Abstract
Gymnanthes klotzschiana is a tree native to the Atlantic Forest Biome. The objective of this study was to analyze the synchronization of five phenophases and their correlation with environmental factors during two years of survey in two distinct areas (Area 1: Mixed Alluvial Ombrophilous Forest; Area 2: Montane Semi-deciduous Seasonal Forest). Ten individuals were randomly selected in each area, with the following variables being studied: leaf fall and budding, flowering, and immature and ripe fruit production. The activity index and the intensity index were determined for each phenological event. Timing and interactions with environmental factors were tested using Spearman’s correlation. The photoperiod correlated with all phenophases and precipitation did not correlate with any. There was synchronization in the foliar fall and foliage in the two areas. The species can be characterized as semi-deciduous. Flowering and fruiting were annual, with the highest intensity of fruit production occurring in Area 1.
Keywords:
phenophases; environmental factors; activity index; Fournier index
1. INTRODUCTION AND OBJECTIVES
The branquilho (Gymnanthes klotzschiana Müll.Arg) is a pioneer tree species in the Euphorbiaceae family (Klein, 1960Klein RM. O aspecto dinâmico do pinheiro brasileiro. Sellowia 1960; 12(12): 17-44.). Mixed Alluvial Ombrophilous Forest (MOF) and Montane Semi-deciduous Seasonal Forest (SSF) are among the forest formations in which branquilho can be found (IBGE, 2012Instituto Brasileiro de Geografia e Estatística - IBGE. Manual técnico da vegetação brasileira: sistema fitogeográfico inventário das formações florestais e campestres técnicas e manejo de coleções botânicas procedimentos para mapeamentos. Rio de Janeiro; 2012.). Its preference for wet and alluvial soils makes this species occur with greater density in riparian areas (Silva et al., 2012Silva AC, Higuchi H, Van Den Berg E, Nunes MH, Carvalho DA. Florestas inundáveis: ecologia, florística e adaptações das espécies. Lavras: Editora UFLA; 2012.).
Phenology studies the occurrence of repetitive biological events such as leaf sprouting, flowering and fruiting, and these are correlated with the biotic and abiotic factors of the environment (Roberts et al., 2015Roberts AMI, Tansey C, Smithers RJ, Phillimore AB. Predicting a change in the order of spring phenology in temperate forests. Global Change Biology 2015; 21(7): 2603-2611. 10.1111/gcb.12896
https://doi.org/10.1111/gcb.12896...
). This research field generates data which support degraded land reclamation and protected area management programs (Muhanguzi & Ipulet, 2011Muhanguzi HDR, Ipulet P. Fruiting phenology of fig trees in Kalinzu Forest, Uganda. African Journal of Ecology 2011; 50(1): 90-101. 10.1111/j.1365-2028.2011.01301.x
https://doi.org/10.1111/j.1365-2028.2011...
; Rego & Lavaronti, 2007Rego GM, Lavaronti OJ. Atividades fenológicas de Imbuia (Octea porosa (Nees et. Martius ex. Ness) em áreas de floresta ombrófila mista, no estado do Paraná. In: Rego GM, Negrelle RRB, Morellato LPC, editors. Fenologia: ferramenta para conservação, melhoramento e manejo de recursos vegetais arbóreos. Colombo: Embrapa Florestas; 2007. p. 181-202.), and is currently being used as a tool to monitor climate change (Melaas et al., 2016Melaas EK, Friedl MA, Richardson AD. Multiscale modeling of spring phenology across Deciduous Forests in the Eastern United States. Global Change Biology 2016; 22(2): 792-805. 10.1111/gcb.13122
https://doi.org/10.1111/gcb.13122...
; Richardson et al., 2012Richardson AD, Anderson RS, Arain MA, Barr AG, Bohrer G, Chen G, et al. Terrestrial biosphere models need better representation of vegetation phenology: results from the North American Carbon Program Site Synthesis. Global Change Biology 2012; 18(2): 566-584. 10.1111/j.1365-2486.2011.02562.x
https://doi.org/10.1111/j.1365-2486.2011...
).
Abiotic factors such as temperature, precipitation and photoperiod are essential for triggering phenological events during plant growth (Tesfaye et al., 2011Tesfaye G, Teketay D, Fetene M, Beck E. Phenology of seven indigenous tree species in a dry Afromontane forest, southern Ethiopia. Tropical Ecology 2011; 52(3): 229-241.), as well as the genetic character of species (Kozlov et al., 2019Kozlov K, Singh A, Berger J, Wettberg EB, Kahraman A, Aydogan A et al. Non-linear regression models for time to flowering in wild chickpea combine genetic and climatic factors. BMC Plant Biology 2019; 19(Suppl 2) (94): 1-14. 10.1186/s12870-019-1685-2
https://doi.org/10.1186/s12870-019-1685-...
). Nevertheless, soil moisture is the main variable that influences the phenological patterns of a species (Singh & Singh, 1992Singh JS, Singh VK. Phenology of seasonally dry tropical forest. Current Science 1992; 63: 684-688.). As seasons are more evident in temperate regions than in tropical regions, greater seasonality in vegetative and reproductive phenology can be perceived in colder environments (Singh & Kushwaha, 2006Singh KP, Kushwaha CP. Diversity of flowering and fruiting phenology of trees in a tropical deciduous forest in India. Annals of Botany 2006; 97(2): 265-276. 10.1093/aob/mcj028
https://doi.org/10.1093/aob/mcj028...
). Seasonal rainfall associated with temperature and photoperiod can generate a phenological pattern in forest species in Southern Brazil, as observed by Bianchini et al. (2006Bianchini E, Pimenta JA, Santos FAM. Fenologia de Chrysophyllum gonocarpum (Mart. & Eichler) Engl. (Sapotaceae) em floresta semidecídua do Sul do Brasil. Revista Brasileira de Botânica 2006; 29(4): 595-602. 10.1590/S0100-84042006000400009
https://doi.org/10.1590/S0100-8404200600...
