Abstract
Meiotic and mitotic chromosomes of Isocopris inhiata and Diabroctis mimas were studied by standard staining procedures, C-banding, silver nitrate staining and FISH using Apis mellifera 28S rDNA as probe. Isocopris inhiata presented a 2n = 18 (8II+ Xy p) karyotype, composed of meta-submetacentric chromosomes with gradual reduction in size. The karyotype of D. mimas was 2n = 20 (9II+ Xy p), composed of meta-submetacentric (pairs 1, 2, 3, 4 and 7) and acrocentric (pairs 5, 6, 8 and 9) chromosomes, with gradual reduction in size. Analysis of constitutive heterochromatin revealed similar C-banding patterns for both species, showing pericentromeric and telomeric bands and diphasic chromosomes. In addition, the X chromosomes of these species were found to be almost completely heterochromatic. The presence of chromocenters was checked in one or more phases of prophase I of these species. All heterochromatin reacted positively for the silver stain. By FISH analysis we were able to locate the rDNA in medium-size autosome pairs in both species and in the X chromosome of D. mimas.
Coleoptera; heterochromatin; NORs; FISH
ANIMAL GENETICS
RESEARCH ARTICLE
A study of the constitutive heterochromatin and nucleolus organizer regions of Isocopris inhiata and Diabroctis mimas (Coleoptera: Scarabaeidae, Scarabaeinae) using C-banding, AgNO3 staining and FISH techniques
Edgar BioneI; Marjori Leiva CamparotoI; Zilá Luz Paulino SimõesII
IUniversidade de São Paulo, Faculdade de Medicina de Ribeirão Preto, Ribeirão Preto, SP, Brazil
IIUniversidade de São Paulo, Escola de Filosifia, Ciências e Letras, Ribeirão Preto, SP, Brazil
Correspondence Correspondence to Edgar Bione Universidade de São Paulo, Escola de Medicina de Ribeirão Preto, Departamento de Genética Av. Bandeirantes 3900, Monte Alegre 14049-900 Ribeirão Preto, SP, Brazil E-mail: edgarbione@rge.fmrp.usp.br
ABSTRACT
Meiotic and mitotic chromosomes of Isocopris inhiata and Diabroctis mimas were studied by standard staining procedures, C-banding, silver nitrate staining and FISH using Apis mellifera 28S rDNA as probe. Isocopris inhiata presented a 2n = 18 (8II+ Xyp) karyotype, composed of meta-submetacentric chromosomes with gradual reduction in size. The karyotype of D. mimas was 2n = 20 (9II+ Xyp), composed of meta-submetacentric (pairs 1, 2, 3, 4 and 7) and acrocentric (pairs 5, 6, 8 and 9) chromosomes, with gradual reduction in size. Analysis of constitutive heterochromatin revealed similar C-banding patterns for both species, showing pericentromeric and telomeric bands and diphasic chromosomes. In addition, the X chromosomes of these species were found to be almost completely heterochromatic. The presence of chromocenters was checked in one or more phases of prophase I of these species. All heterochromatin reacted positively for the silver stain. By FISH analysis we were able to locate the rDNA in medium-size autosome pairs in both species and in the X chromosome of D. mimas.
Key words: Coleoptera, heterochromatin, NORs, FISH.
Introduction
Scarabaeidae constitutes the largest family within the superfamily Scarabaeoidea, forming a cosmopolitan group and one of the richest in species number within the order Coleoptera. It comprises approximately 2,300 genera and 27,000 species of worldwide distribution. About 204 genera and 1,800 species have been described for Brazil (Crowson, 1967; Costa et al., 1988). The Scarabaeinae constitute a highly diverse subfamily that comprises about 5,000 described species belonging to 234 genera spread widely in the world (Hanski and Cambefort, 1991).
Representatives of about 320 species comprising 21 Scarabaeidae subfamilies are cytogenetically characterized to date. The chromosome numbers and morphology in this family are highly conserved, predominantly presenting a diploid number of 2n = 20, a sex chromosome mechanism of the "parachute" Xy type (Xyp), and metacentric, submetacentric and acrocentric chromosomes (Smith and Virkki, 1978; Yadav and Pillai, 1979; Colomba et al., 1996; Bione, 1999; Moura et al., 2003). This chromosome number is considered as being the most primitive within the order Coleoptera. Most of the 85 Scarabaeinae species which are known cytogenetically have a chromosome number varying from 2n = 12 to 2n = 20, with the Xyp type being the most prevalent sex chromosome determination system (Smith and Virkki, 1978; Vidal, 1984; Colomba et al., 2000a).
