of information presented on health and medication websites showed significant differences, suggesting the need for a more systematic organization of these topics on the Internet.
Keywords: Internet; Consumer health information; Information products and services
INTRODUCTION
In the last two decades, the number of people using the Internet to retrieve information in all areas, particularly health, has grown exponentially. It is estimated that 4.5% of all Internet search engine queries related to health were queries about health problems(1,2).
In the United States, two surveys of Internet users have revealed that more than 63% use it at least once a year to obtain information about health(3,4). Reasons for this use include the direct possibility improving health and challenging medical databases by confronting them with "stories" from the Internet. Studies conducted in the United States and Europe show that a small proportion of doctors and health professionals use commercial search engines for information on health-related issues(5).
In Brazil, a study has shown that Brazilian online pharmacies enable access to unregistered medication and circumvent restrictions on the sale of controlled drugs, thus permitting the inappropriate and indiscriminate use of drugs. That study indicated a lack of human resources and a regulatory failure of the National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária - ANVISA). The study also found that the primary source of information on the sites was pharmaceutical inserts(6).
The presence of medication and health information on the Internet leads to changes in the doctor-patient relationship regarding the patient's actions of toward his or her illness: more than 70% of users claim that information contained on the Internet influences their decisions regarding prescribed treatment(7). Furthermore, the pharmaceutical industry encourages the use of information on the Internet by promoting credibility strategies for their products, thereby encouraging higher consumption, which in turn can lead to adverse effects or drug intoxication(8).
In the United States, an estimated US$800 billion growth in pharmaceuticals occurred in 2010, and that figure could be even higher by 2013(9). In Brazil, an estimated increase of US$15.4 billion(10) has been reported. This finding highlights the need to constantly evaluate the quality of health information on the Internet. Ethical issues are inarguably essential to the provision of good quality health care, and the dissemination of information through the Internet presents a challenge for clinicians(11). The International Code of Ethics for health sites and services on the Internet was passed by the U.S. Senate in 2000(12). This code was also adopted in Europe and other countries as recommended voluntary standards; the recommendations are not tied to an organization and thus merely guide ethical and social activities(13).
In the healthcare industry, there is an every-present concern for quality. Since 1997, the Agency for Health Care Policy and Research, along with the Health Information Technology Institute (HiTi) and the Mitretek Systems company, through the intermediary Health Summit Working Group, have prepared a document entitled Criteria for the Quality of Health Information on the Internet, which presents seven criteria for evaluating the quality of information on health websites: credibility, content, site presentation, links, design, interactivity and advertisements. This document also aims to assist the Internet consumer with choosing and identifying reliable and fraudulent websites(14).
One of the best-known institutions, the Internet Healthcare Coalition, advocates the promotion of quality health resources on the Internet. The coalition includes American consumers, patient advocates, commercial health information creators, health professionals, medical librarians, government officials and pharmaceutical manufacturers, and it aims to educate consumers, health professionals and others on issues relating to the evolving quality of Internet health resources and information(15).
The World Health Organization (WHO) states that information available on the Internet should establish the purpose of the site (whether it has educational or business purposes), thereby defining its target audience(16). Information should include the date and source of publication (institutions, organizations or manufacturers that are responsible legally and ethically for providing the information), clarify whether there are references to clinical studies and mention groups or entities of sufferers of a particular disease that support the publication of such information.
Some entities, such as the American Medical Association (AMA), have developed guidelines for information searches on websites, from site acquisition to online advertising and sponsorship of information and the product, ensuring the privacy and confidentiality of visiting patients. Furthermore, sites must provide safe and effective means for marketing products. These guidelines favor the AMA's own site and the sites of other providers. The AMA website is frequently updated in accordance with technological evolution and new Internet practices(17).
The Brazilian Federal Medical Council has produced a document with nine standards to ensure the quality of information on the Internet: information and service integrity, privacy, confidentiality, authentication, auditing, honesty, safety, transparency and free and informed consent(18). It is also noteworthy that the Regional Medical Council of the State of São Paulo (Conselho Regional de Medicina do Estado de São Paulo - CREMESP) has published a manual of ethical principles for medical and health websites that offers guidance to the user in searching for health information, products and services online. Moreover, it informs the user of his/her right to demand the following from the organizations and individuals responsible for the sites: transparency, honesty, quality, free and informed consent, privacy, medical ethics, responsibility and origin(19).
The importance of cyberspace as source and provider of information about health and medication should encourage reflection upon a consensus for both health professionals and consumers who use the Internet as an information source.
OBJECTIVE
This article aims to assess differences in the quality of information available on Brazilian websites related to health and medication.