).
The objective of this study was to analyze the synchronization between the phenophases and to correlate vegetative and reproductive phenological aspects with abiotic factors in two forest formations (SSF and MOF) of the Atlantic Forest Biome where Gymnanthes klotzschiana species naturally occur.
2. MATERIALS AND METHODS
The phenological study was restricted to two areas. The first, in the municipality of Lages, SC (Area 1), is a forest fragment characterized as Mixed Alluvial Ombrophilous Forest (IBGE, 2012Instituto Brasileiro de Geografia e Estatística - IBGE. Manual técnico da vegetação brasileira: sistema fitogeográfico inventário das formações florestais e campestres técnicas e manejo de coleções botânicas procedimentos para mapeamentos. Rio de Janeiro; 2012.; Klein, 1960Klein RM. O aspecto dinâmico do pinheiro brasileiro. Sellowia 1960; 12(12): 17-44.), and located at the geographical coordinates: Latitude: −27.841221° and Longitude: −50.234878°. Area 2 is in the municipality of Capão Alto, SC, and is a forest fragment characterized as Montane Semi-deciduous Seasonal Forest (IBGE, 2012Instituto Brasileiro de Geografia e Estatística - IBGE. Manual técnico da vegetação brasileira: sistema fitogeográfico inventário das formações florestais e campestres técnicas e manejo de coleções botânicas procedimentos para mapeamentos. Rio de Janeiro; 2012.; Klein, 1960Klein RM. O aspecto dinâmico do pinheiro brasileiro. Sellowia 1960; 12(12): 17-44.), being located at the following geographical coordinates: Latitude: −28.195956° and Longitude: −50.751073°.
Ten individuals were studied in each research area, within the number suggested by Fournier & Charpantier (1975Fournier LAO, Charpantier C. El tamaño de la muestra y la frecuencia de las observaciones en el estudio da las características de los árboles tropicales. Turrialba 1975; 25(1): 45-48.). Only individuals above 20 cm in circumference at breast height (CBH) and at least 30 m apart between specimens were randomly selected in the two research areas. It is noteworthy that these individuals were at least 100 m from the edge in the two forest fragments studied.
Observations were performed monthly using Nikon 10 × 42 16031 Prostaff 3s binoculars, extending from May 2014 to May 2016. The following phenophases were assessed: (1) leaf fall: when the leaves were yellowish and fell easily by the wind or shaking the tree; (2) leaf budding: when there were small leaves (≤ 1 cm long) of green color; (3) flowering: inflorescences with flowers closed and open at the same time; (4) immature fruit: when the capsules were greenish; (5) ripe fruit: when the fruits were dark brown. The determination of leaf yield and fall patterns followed the classification proposed by Morellato et al. (1989Morellato LPC, Rodrigues RR, Leitão-Filho HF, Joly CA. Estudo comparativo da fenologia de espécies arbóreas de floresta de altitude e floresta mesófila semidecídua na Serra do Japi, Jundiaí, São Paulo. Revista Brasileira de Botânica 1989; 12(1/2): 85-98.), consisting of three categories: deciduous, semi-deciduous and perennial.
Phenological events were analyzed by the Activity Index (qualitative method), which assesses the presence or absence of phenophase in the individual, as well as the synchronization of each individual in the population in relation to each phenophase; and the Fournier Intensity Index (Fournier, 1974Fournier LA. Un método cuantitativo para la medición de características fenológicas en árboles. Turrialba 1974; 24: 422-423.), with values being within a semi-quantitative scale of five categories (0 to 4) and with a 25% interval between them. For this method, each month is the sum of the intensity values per individual and divided by the maximum possible value (number of individuals multiplied by four). The final value is multiplied by 100 to make it a percentage.
The Shapiro and Wilks test was performed to test the type of data distribution. The synchronization of the phenophases between consecutive 12-month periods was tested by the Spearman correlation (Zar, 2010Zar JH. Biostatistical analysis. New Jersey: Prentice-Hall; 2010.) using activity index data (p < 0.05). The relationships between different climatic variables and the activity index of each phenophase were also tested by the Spearman correlation (p < 0.05). The climatic data was obtained monthly from the Lages weather station, from May/2014 to May/2016 of INMET ([2016?]Instituto Nacional de Meteorologia - INMET. BDMEP [Internet]. [2016?] [cited 2016 July 9]. Available from: Available from: https://bit.ly/2LThkVG
https://bit.ly/2LThkVG...
). The length of the day was calculated by the formula N = 2hn/15, where N is the length of the day, and hn the angle of the sunrise time, which is determined by: hn = acos[−tan(ø)tan(δ)], where ø is the latitude of the site and δ is the solar declination. Solar declination is calculated by δ = 23.45sen[360/365(284 + NDY)] in which NDY is the number of days of the year (Ometto, 1981Ometto JC. Bioclimatologia vegetal. São Paulo: Ceres; 1981.). BioEstat 5.0 software was used for data analysis.
3. RESULTS
3.1. Mixed Alluvial Ombrophilous Forest (Area 1)
Leaf loss in G. klotzschiana showed synchronization between the two phenological periods (Table 1), and this result is reinforced by the presented activity peaks, which coincided between June and August of 2014 and 2015 (Figure 1c). This phenophase lasted from between May/2014 to October/2014, February/2015 and January/2016, and March to May of 2016. Peak intensity occurred in September/2014 (80%), August/2015 (80%) and May/2016 (47.5%) (Figure 1c). Leaf fall showed a significant (p < 0.0001) and negative (rs = −0.7860) correlation with day length, meaning that as days became longer, leaf loss became less expressive (Figure 1d). This phenophase also showed a negative and significant correlation with the mean maximum temperature (rs = −0.6308; p = 0.0007) and mean minimum temperature (rs = −0.6319; p = 0.0007), showing that there was an influence of temperature on leaf loss or maintenance (Table 2).