The order Coleoptera has the highest species diversity within the animal kingdom, yet cytogenetic data using specific banding techniques are still scarce. C-banding data have revealed a preferential localization of autosomal constitutive heterochromatin (CH) in the centromeric area and less so observed in interstitial and telomeric areas. Sex chromosomes also show a variable CH distribution, as it has been observed in the pericentromeric region or along the entire chromosome (Ennis, 1974; Vidal et al., 1977; Angus, 1983; Drets et al., 1983; Virkki, 1983; Juan and Petitpierre, 1989).
AgNO3 staining of both mitotic and meiotic chromosomes of eukaryotic species has been a very useful approach for the analysis of the structure and variability of nucleoli, nucleolar organizer regions (NORs) and kinetochores (Goodpasture and Bloom, 1975; Virkki and Denton, 1987; Virkki et al., 1991). Although in Coleoptera this technique has been considered unsuitable to identify active rDNA clusters (Colomba et al., 2000a), it is generally accepted that silver impregnation is indicative of rRNA synthesis and that it stains functionally active NORs (Miller et al., 1976; Hubbell, 1985). However, in most genomes transcriptionally active and inactive rRNA genes usually coexist (López-León et al., 1999).
Recently, the FISH method has been employed in a great number of species to locate specific DNA sequences directly on the chromosomes. This approach has been used mainly for mapping different types of repetitive DNA sequences, including ribosomal DNA (Vitturi et al., 1999; Colomba et al., 2000a, 2000b; Moura, 2002). Due to its high resolution, the use of in situ hybridization with a ribosomal DNA as probe makes the precise detection of active and inactive rDNA clusters possible, even in cases with minute amounts of ribosomal genes (López-León et al., 1999).
In the present study, meiotic and mitotic chromosomes of male Isocopris inhiata and Diabroctis mimas specimens were analyzed, in order to better characterize the chromosomes of the Scarabaeinae subfamily, especially in terms of distribution and variability of CH and NORs.
Material and Methods
Meiotic chromosomes of 15 Isocopris inhiata (Germar, 1824) and 2 Diabroctis mimas (Linnaeus, 1758) male specimens were analyzed. The material was collected in Ribeirão Preto (I. inhiata), State of São Paulo (21°10'24"S and 47°48'24"W), and Igarassú (D. mimas), State of Pernambuco (7°50'20''S and 35°00'10''W), a south-eastern and a north-eastern region of Brazil, respectively. The insects were anaesthetised with ether [(C2H5) 2O], and their testes were removed and fixed in ethanol-acetic acid (3:1), using the classic technique of testicular follicle squashing and staining with 2% lacto-acetic orcein for standard karyotype analysis.
C-banding was performed by the method described by Sumner (1972). The material was incubated with 0.2 N HCl, followed by treatment with 5% barium hydroxide and 2 x SSC at 60ºC. Silver nitrate staining was performed as described by Rufas et al. (1987), with the slides being pre-treated with 2 x SSC at 60 °C for 10 min and stained with silver nitrate (1 g/L mL) at 70-80 °C.
FISH was performed according to the methodology described by Natarajan et al. (1998) and Sakamoto-Hojo et al. (1999). The rDNA fragment used as probe for NOR detection was obtained from an Apis mellifera 28S clone (GenBank accession number AJ302936). First this fragment was amplified by polymerase chain reaction (PCR) and then used as template to amplify biotinylated products by another PCR round.
Results
Standard staining and C banding
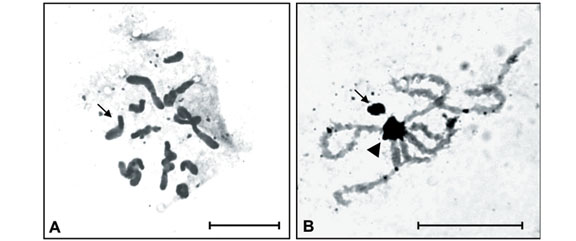
This was the first time Isocopris inhiata and Diabroctis mimas were analyzed cytogenetically. The I. inhiata males presented a 2n = 18 karyotype, with a meioformula of 8II + Xyp. The chromosomes of this species were meta-submetacentric and showed a gradual reduction in size. Pair 1 was considerably larger than the other chromosomes (Figure 1). The chromosome number of the D. mimas males was 2n = 20, with a meioformula of 9II + Xyp (Figure 2). This species presented both meta-submetacentric (pairs 1, 2, 3, 4, and 7) and acrocentric (pairs 5, 6, 8, and 9) chromosomes, with gradual size reduction. The sex determination mechanism turned out to be of the "parachute" type, and the y chromosome had a metacentric dot configuration in both species (Figure 1).