METHODS
This is a descriptive, qualitative study of Brazilian websites that provide information about health and medication. Microsoft Internet Explorer® Version 6.0 was used to view websites via their uniform resource locator (URL) addresses. The Google search engine was selected, as it is the most widely used search engine worldwide(20).
The sites were surveyed between January and March 2011. Two hundred eighty-six health and medication sites were identified using two key phrases: "medication information" and "health information." The sites with "health information" contained health, wellness, health promotion or disease-specific images or text. The sites with "medication information" presented images or text of pharmaceutical products or medication.
Based on the criteria adopted (URL addresses with a.br suffix and containing information on health and medication targeted at healthcare professionals and users), 37 Brazilian sites were identified. The criteria for exclusion were the following: pages containing only advertisements and sales of products and medication; hospital sites; the sites of nongovernmental organizations, councils, government associations and agencies; sites providing information on veterinary medication or cosmetics; and pages that were unavailable or that did not contain information about health and medication.
This study analyzed seven variables based on the WHO criteria for information quality on the Internet(16) and the International Code of Ethics for health sites and services on the Internet(12), and a form was developed for data collection (Appendix 1).
The dependent variable was the site itself. The independent variables were as follows: access, defined as easy access; links to other relevant sites related to some specialty and having a number of users who access them; appearance, defined as the visibility of a publication or revision date, the citation of sources used, the ease of understanding the site and the use of objective language, accuracy and scientific grounding; organization, defined as how the website was updated and the logical organization of information; honesty, defined as the purpose of the site being truthfully stated and not misleading, through the intervention of the authors and sponsors; transparency, defined as taking responsibility for selecting content that does not jeopardize the privacy of users' information, nor having chat rooms, spaces for questions, complaints and suggestions or visual or audible effect aimed at some specialty that would distract users from understanding information; responsibility, defined as taking responsibility for creating and updating the site, indicating the presence of sponsors, identifying the responsible technician, avoiding illogical information for good pharmacokinetic and therapeutic understanding, and submitting articles to a publisher for review; and origin, defined as the involvement of professional sources, organizations, universities, government agencies (municipality, state and federal), private bodies and other institutions with recognized qualifications.
The data were entered into a database and analyzed with the Statistical Package for the Social Sciences 14.0 for Windows, Student Version® (USA). The statistical analysis provided results that were expressed as a proportion. The χ2 test and Fisher's exact test were applied with a significance level of 5% to evaluate the differences between the sites in terms of health information and medication information. The items comprising each variable were assigned values of "yes" or "no".
RESULTS
Of the 37 Brazilian sites analyzed, 24 (64.9%) contained health information, and 13 (35.1%) contained medication information.
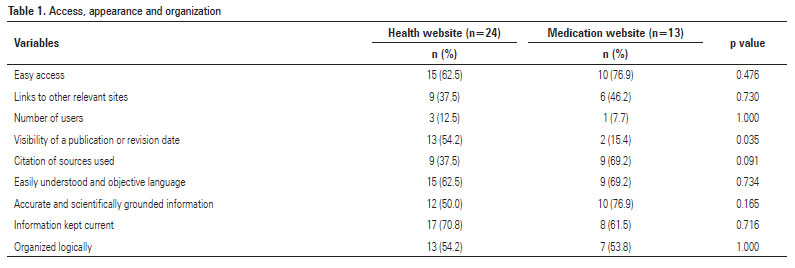
Regarding appearance, organization and access criteria, most health and medication sites were easily accessible, easy to understand, used objective language and accurate and scientifically grounded information and were updated and logically organized. Publication or revision dates were visible on few of the medication sites (15.4%). Citations of sources were visible on a high proportion of the health sites (69.2%) (Table 1).
Access numbers varied across the sites, with some reaching 74,000 users per day.
None of the access criteria differed significantly among the sites investigated. For the appearance criteria, the visibility of the publication or revision date (p=0.035) differed significantly among the sites surveyed (Table 1).
Regarding honesty, transparency and accountability criteria, most health and medication sites selected content responsibly and did not put the privacy of users at risk. The majority (62.5%) of the health sites had no chat options, spaces for questions, complaints or suggestions and had links to other relevant sites, such as those focused on a specialty. Most of the medication sites had a high proportion of sponsors' messages (92.3%) and intervention from sponsors (69.2%). A very low proportion (8.3%) of the health sites showed the purpose of the site. Twenty-five percent of the health sites showed the technician responsible, as did 46.2% of the medication sites. More than 15% of the information on both the health and medication sites was incorrect with regard to proper therapeutic and pharmacokinetic understanding (Table 2).