Association of climatic and phenological data in Gymnanthes klotzschiana for Area 1 (Lages, SC) using the Activity Index and the Fournier Intensity Index.
Leaf budding occurred between September and March in the first evaluation period (2014/2015) and from September to February in the second period (2015/2016), with correlation (Table 1). Peak activity was observed in October/2014, December/2014, September/2015 and October/2015 (100%). The maximum intensity observed was 35% in October/2014 and 42.5% in November/2015 (Figure 1c). There was a positive and significant correlation for day length (rs = 0.7240; p = < 0.0001), mean maximum temperature (rs = 0.4960; p = 0.0116) and mean minimum temperature (rs = 0.4799; p = 0.0152) in this phenophase (Table 2).
Regarding flowering, there was no correlation between the two study periods (Table 1), as the activity peaks occurred in October/2014 (90%), December/2015 (100%) and January/2016 (100%). The highest intensity indices were recorded in October/2014 (25%) and December/2015 (47.5%) (Figure 1e). There was a positive and significant correlation with the mean maximum temperature (rs = 0.5118; p = 0.0089), mean minimum temperature (rs = 0.5645; p = 0.0033) and day length (rs = 0.6510; p = 0.0004) (Table 2).
Immature and ripe fruits showed significant correlation (Table 1), indicating that fruiting occurred at regular intervals. Peak activity in immature fruits was identified in January/2015 (60%) and December/2015 (70%), and the maximum intensity reached was recorded in January/2015 (25%) and December/2015 (22.5%) (Figure 1f). The largest production of ripe fruits was concentrated in January/2015 (20%) and January/2016 (15%). The activity index achieved was 60% for these same previously mentioned periods (Figure 1g). These two phenophases only had no significant correlation with precipitation (Table 2).
3.2. Montane Semi-deciduous Seasonal Forest (Area 2)
Both cycles in this area presented synchronization regarding leaf loss (Table 3). Peak activity occurred between June and August 2014 (100%), and in 2015 between April and July (100%). Intensity peaks were recorded in August/2014 (72.5%) and June/2015 (70%) (Figure 2c). Leaf fall had a significant negative correlation with day length (rs = −0.8730; p = <0.0001), mean maximum temperature (rs = −0.7791; p = < 0.0001) and mean minimum temperature (rs = −0.7814; p = < 0.0001) (Table 4).
Association of climatic and phenological data in Gymnanthes klotzschiana for Area 2 (Capão Alto, SC) using the Activity Index and the Fournier Intensity Index.
Leaf budding showed correlation between the two study periods (Table 3). The activity and intensity peaks coincided for the same periods, which was 100% and 52.5% for October/2014, respectively, while for September/2015 it was 90% (activity) and 40% (intensity) (Figure 2d). Day length was the only environmental factor that showed a significant correlation with leaf budding (rs = 0.4613; p = 0.0202) (Table 4).
Flowering showed no correlation between the two years of evaluation (Table 3). Both activity and intensity peaks were low in this phenophase and coincided over time, with 30% for activity and 7.5% for intensity in November/2014, and 20% and 12.5% in December/2015, respectively (Figure 2e). Flowering was positively correlated with day length (rs = 0.5829; p = 0.0022), and with mean, maximum (rs = 0.4593; p = 0.0209) and minimum (rs = 0.4955; p = 0.0117) temperatures (Table 4).
There was no correlation for immature and ripe fruit (Table 3). Activity rates were low for both ripening stages, reaching immature fruit peaks of 50% (December/2014) and 10% (February and March/2016). The highest activity achieved for ripe fruit was 30% in January/2015 and 10% in March/2016 (Figure 2f; Figure 2g). Intensity peaks occurred for immature fruit in December/2014 (15%), and February and March/2016 (5%), while the highest rates for ripe fruit were found in January/2015 (12.5%) and March/2016 (2.5%). Immature fruit was only correlated with day length (rs = 0.4504; p = 0.0238). However, there was no correlation in ripe fruit, only with total precipitation (Table 4).
4. DISCUSSION
Except for precipitation, the phenophases showed significant correlations with abiotic factors, indicating that the climatic variables had seasonal behavior in both areas, i.e. they had a direct influence on the G. klotzschiana phenological pattern. Morellato & Leitão Filho (1990Morellato LPC, Leitão Filho HF. Estratégias fenológicas de espécies arbóreas em floresta mesófila na Serra do Japi, Jundiaí, São Paulo. Revista Brasileira de Biologia 1990; 50: 163-173.) point out that environmental factors have less influence on the phenophases in non-seasonal environments. Marques (2007Marques MCM. Fenologia no limite sul da região tropical: padrões e algumas interpretações. In: Rego GM, Negrelle RRB, Morellato LPC, editors. Fenologia: ferramenta para conservação, melhoramento e manejo de recursos vegetais arbóreos. Colombo: Embrapa Florestas; 2007. p. 101-112.) comments that Southern Brazil is an ecotone between the tropical and subtropical climate, which presents well-distributed rainfall during the year. Thus, phenological patterns in this region are more related to temperature and photoperiod (Morellato et al., 2000Morellato LPC, Talora DC, Takahasi A, Bencke CSC, Romera EC, Zipparro V. Phenology of Atlantic rain forest trees: a comparative study. Biotropica 2000; 32(4b): 811-823. 10.1111/j.1744-7429.2000.tb00620.x
https://doi.org/10.1111/j.1744-7429.2000...
).