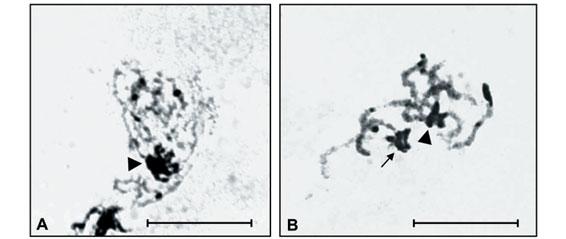
Constitutive heterochromatin (CH) analysis revealed similar C-banding patterns for both species, despite the diphasic chromosomes, i.e., large paracentromeric blocks observed on each short arm of pairs 3, 4, 5 and 7 of I. inhiata and pairs 2, 4 and 7 of D. mimas (Figure 1A and 1B). In addition, I. inhiata showed telomeric blocks on pair 6 (Figure 1A), and D. mimas on pairs 1 and 3 (Figure 1B). The X chromosomes of these species were found to be almost completely heterochromatic. Non-homologous heterochromatic associations forming chromocenters between the bivalent autosomes were visible during the meiotic prophase and persisted until the end of pachytene (Figure 1C).
AgNO3 staining
Amorphous masses corresponding to argyrophilous proteins were observed upon AgNO3 staining in the Xyp bivalent of the two species studied. These masses were observed from the beginning of prophase until the end of pachytene or the beginning of diplotene. AgNO3 staining also detected blocks similar to those observed by C-banding during the different phases of meiosis (Figures 2A and 3B).
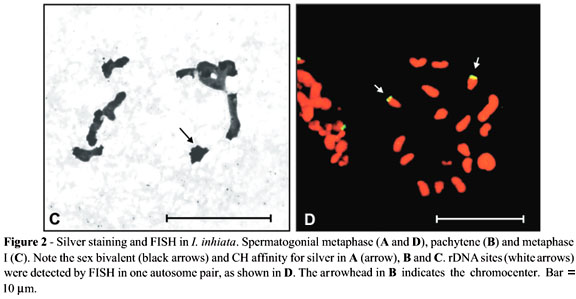
FISH analysis revealed rDNA clusters in one autosome pair of Isocopris inhiata (Figure 2D) and in two autosome pairs plus in the X chromosome of Diabroctis mimas (Figure 3C and D). In both species, these clusters were located in chromosomes of medium size.
Discussion
The chromosome number found in D. mimas was in accordance to that observed in most species of the suborder Polyphaga. Therefore, the presence of acrocentric chromosomes in this karyotype (Figure 1B) suggests the occurrence of pericentric inversions. The reduction in chromosome number and the presence of a larger pair observed in I. inhiata (Figure 1A) may be due to inversion followed by fusion mechanisms between autosomes, a fact that may explain the alteration in karyotype without modifying the type of sex-determining system. Such changes have been widely reported in the literature, including the karyotype evolution types proposed for Coleoptera by Yadav and Pillai (1979).
D. mimas and I. inhiata presented an interesting distribution pattern of CH blocks, as well as a type of heterochromatic association forming chromocenters between some bivalent autosomes (Figure 1C). Non-homologous heterochromatic associations and chromocenter fusions involving various or all chromosomes seem to be a very common phenomenon in insect meiosis. The heterochromatic segments of several coleopteran species present different degrees of ectopic pairing, leading to the formation of chromocenters. This type of association seems to play an important role in nuclear organization and segregation of meiotic chromosomes in beetles (Smith and Virkki, 1978; Drets et al., 1983).
