Abstract
Cytogenetic studies were performed on five closely related microteiid lizards (Gymnophthalmini), three Calyptommatus species and Psilophthalmus paeminosus from the sand dunes of the middle São Francisco river in the semiarid caatinga of the Brazilian state of Bahia and Tretioscincus oriximinensis from the Brazilian Amazon region. The diploid chromosome number in Calyptommatus species was 2n = 58 in females and 2n = 57 in males due to a multiple X1X1X2X2:X1X2Y sex chromosomes system, while P. paeminosus was 2n = 44 (20M+24m): where M = macrochromosomes and m = microchromosomes) and T. oriximinensis 2n = 42 (18M+24m). A single pair of silver staining nucleolar organizing regions (Ag-NORs) characterizes all five species. Incorporation of 5-BrdU (Bromodeoxyuridine) followed by replication R-banding (RBG) karyotyping allowed the identification of the larger pairs of chromosomes through longitudinal bands and the late replicating regions. Our data reinforce the remarkable chromosomal variability that has been found in the Gymnophthalmidae and the importance of using differential staining for comparative cytogenetics within this group of lizards. Chromosomal evolution in Gymnophthalmini seems to have included chromosomal fission and fusion, pericentric inversions and variation in the amount and localization of constitutive heterochromatin and the Ag-NOR pattern. Different mechanisms of sex determination also evolved independently in this radiation.
Gymnophthalmidae; banding patterns; karyotypes; multiple sex chromosomes
ANIMAL GENETICS
RESEARCH ARTICLE
Chromosomal banding patterns in the eyelid-less microteiid lizard radiation: The X1X1X2X2:X1X2Y sex chromosome system in Calyptommatus and the karyotypes of Psilophthalmus and Tretioscincus (Squamata, Gymnophthalmidae)
Yatiyo Yonenaga-YassudaI; Miguel Trefaut RodriguesII; Katia Cristina Machado PellegrinoIII
IUniversidade de São Paulo, Instituto de Biociências, Departamento de Genética e Biologia Evolutiva, São Paulo, SP, Brazil
IIUniversidade de São Paulo, Instituto de Biociências, Departamento de Zoologia, São Paulo, SP, Brazil
IIIUniversidade Católica de Goiás, Programa de Pós-Graduação em Ciências Ambientais e Saúde, Goiânia, GO, Brazil
Send correspondence to Send correspondence to Yatiyo Yonenaga-Yassuda Universidade de São Paulo, Instituto de Biociências, Departamento de Genética e Biologia Evolutiva Caixa Postal 11461 05422-970 São Paulo, SP, Brazil E-mail: yyassuda@usp.br
ABSTRACT
Cytogenetic studies were performed on five closely related microteiid lizards (Gymnophthalmini), three Calyptommatus species and Psilophthalmus paeminosus from the sand dunes of the middle São Francisco river in the semiarid caatinga of the Brazilian state of Bahia and Tretioscincus oriximinensis from the Brazilian Amazon region. The diploid chromosome number in Calyptommatus species was 2n = 58 in females and 2n = 57 in males due to a multiple X1X1X2X2:X1X2Y sex chromosomes system, while P. paeminosus was 2n = 44 (20M+24m): where M = macrochromosomes and m = microchromosomes) and T. oriximinensis 2n = 42 (18M+24m). A single pair of silver staining nucleolar organizing regions (Ag-NORs) characterizes all five species. Incorporation of 5-BrdU (Bromodeoxyuridine) followed by replication R-banding (RBG) karyotyping allowed the identification of the larger pairs of chromosomes through longitudinal bands and the late replicating regions. Our data reinforce the remarkable chromosomal variability that has been found in the Gymnophthalmidae and the importance of using differential staining for comparative cytogenetics within this group of lizards. Chromosomal evolution in Gymnophthalmini seems to have included chromosomal fission and fusion, pericentric inversions and variation in the amount and localization of constitutive heterochromatin and the Ag-NOR pattern. Different mechanisms of sex determination also evolved independently in this radiation.
Key words: Gymnophthalmidae, banding patterns, karyotypes, multiple sex chromosomes.
Introduction
The family Gymnophthalmidae is composed of a morphologically and ecologically diverse group of small to medium-sized lizards referred to as microteiids. At present 35 genera are known containing about 180 species, although the Gymnophthalmidae is still far from taxonomically well-described at the suprageneric, generic or specific level (Pellegrino et al., 2001; Castoe et al., 2004) and only a few species have been karyotyped because the members of this family are rare, small and difficult to collect.