The defoliation pattern in G. klotzschiana was characterized as semi-deciduous for both analyzed areas. This leaf loss pattern had already been observed in this species by Athayde et al. (2009Athayde EA, Giehl ELH, Budke JC, Gesing JPA, Eisinger SM. Fenologia de espécies arbóreas em uma floresta ribeirinha em Santa Maria, sul do Brasil. Revista Brasileira de Biociências 2009; 7(1): 43-51.) in a riverside forest, where the loss of 50% of leaves in the analyzed individuals occurred between June and September. Leaf fall presented seasonality, where it was shown that this event predominantly occurs with greater activity and intensity in the winter; however, in autumn and spring it happens more mildly. Seasonality for leaf fall was strongly correlated with mild temperatures and a shorter photoperiod, suggesting that these factors may act as triggers for leaf loss in G. klotzschiana. Marchioretto et al. (2007Marchioretto MA, Mauhs J, Budke JC. Fenologia de espécies arbóreas zoocóricas em uma floresta psamófila no sul do Brasil. Acta Botanica Brasilica 2007; 21(1): 193-201. 10.1590/S0102-33062007000100018
https://doi.org/10.1590/S0102-3306200700...
) and Athayde et al. (2009Athayde EA, Giehl ELH, Budke JC, Gesing JPA, Eisinger SM. Fenologia de espécies arbóreas em uma floresta ribeirinha em Santa Maria, sul do Brasil. Revista Brasileira de Biociências 2009; 7(1): 43-51.) also observed this scenario in several tree species, where leaf fall occurs when there are low temperatures and shorter day length. Marques et al. (2004Marques MCM, Roper JJ, Salvalaggio APB. Phenological patterns among plant life-forms in a subtropical forest in southern Brazil. Plant Ecology 2004; 173(2): 203-213. 10.1023/B:VEGE.0000029325.85031.90
https://doi.org/10.1023/B:VEGE.000002932...
) observed similar behavior for a Mixed Ombrophilous Forest area in the state of Paraná.
Even though precipitation did not significantly correlate with leaf loss, this environmental factor influenced defoliation, especially for Area 1, an alluvial environment. Defoliation was more pronounced in 2015 when compared to 2014 due to harsher rainfall, especially between September and October, which caused almost permanent flooding until December of 2015. Although rainfall was intense during the aforementioned period for Area 2, leaf fall did not occur at the same intensity and moment, because water did not accumulate in the soil due to the slope of the land. Leaf fall can be explained by a lack of oxygen in the soil and is understandable because photosynthesis requires oxygen for energy production and other compounds (Taiz & Zeiger, 2013Taiz L, Zeiger E. Fisiologia vegetal. 5. ed. Porto Alegre: Artmed; 2013.).
Budding occurred more intensely after the leaf fall period. The emission of new leaves was seasonal during both cycles and showed no significant positive correlations with rainfall, as the study region is characterized by the presence of well-distributed rainfall (Leite, 2002Leite PF. Contribuição ao conhecimento fitoecológico do Sul do Brasil. Ciência e Ambiente 2002; 24(1): 51-73.). Some phenological studies report that photoperiod and higher temperatures may induce budding (Marchioretto et al., 2007Marchioretto MA, Mauhs J, Budke JC. Fenologia de espécies arbóreas zoocóricas em uma floresta psamófila no sul do Brasil. Acta Botanica Brasilica 2007; 21(1): 193-201. 10.1590/S0102-33062007000100018
https://doi.org/10.1590/S0102-3306200700...
) with the emergence of new leaves signaling the transition from winter to spring and the beginning of the growing season in temperate forests (Polgar & Primack, 2011Polgar CA, Primack RB. Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytologist 2011; 191: 926-941. 10.1111/j.1469-8137.2011.03803.x
https://doi.org/10.1111/j.1469-8137.2011...
). The day length was the environmental variable that most influenced the leaf budding in both evaluation areas, as this tends to optimize the photosynthetic production rates (DaMatta et al., 2007DaMatta FM, Ronchi CP, Maestri M, Barros RS. Ecophysiology of coffee growth and production. Brazilian Journal of Plant Physiology 2007; 19(4): 485-510. 10.1590/S1677-04202007000400014
https://doi.org/10.1590/S1677-0420200700...
) and consequently accumulates reserves aiming at higher quality seed production (Santos et al., 2012Santos SB, Martins MA, Aguilar PRM, Caneschi AL, Carneiro ACO, Dias LAS. Acúmulo de matéria seca e óleo nas sementes de pinhão-manso e qualidade do óleo extraído. Revista Brasileira de Engenharia Agrícola e Ambiental 2012; 16(2): 209-215. 10.1590/S1415-43662012000200012
https://doi.org/10.1590/S1415-4366201200...
).
There was intense leaf renewal, as both leaf fall and budding occurred during most studies. This probably demonstrates that the branquilho is an important species in providing food for herbivores (Araújo, 2013Araújo WS. A importância de fatores temporais para a distribuição de insetos herbívoros em sistemas neotropicais. Revista da Biologia 2013; 10(1): 1-7. 10.7594/revbio.10.01.01
https://doi.org/10.7594/revbio.10.01.01...
; Farias & Xavier, 2011Farias RP, Xavier SRS. Fenologia e sobrevivência de três populações de samambaias em remanescente de Floresta Atlântica Nordestina, Paraíba, Brasil. Revista Biotemas 2011; 24(2): 13-20. 10.5007/2175-7925.2011v24n2p13
https://doi.org/10.5007/2175-7925.2011v2...
; Nunes et al., 2008Nunes YRF, Fagundes M, Almeida HS, Veloso MDM. Aspectos ecológicos da aroeira (Myracrodruon urundeuva Allemão - anacardiaceae): fenologia e germinação de sementes. Revista Árvore 2008; 32(2): 233-243. 10.1590/S0100-67622008000200006
https://doi.org/10.1590/S0100-6762200800...