Although the CH in these species was seen mostly in pericentromeric regions, as in other beetles analyzed by the C-banding technique (Rees et al., 1976; Vidal and Giacomozzi, 1978; Virkki, 1983; Vidal and Nocera, 1984; Postiglioni and Brum-Zorrilla, 1988; Colomba, et al., 1996; Bione, 1999; Moura, 2002), Diabroctis mimas and Isocopris inhiata showed some telomeric blocks and diphasic chromosomes with one heterochromatic and one euchromatic arms (Figures 1A and 1B). According to previous reports, in addition to pericentromeric C-bands, distal C-bands were also observed in the tenebrionids Misolampus goudoti (Juan and Petitpierre, 1989) and Palembus dermestoides (Almeida et al., 2000), while exclusively telomeric blocks were observed in the carabid Bembidion minimum (Rozek and Rudek, 1992). The additional C-bands in these beetles and in D. mimas and I. inhiata probably arose by small tandem duplications, as proposed by King and John (1980).
Data on localization of NORs in Coleoptera suggest that an autosome pair functioning as nucleolus organiser appears to be widely distributed in this order (Virkki, 1983; Virkki et al., 1984; Postiglioni and Brum-Zorrilla, 1988; Colomba et al., 2000a; Moura et al., 2003). This stands in contrast to most Scarabaeidae, where the NOR is found in the sex bivalent (Bione, 1999; Moura, 2002)
NOR activity at the beginning of the meiotic prophase is widely observed in a large number of organisms, including Coleoptera species. However, this activity was observed during a restricted period of time only, declining rapidly and disappearing in the middle of the diplotene phase. Nevertheless, the nucleolar masses produced can persist for a longer period of time, especially in species with a prolonged diplotene (Virkki and Denton, 1987; Virkki et al., 1991). This phenomenon was clearly observed in the two species studied here.
Studies on some curculionid beetles investigating the development and segregation of the Xyp showed that, even when the NORs are autosomal, the lumen of the sex bivalent is filled with a proteinaceous substance with affinity for silver, and this affinity persists from diakinesis to anaphase I. This substance is probably similar to that present in the synaptonemal complex and in the chromosome skeleton. It has been suggested that this substance may play an adhesive role, thus controlling the correct separation of the sex chromosomes (Virkki et al., 1990, 1991). The fact that the sex bivalents of the species analyzed here continued to be labelled with silver after the disappearance of the nucleolus, as well as the high affinity of their heterochromatin for silver, suggests that the Xyp association is not necessarily due to the NOR, but possibly to the presence of argyrophilic proteins associated with the heterochromatin of these species. These results are supported by data obtained for representatives of Geotrupinae (Vitturi et al., 1999), Scarabaeinae (Colomba et al., 2000b), Rutelinae and Dynatinae (Bione, 1999) and Melolonthinae (Moura et al., 2003) showing that the entire heterochromatin stained by C-banding possessed high affinity for silver, regardless of its base pair composition.
Juan et al. (1993) demonstrated that in Tenebrio molitor only the Xyp bivalent was labelled upon AgNO3 staining. However, FISH analysis using an rDNA as probe revealed 6 NORs in the mitotic metaphase chromosomes of this species, located on two different autosome pairs and on the Xp and yp chromosomes. Similarly, D. mimas presented two pairs of autosomes and an X chromosome carrying nucleolus organizer regions. On the other hand, due to the great affinity of the constitutive heterochromatin of I. Inhiata and D. mimas for the AgNO3 substrate (Figures 2 and 3), we were unable to determine which NORs were active during meiosis in these species.
I. inhiata and D. mimas showed differences in number and localization of rDNA clusters, as evidenced by the FISH method. At least for I. Inhiata, our data are in concordance with the hypothesis that an autosome pair functions as nucleolus organizer in Coleoptera. The fact that the nucleolus organizer of D. mimas is localized in the chromocenter could have favoured the "dislocation" of the rDNA clusters and its subsequent amplification on the other chromosomes that form the chromocenter. A plausible explanation for the presence of an rDNA cluster in the X chromosome could be the occurrence of an autosome fission followed by the translocation of the cluster to this chromosome.
Although I. inhiata and D. mimas belong to distinct tribes (Coprini and Phanaeini, respectively) and present differences in their chromosome number and morphology, the data collected here show similarities between the karyotypes of these two species in respect to the amount, localization and behavior of the CH during meiosis, as well as to the presence of diphasic chromosomes. Our results suggest that a similar evolutionary pathway was followed by these two species. The use of banding and FISH methods is necessary to establish a real karyotype overview in beetles. Further on, such studies will shed more light on the chromosome evolution in this subfamily.