The tribe Gymnophthalmini consists of a group of eight South American genera sharing several characters apparently unique in gymnophthalmids and is now considered monophyletic based on morphology (Rodrigues, 1995), DNA sequences (Pellegrino et al., 2001) and mitochondrial DNA restriction site data (Benozzati and Rodrigues, 2003). Admitted relationships (Rodrigues, 1995, 1996) for this radiation are (Tretioscincus (Micrablepharus (Gymnophthalmus (Procellosaurinus, Vanzosaura) (Psilophthalmus (Nothobachia, Calyptommatus))))), the habitats of theses species being as follows: Tretioscincus occurs in the Brazilian Amazon; Micrablepharus and Vanzosaura in open areas of Brazilian cerrado (woodland savannas) and caatinga (semi-arid bush) and the Chaco (tropical and subtropical dry broadleaf forests); Gymnophthalmus in Central America, some Caribbean islands and on the northern bank of the Amazon river in the Brazilian Amazon; and Calyptommatus, Nothobachia, Procellosaurinus and Psilophthalmus are sand-adapted lizards endemic to a small area of Quaternary sand dunes on the banks of the middle São Francisco river in the semiarid caatinga of the Brazil state of Bahia (Rodrigues, 1991a; 1991b; 1996; 2003). An interesting feature of members of the Gymnophthalmini is progressive limb reduction and body elongation associated with the habitation of sandy environments and fossoriality. Members of the genus Tretioscincus have distinctive eyelids but all the remaining genera of the Gymnophthalmini radiation lack eyelids.
During the last few years we have been studying the chromosomes of the Gymnophthalmini genera Gymnophthalmus (Yonenaga-Yassuda et al., 1995), Procellosaurinus and Vanzosaura (Yonenaga-Yassuda et al., 1996), Nothobachia (Pellegrino et al., 1999a) and Micrablepharus (Yonenaga-Yassuda and Rodrigues, 1999). As far as we know this group presents the largest range and diversity of chromosome rearrangements described for microteiids, with the diploid number varying from 2n = 40 (16M+24m: where M = macrochromosomes and m = microchromosomes), in Procellosaurinus erythrocercus, Procellosaurinus tetradactylus and Vanzosaura rubricauda (Yonenaga-Yassuda et al., 1996) to 2n = 62-64 in Nothobachia ablephara (Pellegrino et al., 1999a). Furthermore, an exceptional diversity in chromosomal repatterning also characterizes the group.
Our previous cytogenetic findings based on analyses of fibroblast cultures suggested the occurrence of species-specific karyotypes in the Gymnophthalmini. The Gymnophthalmus species leucomystax, vanzoi and underwoodi share the same diploid number (2n = 44, 20M+24m) but exhibit three different karyotypes. The species G. leucomystax and G. vanzoi are very similar but can be distinguished after conventional staining by the difference in morphology of two macrochromosomes, which are subtelocentric in G. leucomystax but submetacentric in G. vanzoi. Furthermore, G. vanzoi has more biarmed microchromosomes than G. leucomystax and the C-band patterns of the microchromosomes differ in the two species. The karyotype of the unisexual G. underwoodi shows chromosome differences that cannot be explained by simple structural rearrangements in relation to the other two species. These three Gymnophthalmus species also differ with respect to the number and position of their nucleolar organizer regions (NORs) (Yonenaga-Yassuda et al., 1995). Yonenaga-Yassuda et al. (1996) have shown that P. erythrocercus, P. tetradactylus and V. rubricauda have a similar 2n = 40 (16M+24m) karyotype but each species can be easily differentiated by the position and amount of C-heterochromatin and silver staining NORs (Ag-NORs). Variation in diploid number due to a supernumerary chromosome system was detected in Nothobachia ablephara (2n = 62-64) (Pellegrino et al., 1999a) and in Micrablepharus maximiliani and M. atticolus (2n = 50-53) (Yonenaga-Yassuda and Rodrigues, 1999). The two species of Micrablepharus have the same basic diploid number of 2n = 50 without distinction between macro- and microchromosomes, but with differences in the number of bi- and uniarmed chromosomes. Sex determination of the XX:XY type has also been observed in both species of Micrablepharus and in Nothobachia ablephara.