), and can also use this strategy to improve seed germination because there would be less restriction on the solar radiation entry in this condition and consequently the formation of a faster seedling bank.
The flowering pattern can be considered as regular or seasonal and annual according to the classification of Newstrom et al. (1994Newstrom LE, Frankie GW, Baker HGA. A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La selva, Costa Rica. Biotropica 1994; 26(2): 141-159. 10.2307/2388804
https://doi.org/10.2307/2388804...
). This is because flowering occurred in similar periods in the two analyzed areas, especially in October and November in the analyzed cycles. Athayde et al. (2009Athayde EA, Giehl ELH, Budke JC, Gesing JPA, Eisinger SM. Fenologia de espécies arbóreas em uma floresta ribeirinha em Santa Maria, sul do Brasil. Revista Brasileira de Biociências 2009; 7(1): 43-51.) reported G. klotzschiana flowering from August to October in 75% of the individuals evaluated. Annual flowering behavior was observed in other species of the Atlantic Forest Biome, such as Inga edulis and Trema micrantha (Pereira et al., 2008Pereira TS, Costa MLMN, Moraes LFD, Luchiari C. Fenologia de espécies arbóreas em Floresta Atlântica da Reserva Biológica de Poço das Antas, Rio de Janeiro, Brasil. Iheringia 2008; 63(2): 329-339.). There was no correlation between flowering and precipitation, as this phenophase occurred after the rainy season. This behavior may be associated with greater protection of reproductive organs and pollinator efficiency, as intense rainfall can damage floral parts, affecting seed production (Guedes et al., 2009Guedes RS, Alves EU, Gonçalves EP, Bruno RLA, Braga JM Jr, Medeiros MS. Germinação de sementes de Cereus jamacaru DC. em diferentes substratos e temperaturas. Acta Scientiarum. Biological Sciences 2009; 31(2): 159-164. 10.4025/actascibiolsci.v31i2.635
https://doi.org/10.4025/actascibiolsci.v...
; Tonini, 2013Tonini H. Amostragem para a estimativa de produção de sementes de castanheira-do-brasil em floresta nativa. Pesquisa Agropecuária Brasileira 2013; 48(5): 519-527.). Longer day length and higher temperatures are determinant in flowering induction (Ziparro & Morellato, 2007Ziparro VB, Morellato LPC. Fenologia reprodutiva da comunidade arbórea em Floresta Atlântica no Sudeste do Brasil: um estudo de seis anos. In: Rego GM, Negrelle RRB, Morellato LPC, editors. Fenologia: ferramenta para conservação, melhoramento e manejo de recursos vegetais arbóreos. Colombo: Embrapa Florestas; 2007. p. 113-126.), which in the case of branquilho was positively and significantly correlated in both study areas. Liebsch & Mikich (2009Liebsch D, Mikich SB. Fenologia reprodutiva de espécies vegetais da Floresta Ombrófila Mista do Paraná, Brasil. Revista Brasileira de Botânica 2009; 32(2): 375-391. 10.1590/S0100-84042009000200016
https://doi.org/10.1590/S0100-8404200900...
), Cascaes et al. (2013Cascaes MF, Citadini-Zanette V, Harter-Marques B. Reproductive phenology in a riparian rainforest in the south of Santa Catarina state, Brazil. Anais da Academia Brasileira de Ciências 2013; 85(4): 1449-1460. 10.1590/0001-37652013105112
https://doi.org/10.1590/0001-37652013105...
) and Roberts et al. (2015Roberts AMI, Tansey C, Smithers RJ, Phillimore AB. Predicting a change in the order of spring phenology in temperate forests. Global Change Biology 2015; 21(7): 2603-2611. 10.1111/gcb.12896
https://doi.org/10.1111/gcb.12896...
), pointed to a seasonal pattern for flowering, especially when there is a transition from colder to higher temperatures. These abiotic factors may also be related to the presence of pollinators, dispersers and predators, which exert a strong direct influence on phenological behavior (Morellato et al., 1989Morellato LPC, Rodrigues RR, Leitão-Filho HF, Joly CA. Estudo comparativo da fenologia de espécies arbóreas de floresta de altitude e floresta mesófila semidecídua na Serra do Japi, Jundiaí, São Paulo. Revista Brasileira de Botânica 1989; 12(1/2): 85-98.).
There was low flower production in Area 1 between September and December of 2015, as the environment was competently flooded in this time interval. There was higher flower production after the end of the flood in this area when compared to the previous cycle (2014), showing high adaptability of this species to this lack of oxygen. This higher flower production may be linked to the urgency of the species to leave more descendants, since the production of ripe fruits after flowering takes around three months, which coincided with the beginning of leaf loss, i.e. with the lowest physiological metabolism of G. klotzschiana individuals.
The production of immature and ripe fruits indicated that the species presents an annual fruiting pattern (Newstrom et al., 1994Newstrom LE, Frankie GW, Baker HGA. A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La selva, Costa Rica. Biotropica 1994; 26(2): 141-159. 10.2307/2388804
https://doi.org/10.2307/2388804...
) in both analyzed areas. There was higher numeric activity and intensity of fruit production in Area 1, which highlights the greater adaptability of this species to alluvial environments. These phenophases also show a seasonal pattern, and there was a positive correlation for longer days and higher temperatures (especially for Area 1); however, there was no relationship with rainfall in the period. This same behavior has already been observed in some tree species located in an Atlantic Forest of Southern Brazil (Marchioretto et al., 2007Marchioretto MA, Mauhs J, Budke JC. Fenologia de espécies arbóreas zoocóricas em uma floresta psamófila no sul do Brasil. Acta Botanica Brasilica 2007; 21(1): 193-201. 10.1590/S0102-33062007000100018
https://doi.org/10.1590/S0102-3306200700...