Significant differences were observed among the sites on all honesty criteria, including the involvement of the information's authors and the sponsors of the content (p<0.001) and the site's editorial angle (0.002). There were no significant differences among the sites regarding transparency criteria. For responsibility criteria, there were significant differences for the site creation responsibility criterion (p=0.005) and the content sponsors criterion (p<0.001; Table 2).
Regarding origin criteria, university information was infrequent on both the health (8.3%) and the medication (7.7%) sites. There was a higher frequency of public health institution information on the health sites than on the medication sites. A higher frequency of involvement from other known qualified institutions (70.8%) was observed on the health sites compared with the medication sites (Table 3).
On the health sites, in terms of public spaces and advice pages, information about products, services and personalized medical care were provided, while offers of specialized services were very low. Some sites offered medical consultations and drug prescriptions.
No significant differences were found among the sites regarding source criteria (Table 3).
DISCUSSION
Based on the criteria adopted for access, appearance, organization, honesty, transparency, responsibility and origin, only the honesty criteria showed significant differences on all items between the health and medication sites.
Furthermore, our study revealed that the information presented on the health and medication websites is directly related to the marketing and commercialization of pharmaceutical products. The medication sites use commercial sources, such as pharmaceutical companies, distributors and virtual pharmacies, for information, and the information presented on these sites originated from informational inserts or leaflets produced by the company. The quality of the text taken from inserts for medical products marketed in Brazil is debatable because it may omit or exclude important information(21).
The results for the medication sites are worrisome, not only from a legal perspective(22), but particularly because of market influence: the sites contain the names of sponsors, drawing attention to their influence and showing them as willing to offer help to users. Product ads are considered unfavorable in the United States, as they might indicate that information for American users is subject to commercialization(23).
Users should be made aware that information about medication must go beyond the interest of increasing sales; i.e., adequate information about the medications must also be provided(24).
It was evident that some sites offered medical consultations and drug prescriptions; however, it is extremely important to recognize that medical advice is a complementary approach and does not replace the doctor-patient relationship(19).
The lack of identification of technical responsibility calls into question the role of health surveillance agencies(18,22), which are responsible for overseeing health and medication websites. Greater supervision by health surveillance agencies in these spaces is needed to avoid abuse. One of the roles of these agencies is to monitor sites to ensure that they provide safe, accurate, quality information about medications and health for the population.
The current situation could be very dangerous to the user because some sites do not employ qualified professionals who can provide reliable information about health-disease processes or drugs. This situation may lead the user to misuse drugs and may aggravate health problems. A study has shown that American users with chronic diseases are 1.3 times more likely to use the Internet to find information about their problem compared with those without chronic disease(23).
At present, basic legislative criteria that ensure quality of information(18,22) are not always followed. These criteria's recommendations include providing links with alert messages or that take the user directly to the ANVISA site, constantly updating information and making provider information visible.
Spaces for questions and suggestions are insufficient. Some sites, however, do provide opportunities for users to ask questions about their pathology and the drugs they use. There are even sites that host discussion forums that can be viewed by any other user, potentially jeopardizing the privacy of users who do not want exposure(18).
Regarding the quality of information on the Internet, the sites varied on criteria regarding the site's origin, updates, editorial development processes and the interests of the provider and others(25). When these criteria are adopted, they can help health professionals meet their duty to care for the patient. However, it is extremely important that this information does not suffer from external influences and that it be conveyed in a clear and precise manner and come from reliable sources. In summary, the risks inherent in the availability of health and medication information on the Internet can be clearly observed.
CONCLUSIONS
The findings of this study confirm that the honesty criterion is the key element in ensuring the quality of the information presented on health and medication websites. It is also necessary to consider such criteria as access, appearance, organization, transparency, responsibility and origin.
There are important differences in the quality of information generated on health and medication sites, and a more systematic organization of the way these themes are presented in cyberspace should be required. Quality-of-information parameters should be established for such parameters as precision, durability, updates and the authorship of information disseminated on the Internet.
Ultimately, those who provide information about health and drugs should be required to make an ethical commitment to the user of that information.
REFERENCES
- 1. Eysenbach G, Diepgen TL. Labeling and filtering of medical information on the internet. Methods Inf Med. 1999;38(2):80-8.
- 2. Eysenbach G, Kohler Ch. What is the prevalence of health-related searches on the World Wide Web? Qualitative and quantitative analysis of search engine queries on the Internet. AMIA Annu Symp Proc. 2003:225-9.
- 3. Madden M. The changing picture of who's online and what they do [Internet]. Pew Internet & American Life Project. 2003 Dec 22. [cited 2012 Sep 8]. Available from: http://www.pewinternet.org/~/media//Files/Reports/2003/PIP_Online_Pursuits_Final.PDF.PDF
- 4. Cline RJW, Haynes KM. Consumer health information seeking on the Internet: the state of the art. Health Educ Res. 2001;16(6):671-92.