Acknowledgments
This work was supported by grants from the Brazilian agency Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). We are grateful to Dr. Sérgio Ide from Instituto Biológico, São Paulo, and Fernando Vaz de Melo from Universidade Federal de Lavras (UFLA), for the taxonomic identification of the species used in this study. We also thank Angel Roberto Barchuk for help in preparing the rDNA probe and Dr. Adriana Mendes Nascimento and Dr. Rita de Cássia de Moura for critically reading and commenting on a previous version of this manuscript.
Received: December 19, 2003; Accepted: June 25, 2004.
Associate Editor: Yatiyo Yonenaga-Yassuda
- Almeida MC, Zacaro AA and Cella DM (2000) Cytogenetic analysis of Epicauta atomaria (Meloidae) and Palembus dermestoides (Tenebrionidae) with Xyp sex determination system using standard staining, C-bands, NOR and synaptonemal complex microspreading techniques. Hereditas 133:147-157.
- Angus RB (1983) Separation of Helophorus grandis, maritimus and occidentalis sp. n. (Coleoptera, Hydrophilidae) by banded chromosome analysis. Syst Entomol 8:1-13.
- Bione EG (1999) Citogenética de coleópteros das subfamílias Dynastinae e Rutelinae (Polyphaga, Scarabaeoidea, Scarabaeidae). Master's Thesis, UFPE, Brazil, 76 pp.
- Colomba MS, Monteresino E, Vitturi R and Zunino M (1996) Characterisation of mitotic chromosomes of the scarab beetle Glyphoderus sterquilinus (Westwood) and Bubas bison (L.) (Coleoptera: Scarabaeidae) using conventional and banding techniques. Biol Zentbl 115:58-70.
- Colomba, MS, Vitturi R and Zunino M (2000a) Karyotype analysis, banding, and fluorescent in situ hybridization in the scarab beetle Gymnopleurus sturmi McLeay (Coleoptera: Scarabaeoidea: Scarabaeidae). J Hered 91:260-264.
- Colomba MS, Vitturi R and Zunino M (2000b) Chromosome analysis and rDNA FISH in the stag beetle Dorcus parallelipipedus L. (Coleoptera: Scarabaeoidea: Lucanidae). Hereditas 133:249-253.
- Costa C, Vanin SA and Casari-Chen SA (1988) Larvas de coleopteros do Brasil. Museu de Zoologia, Universidade de São Paulo, Brasil, 282 pp.
- Crowson RA (1967) The Natural Classification of the Families of Coleoptera. EW Classey, Middlesex, pp 213.
- Drets ME, Corbella E, Panzera F and Folle GA (1983) C-banding and non-homologous association II. The "parachute" Xyp sex bivalent and the behavior of heterochromatic segments in Epilachna paenulata Chromosoma 88:249-255.
- Ennis TJ (1974) Chromosome structure in Chilocorus (Coleoptera: Coccinelidae). I. Fluorescent and Giemsa banding patterns. Can J Genet Cytol 16:651-661.
- Goodpasture C and Bloom SE (1975) Visualization of nucleolar organizer regions in mammalian chromosomes using silver staining. Chromosoma 53:37-50.
- Hanski I and Cambefort Y (1991) Dung Beetle Ecology. Princeton Univ. Press, Princeton, 481 pp.
- Hubbell HR (1985) Silver staining as an indicator of active ribosomal genes. Stain Technol 60:285-94.
- Juan C and Petitpierre E (1989) C-banding and DNA content in seven species of Tenebrionidae (Coleoptera). Genome 32:834-839.
- Juan C, Vazquez P, Rubio JM, Petitpierre E and Hewitt GM (1993) Presence of highly repetitive DNA sequences in Tribolium flour-beetles. Heredity 70:1-8.
- King M and John B (1980) Regularities and restrictions governing C-band variation in acridoid grasshopper. Chromosoma 76:123-150.
- López-León MD, Cabrero J and Camacho JP (1999). Unusually high amount of inactive ribosomal DNA in the grasshopper Stauroderus scalaris Chromosome Res 7:83-8.
- Miller DA, Dev VG, Tantravahi R and Miller OJ (1976) Suppression of human nucleolus organizer activity in mouse-human somatic hybrid cells. Exp Cell Res 101:235-243.
- Moura, RC (2002) Análise citogenética comparativa em coleópteros da familia Scarabaeidae e caracterização de polimorfismo autossômico em Euchroma gigantea - Buprestidae (Polyphaga). Doctor's Thesis, UFPE, Brazil, 124 pp.