). High temperatures associated with low rainfall may be fundamental in seed dispersal. Since branquilho is an autochorous species, this climatic condition could favor natural opening of the capsules and consequently more efficient propagule dispersion. Branquilho trees can be an important management tool to support the diversity of frugivores (Bianchini et al., 2015Bianchini E, Emmerick JM, Messetti AVL, Pimenta JA. Phenology of two Ficus species in seasonal semi-deciduous forest in Southern Brazil. Brazilian Journal of Biology 2015; 75(4): 206-214. 10.1590/1519-6984.10614
https://doi.org/10.1590/1519-6984.10614...
), especially in MOF in which the relative density of this species is usually high (Silva et al., 2012Silva AC, Higuchi H, Van Den Berg E, Nunes MH, Carvalho DA. Florestas inundáveis: ecologia, florística e adaptações das espécies. Lavras: Editora UFLA; 2012.).
Regarding fruit and seed production, the results show an important strategy developed by the species as a way to provide bird fauna with food for a longer period of time, since the trees remained with ripe fruits for up to two months in both areas. Phenological studies which include an evaluation of avifauna resource availability may contribute to understanding the animal-plant relationship and are fundamental for biodiversity conservation (Patricio et al., 2014Patricio RS, Pereira AS, Elias SG, Furlanetto CB, Cascaes MF, Vinholes AR et al. Apifauna (Hymenoptera: Apidae) em área de restinga no Sul de Santa Catarina, Brasil. Revista Tecnologia e Ambiente 2014; 21: 248-269. 10.18616/ta.v20i0.1761
https://doi.org/10.18616/ta.v20i0.1761...
; Pereira et al., 2008Pereira TS, Costa MLMN, Moraes LFD, Luchiari C. Fenologia de espécies arbóreas em Floresta Atlântica da Reserva Biológica de Poço das Antas, Rio de Janeiro, Brasil. Iheringia 2008; 63(2): 329-339.).
5. CONCLUSIONS
All analyzed phenophases showed significant correlation with day length and none correlated with precipitation, showing that the photoperiod was the climatic variable with the greatest influence on the phenological behavior of G. klotzschiana in both study areas.
Leaf loss characterized the species as semi-deciduous. Leaf fall and budding at both sites were synchronized during the two years of evaluation, being more active and intense in Area 1.
Flowering and fruiting were characterized as annual in both study areas. The highest activity in flowering was observed in Area 1, especially for the second cycle when there was a longer flooding period, suggesting that this species is more adaptable to oxygen restriction conditions.
The highest activity of immature and ripe fruits occurred for the area most prone to flooding (Area 1), indicating that G. klotzschiana prefers wet soils to develop and reproduce.
ACKNOWLEDGEMENTS
To the entire Centro de Ciências Agroveterinárias of Universidade do Estado de Santa Catarina (Udesc) forest seed laboratory team that was involved in this project.
REFERENCES
- Araújo WS. A importância de fatores temporais para a distribuição de insetos herbívoros em sistemas neotropicais. Revista da Biologia 2013; 10(1): 1-7. 10.7594/revbio.10.01.01
» https://doi.org/10.7594/revbio.10.01.01 - Athayde EA, Giehl ELH, Budke JC, Gesing JPA, Eisinger SM. Fenologia de espécies arbóreas em uma floresta ribeirinha em Santa Maria, sul do Brasil. Revista Brasileira de Biociências 2009; 7(1): 43-51.
- Bianchini E, Emmerick JM, Messetti AVL, Pimenta JA. Phenology of two Ficus species in seasonal semi-deciduous forest in Southern Brazil. Brazilian Journal of Biology 2015; 75(4): 206-214. 10.1590/1519-6984.10614
» https://doi.org/10.1590/1519-6984.10614 - Bianchini E, Pimenta JA, Santos FAM. Fenologia de Chrysophyllum gonocarpum (Mart. & Eichler) Engl. (Sapotaceae) em floresta semidecídua do Sul do Brasil. Revista Brasileira de Botânica 2006; 29(4): 595-602. 10.1590/S0100-84042006000400009
» https://doi.org/10.1590/S0100-84042006000400009 - Cascaes MF, Citadini-Zanette V, Harter-Marques B. Reproductive phenology in a riparian rainforest in the south of Santa Catarina state, Brazil. Anais da Academia Brasileira de Ciências 2013; 85(4): 1449-1460. 10.1590/0001-37652013105112
» https://doi.org/10.1590/0001-37652013105112 - DaMatta FM, Ronchi CP, Maestri M, Barros RS. Ecophysiology of coffee growth and production. Brazilian Journal of Plant Physiology 2007; 19(4): 485-510. 10.1590/S1677-04202007000400014
» https://doi.org/10.1590/S1677-04202007000400014 - Farias RP, Xavier SRS. Fenologia e sobrevivência de três populações de samambaias em remanescente de Floresta Atlântica Nordestina, Paraíba, Brasil. Revista Biotemas 2011; 24(2): 13-20. 10.5007/2175-7925.2011v24n2p13
» https://doi.org/10.5007/2175-7925.2011v24n2p13 - Fournier LA. Un método cuantitativo para la medición de características fenológicas en árboles. Turrialba 1974; 24: 422-423.
- Fournier LAO, Charpantier C. El tamaño de la muestra y la frecuencia de las observaciones en el estudio da las características de los árboles tropicales. Turrialba 1975; 25(1): 45-48.
- Guedes RS, Alves EU, Gonçalves EP, Bruno RLA, Braga JM Jr, Medeiros MS. Germinação de sementes de Cereus jamacaru DC. em diferentes substratos e temperaturas. Acta Scientiarum. Biological Sciences 2009; 31(2): 159-164. 10.4025/actascibiolsci.v31i2.635
» https://doi.org/10.4025/actascibiolsci.v31i2.635 - Instituto Brasileiro de Geografia e Estatística - IBGE. Manual técnico da vegetação brasileira: sistema fitogeográfico inventário das formações florestais e campestres técnicas e manejo de coleções botânicas procedimentos para mapeamentos. Rio de Janeiro; 2012.