- 5. Spink A, Yang Y, Jansen J, Nykanen P, Lorence DP, Ozmutlu S, et al. A study of medical and health queries to web search engines. Health Info Libr J. 2004;21(1):44-51.
- 6. Gondim, APS, Falcão, CB. Avaliação das farmácias virtuais brasileiras. Rev Saúde Pública. 2007;41(2):297-300.
- 7. Silva EV, Castro, LLC. Infodemiologia: uma abordagem epidemiológica da informação. Rev Espaço Saúde. 2007;8(2):39-43.
- 8. Aquino DS. Por que o uso racional de medicamentos deve ser uma prioridade? Ciênc Saúde Coletiva. 2008;13(Supl):733-6.
- 9. Gatyas G, Savage C. IMS: Forecasts Global Pharmaceutical Market Growth of 4 - 6% in 2010; Predicts 4 - 7% Expansion Through 2013. [cited 2012 set 12]. Available from: http://www.imshealth.com/portal/site/ims/menuitem.d248e29c86589c9c30e81c033208c22a/?vgnextoid=4b8c410b6c718210VgnVCM100000ed152ca2RCRD&vgnextfmt=default
- 10. Ribbink, K. Olá Brazil: Latin America's biggest market accelerates. Pharmavoice. 2011.[cited 2011 Mar 5] Available from: http://www.imshealth.com/imshealth/Global/Content/IMS%20in%20the%20News/Documents/PharmaVoice,%20January,%20Pharmerging-Brazil,%20Nilton%20Paletta.pdf
- 11. Parker M, Gray J. What is the role of clinical ethics support in the era of e- medicine? J Med Ethics. 2001;27(Suppl 1):i33-5.
- 12. e-Health Ethics Initiative. e-Health Ethics Draft Code (Feb 18). J Med Internet Res. 2000;2(1):E2.
- 13. Rodrigues R. Ethical and legal issues in interactive health communications: a call for international cooperation. J Med Internet Res. 2000;2(1):E8.
- 14. Lopes IL. Estudos sobre qualidade da informação em Saúde na Web e a visão de entidades de classe brasileiras [Internet]. [citado 2012 Set 8]. Disponível em: www.tempusactas.unb.br/index.php/tempus/article/viewFile/398/381
- 15. Mack J, Phil MS. The internet healthcare coalition. J Med Internet Res. 2000;2(1):E3.
- 16. World Health Organization. Medical Products and the Internet: A guide to finding reliable information - Regulatory Support Series No. 008 [Internet]. Geneva: WHO; 1999. [cited 2012 Set 12]. Available from: http://apps.who.int/medicinedocs/en/d/Js2277e/
- 17. Winker MA, Flanagin A, Chi-Lum B, White J, Andrews K, Kennett RL, et al. Guidelines for medical and health information sites on the internet: principles governing AMA web sites. JAMA. 2000;283(12):1600-6.
-
18Conselho Federal de Medicina (CFM). Resolução nº 1639 de 10 de Julho de 2002. Normas técnicas para o uso de sistemas informatizados para a guarda e manuseio do prontuário médico. Brasília (DF):CFM; 2002.
-
19Conselho Regional de Medicina do Estado de São Paulo (CREMESP). Resolução nº 097 de 9 de março de 2001. Manual de princípios éticos para sites de medicina e saúde. São Paulo: CRM; 2001.
- 20. Sullivan D. comScore: US has most searches; China Slowest Growth; Google Tops Worldwide in 2009 [Internet]. [cited 2012 Set 8]. Available from: http://searchengineland.com/comscore-us-most-searches-china-slowest-34217
- 21. Gonçalves AS, Melo G, Tokarski MHL, Branco AB. Bula de medicamentos como instrumento de informação técnico-científica. Rev Saúde Pública. 2002;36(1):33-9.
-
22Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Consulta pública nº 20, de 21 de março de 2001. Brasília (DF): MS/ANVISA, mar. 2001.
- 23. Bansil P, Keenan NL, Zlot AI, Gilliland JC. Health-related information on the web: results from the health styles survey, 2002-2003. Prev Chronic Dis. 2006;3(2):A36.
- 24. Barros JAC. Há riscos nas informações sobre saúde e medicamentos disseminados pela internet? Saúde em Debate. 2004;28(66):75-9.
- 25. Risk A, Dzenowagis J. Review of internet health information quality initiatives. J Med Internet Res. 2001;3(4):E28.
Quality of health and medication information on Brazilian websites
Publication Dates
-
Publication in this collection
29 Oct 2012 -
Date of issue
Sept 2012
History
-
Received
27 Nov 2011 -
Accepted
28 Feb 2012