- Moura RC, Souza MJ, Melo NF and Lira-Neto AC (2003) Karyotypic characterization of representatives from Melolonthinae (Coleoptera: Scarabaeidae) Karyotypic analysis, banding and fluorescent in situ hybridization (FISH). Hereditas 138:200-206.
- Natarajan AT, Santos SJ, Darroudi F, Hadjidikova V, Vermeulen S, Chatterjee S, Berg M, Grigorova M, Sakamoto-Hojo ET, Granath F, Ramalho AT and Curado MP (1998) 137Cesium-induced chromosome aberrations analyzed by fluorescence in situ hybridization: Eight years follow-up of the Goiania radiation accident victims. Mutat Res 400:299-312.
- Postiglioni A and Brum-Zorrilla N (1988) Non-relationship between nucleolus and sex chromosomes system Xyp in Chelymorpha variabilis Boheman (Coleoptera: Chrysomelidae). Genetica 77:134-141.
- Rees RW, Fox DP and Maher EP (1976) DNA content, reiteration and satellites in Dermestes In: Jones K and Bradham PE (eds) Current Chromosome Research. Elsevier, North Holland, pp 33-41.
- Rozek M and Rudek Z (1992) Karyotype analysis and C-banding pattern in two species of carabid (Coleoptera, Carabidae). Folia biologica 40:47-52.
- Rufas JS, Giménez-Ábian J, Suja JA and Garcia de la Vega C (1987) Chromosome organisation in meiosis revealed by light microscope analysis of silver-stained "cores". Genome 29:706-712.
- Sakamoto-Hojo ET, Natarajan AT and Curado MP (1999) Chromosome translocations in lymphocytes from individuals exposed to Cs-137 7.5 years after the accident in Goiania (Brazil). Radiat Prot Dosim 86:25-32.
- Smith SG and Virkki N (1978) Coleoptera. In: John B (ed) Animal Cytogenetics. Borntraeger, Berlin, Stuttgart, 366 pp.
- Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:304-306.
- Vidal OR (1984) Coleoptera from Argentina. Genetica 65:235-239.
- Vidal OR and Giaccomozzi RO (1978) Los cromosomas de la subfamilia Dynastinae (Coleoptera, Scarabaeidae). II. Las bandas C en Enema pan (Fabr.). Physis 38:113-119.
- Vidal OR, Giaccomozzi RO and Riva R (1977) Los cromosomas de la subfamilia Dynastinae (Coleoptera, Scarabaeidae). I. Inversión pericéntrica en Diloboderus abderus (Sturm) 1862. Physis 37:303-309.
- Vidal OR and Nocera CP (1984) Citogenética de la tribu Eucranini (Coleoptera, Scarabaeidae). Estudios convencionales y con bandeo C. Physis 42:83-90.
- Virkki N (1983) Banding of Oedionychna (Coleoptera: Alticinae) chromosomes: C- and Ag-bands. J Agric Univ Puerto Rico 67:221-225.
- Virkki N and Denton A (1987) Silver staining of the elements of spermatogenesis in Oedionychna (Crysomelidae: Alticinae). Hereditas 106:37-49.
- Virkki N, Flores M and Escudero J (1984) Structure, orientation and segregation of the sex trivalent in Pyrophorus luminosus (Coleoptera, Elateridae). Can J Genet Cytol 26:326-330.
- Virkki N, Mazzella C and Denton A (1990) Staining of substances adjacent to the Xyp sex bivalent of some weevils (Coleoptera: Curculionidae). J Agric Univ Puerto Rico 74:405-418.
- Virkki N, Mazzella C and Denton A (1991) Silver staining of the coleopteran Xyp sex bivalent. Cytobios 67:45-63.
- Vitturi R, Colomba MS, Barbieri R and Zunino M (1999). Ribosomal DNA location in the scarab beetle Thorectes intermedius (Costa) (Coleoptera: Geotrupidae) using banding and fluorescent in-situ hybridization. Chromosome Research 7:255-260.
- Yadav JS and Pillai RK (1979) Evolution of karyotypes and phylogenetic relationships in Scarabaeidae (Coleoptera). Zool Anz Jena 202:105-118.
Correspondence to
Publication Dates
-
Publication in this collection
08 Sept 2005 -
Date of issue
Mar 2005
History
-
Accepted
25 June 2004 -
Received
19 Dec 2003