- Instituto Nacional de Meteorologia - INMET. BDMEP [Internet]. [2016?] [cited 2016 July 9]. Available from: Available from: https://bit.ly/2LThkVG
» https://bit.ly/2LThkVG - Klein RM. O aspecto dinâmico do pinheiro brasileiro. Sellowia 1960; 12(12): 17-44.
- Kozlov K, Singh A, Berger J, Wettberg EB, Kahraman A, Aydogan A et al. Non-linear regression models for time to flowering in wild chickpea combine genetic and climatic factors. BMC Plant Biology 2019; 19(Suppl 2) (94): 1-14. 10.1186/s12870-019-1685-2
» https://doi.org/10.1186/s12870-019-1685-2 - Leite PF. Contribuição ao conhecimento fitoecológico do Sul do Brasil. Ciência e Ambiente 2002; 24(1): 51-73.
- Liebsch D, Mikich SB. Fenologia reprodutiva de espécies vegetais da Floresta Ombrófila Mista do Paraná, Brasil. Revista Brasileira de Botânica 2009; 32(2): 375-391. 10.1590/S0100-84042009000200016
» https://doi.org/10.1590/S0100-84042009000200016 - Marchioretto MA, Mauhs J, Budke JC. Fenologia de espécies arbóreas zoocóricas em uma floresta psamófila no sul do Brasil. Acta Botanica Brasilica 2007; 21(1): 193-201. 10.1590/S0102-33062007000100018
» https://doi.org/10.1590/S0102-33062007000100018 - Marques MCM, Roper JJ, Salvalaggio APB. Phenological patterns among plant life-forms in a subtropical forest in southern Brazil. Plant Ecology 2004; 173(2): 203-213. 10.1023/B:VEGE.0000029325.85031.90
» https://doi.org/10.1023/B:VEGE.0000029325.85031.90 - Marques MCM. Fenologia no limite sul da região tropical: padrões e algumas interpretações. In: Rego GM, Negrelle RRB, Morellato LPC, editors. Fenologia: ferramenta para conservação, melhoramento e manejo de recursos vegetais arbóreos. Colombo: Embrapa Florestas; 2007. p. 101-112.
- Melaas EK, Friedl MA, Richardson AD. Multiscale modeling of spring phenology across Deciduous Forests in the Eastern United States. Global Change Biology 2016; 22(2): 792-805. 10.1111/gcb.13122
» https://doi.org/10.1111/gcb.13122 - Morellato LPC, Leitão Filho HF. Estratégias fenológicas de espécies arbóreas em floresta mesófila na Serra do Japi, Jundiaí, São Paulo. Revista Brasileira de Biologia 1990; 50: 163-173.
- Morellato LPC, Rodrigues RR, Leitão-Filho HF, Joly CA. Estudo comparativo da fenologia de espécies arbóreas de floresta de altitude e floresta mesófila semidecídua na Serra do Japi, Jundiaí, São Paulo. Revista Brasileira de Botânica 1989; 12(1/2): 85-98.
- Morellato LPC, Talora DC, Takahasi A, Bencke CSC, Romera EC, Zipparro V. Phenology of Atlantic rain forest trees: a comparative study. Biotropica 2000; 32(4b): 811-823. 10.1111/j.1744-7429.2000.tb00620.x
» https://doi.org/10.1111/j.1744-7429.2000.tb00620.x - Muhanguzi HDR, Ipulet P. Fruiting phenology of fig trees in Kalinzu Forest, Uganda. African Journal of Ecology 2011; 50(1): 90-101. 10.1111/j.1365-2028.2011.01301.x
» https://doi.org/10.1111/j.1365-2028.2011.01301.x - Newstrom LE, Frankie GW, Baker HGA. A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La selva, Costa Rica. Biotropica 1994; 26(2): 141-159. 10.2307/2388804
» https://doi.org/10.2307/2388804 - Nunes YRF, Fagundes M, Almeida HS, Veloso MDM. Aspectos ecológicos da aroeira (Myracrodruon urundeuva Allemão - anacardiaceae): fenologia e germinação de sementes. Revista Árvore 2008; 32(2): 233-243. 10.1590/S0100-67622008000200006
» https://doi.org/10.1590/S0100-67622008000200006 - Ometto JC. Bioclimatologia vegetal. São Paulo: Ceres; 1981.
- Patricio RS, Pereira AS, Elias SG, Furlanetto CB, Cascaes MF, Vinholes AR et al. Apifauna (Hymenoptera: Apidae) em área de restinga no Sul de Santa Catarina, Brasil. Revista Tecnologia e Ambiente 2014; 21: 248-269. 10.18616/ta.v20i0.1761
» https://doi.org/10.18616/ta.v20i0.1761 - Pereira TS, Costa MLMN, Moraes LFD, Luchiari C. Fenologia de espécies arbóreas em Floresta Atlântica da Reserva Biológica de Poço das Antas, Rio de Janeiro, Brasil. Iheringia 2008; 63(2): 329-339.
- Polgar CA, Primack RB. Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytologist 2011; 191: 926-941. 10.1111/j.1469-8137.2011.03803.x
» https://doi.org/10.1111/j.1469-8137.2011.03803.x - Rego GM, Lavaronti OJ. Atividades fenológicas de Imbuia (Octea porosa (Nees et. Martius ex. Ness) em áreas de floresta ombrófila mista, no estado do Paraná. In: Rego GM, Negrelle RRB, Morellato LPC, editors. Fenologia: ferramenta para conservação, melhoramento e manejo de recursos vegetais arbóreos. Colombo: Embrapa Florestas; 2007. p. 181-202.
- Richardson AD, Anderson RS, Arain MA, Barr AG, Bohrer G, Chen G, et al. Terrestrial biosphere models need better representation of vegetation phenology: results from the North American Carbon Program Site Synthesis. Global Change Biology 2012; 18(2): 566-584. 10.1111/j.1365-2486.2011.02562.x
» https://doi.org/10.1111/j.1365-2486.2011.02562.x - Roberts AMI, Tansey C, Smithers RJ, Phillimore AB. Predicting a change in the order of spring phenology in temperate forests. Global Change Biology 2015; 21(7): 2603-2611. 10.1111/gcb.12896
» https://doi.org/10.1111/gcb.12896 - Santos SB, Martins MA, Aguilar PRM, Caneschi AL, Carneiro ACO, Dias LAS. Acúmulo de matéria seca e óleo nas sementes de pinhão-manso e qualidade do óleo extraído. Revista Brasileira de Engenharia Agrícola e Ambiental 2012; 16(2): 209-215. 10.1590/S1415-43662012000200012
» https://doi.org/10.1590/S1415-43662012000200012 - Silva AC, Higuchi H, Van Den Berg E, Nunes MH, Carvalho DA. Florestas inundáveis: ecologia, florística e adaptações das espécies. Lavras: Editora UFLA; 2012.
- Singh JS, Singh VK. Phenology of seasonally dry tropical forest. Current Science 1992; 63: 684-688.
- Singh KP, Kushwaha CP. Diversity of flowering and fruiting phenology of trees in a tropical deciduous forest in India. Annals of Botany 2006; 97(2): 265-276. 10.1093/aob/mcj028
» https://doi.org/10.1093/aob/mcj028 - Taiz L, Zeiger E. Fisiologia vegetal. 5. ed. Porto Alegre: Artmed; 2013.
- Tesfaye G, Teketay D, Fetene M, Beck E. Phenology of seven indigenous tree species in a dry Afromontane forest, southern Ethiopia. Tropical Ecology 2011; 52(3): 229-241.
- Tonini H. Amostragem para a estimativa de produção de sementes de castanheira-do-brasil em floresta nativa. Pesquisa Agropecuária Brasileira 2013; 48(5): 519-527.
- Zar JH. Biostatistical analysis. New Jersey: Prentice-Hall; 2010.
- Ziparro VB, Morellato LPC. Fenologia reprodutiva da comunidade arbórea em Floresta Atlântica no Sudeste do Brasil: um estudo de seis anos. In: Rego GM, Negrelle RRB, Morellato LPC, editors. Fenologia: ferramenta para conservação, melhoramento e manejo de recursos vegetais arbóreos. Colombo: Embrapa Florestas; 2007. p. 113-126.
-
1
Associate editor: Henrique Machado Dias 0000-0003-2217-7846
Publication Dates
-
Publication in this collection
01 July 2020 -
Date of issue
2020
History
-
Received
04 Sept 2017 -
Accepted
18 Aug 2019


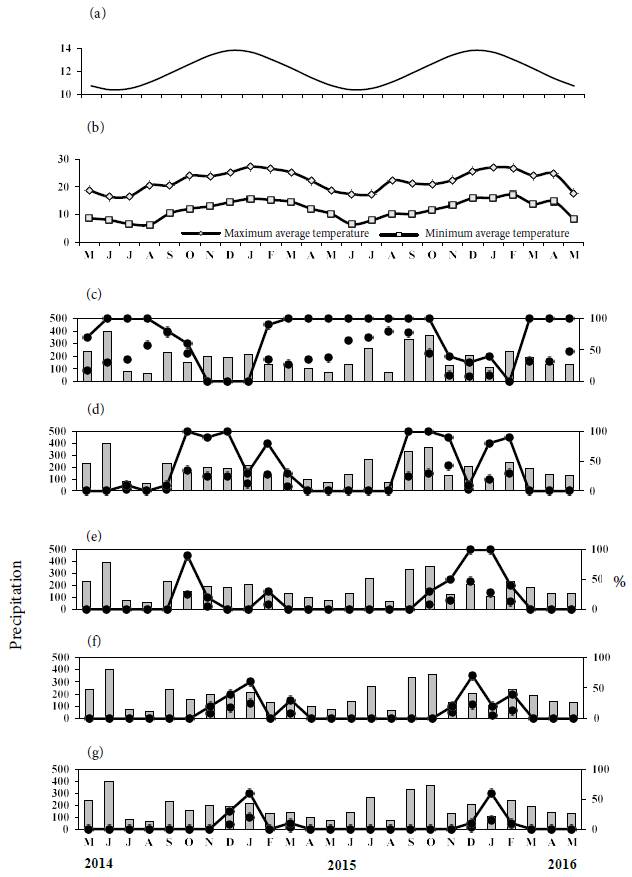 Percentage of activity index (- • ---) and Fournier intensity (- - • - -); Bars: total monthly precipitation in the period; A: day length; B: temperature; C: leaf fall; D: leaf budding; E: flowering; F: immature fruit; G: ripe fruit.
Percentage of activity index (- • ---) and Fournier intensity (- - • - -); Bars: total monthly precipitation in the period; A: day length; B: temperature; C: leaf fall; D: leaf budding; E: flowering; F: immature fruit; G: ripe fruit.
 Percentage of activity index (--- • ---) and Fournier intensity (- - • - -); Bars: Total monthly precipitation in the period; A: Length of Day; B: Temperature; C: Leaf fall; D: Leaf budding; E: Flowering; F: Immature fruit; G: Ripe Fruits
Percentage of activity index (--- • ---) and Fournier intensity (- - • - -); Bars: Total monthly precipitation in the period; A: Length of Day; B: Temperature; C: Leaf fall; D: Leaf budding; E: Flowering; F: Immature fruit; G: Ripe Fruits