In order to complete the cytogenetic study of the Gymnophthalmini we are present data for the remaining genera and species of this interesting monophyletic radiation, i.e. Tretioscincus oriximinensis, Psilophthalmus paeminosus and the three Calyptommatus species leiolepis, nicterus and sinebrachiatus.
Materials and Methods
Cytogenetic analyses were performed on the following specimens: seven male and one female Calyptommatus leiolepis (Figure 1); two female and two male C. nicterus; three male C. sinebrachiatus; five males, two females and five embryos of Tretioscincus oriximinensis; and five male, one female and two immature Psilophthalmus paeminosus. Voucher specimens are deposited in the collection of the Museu de Zoologia da Universidade de São Paulo (MZUSP), Universidade de São Paulo, São Paulo, Brazil. Table 1 lists the localities and identification numbers of the specimens that were cytogenetically studied for each species. For some males only meiotic data were obtained.
For the majority of specimens mitotic chromosomes were obtained from fibroblast cultures grown at 29 ºC in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal calf serum. Giemsa staining, Ag-NORs and C-banding followed routine protocols. For some males, testicular material was analyzed to characterize meiotic phases. Replication R-banding (RBG) was obtained after treatment with 5-BrdU at a final concentration of 25 g/mL for 9-22 h prior to cell harvesting. Cultured cells are stored in liquid nitrogen in the Cell Collection of Departamento de Genética e Biologia Evolutiva, Instituto de Biociências, Universidade de São Paulo (IBUSP).
Results
Calyptommatus leiolepis (2n = 57, male; 2n = 58, female), Calyptommatus nicterus (2n = 57 male; 2n = 58, female), Calyptommatus sinebrachiatus (2n = 57, male)
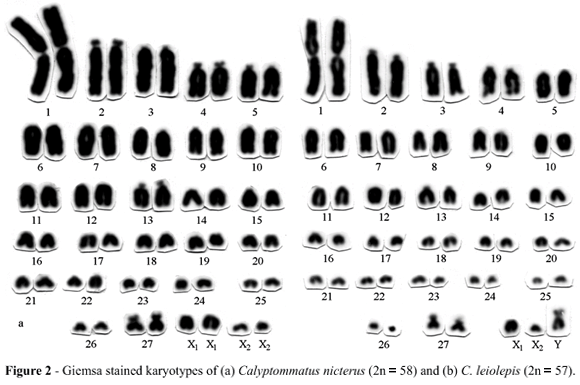
Female specimens of Calyptommatus leiolepis present a karyotype comprised of a metacentric pair 1, distinctly larger than the remaining chromosomes, 26 pairs of acrocentric autosomes decreasing gradually in size and a small pair of submetacentrics (pair 27). Two pairs of acrocentric chromosomes of different sizes, morphologically indistinguishable from autosomes of similar sizes, correspond to the X1 and X2 pairs. The karyotypes of male specimens present an odd medium-sized submetacentric representing the Y chromosome. This male specific biarmed chromosome results from a translocation rearrangement between chromosomes of different sizes, X1 and X2. The reduction of the chromosome number in male cells is due to an X1X1X2X2 female : X1X2Y male or XXAA female : XAA/Y male sex chromosome system (Figure 2b). Analyses of testicular material showed 27 bivalents and one trivalent corresponding to the X1X2Y chromosomes in diplotene cells and metaphases II with 29 (Figure 3b) or 28 chromosomes.
In the majority of the chromosomes C-banding revealed telomeric heterochromatin blocks in both arms (Figure 4a). The Ag-NOR is located on the telomeric region of the short arms of the small submetacentric pair 27. Analyses of 47 Ag-stained metaphases from five animals revealed the presence of only two NORs (Figure 5a). RBG-banding allowed the identification of some chromosomes including the Y and the putative chromosomes involved in the translocation rearrangement. A marker pair of chromosomes always showed a typical pattern with three evident bands (pair 4) and some homologous pairs could be identified by their late replicating pattern. Positive CBG-bands proved to be late-replicating DNA regions (Figure 4b).
The two other Calyptommatus species present the same karyotype as C. leiolepis. The karyotype of the C. nicterus females is presented in the Figure 2a. The male C. sinebrachiatus specimens (Figure 3a) exhibited a 2n = 57 karyotype and probably a X1X1X2X2: X1X2Y type sex determination mechanism, although this needs to be verified by the analyses of female specimens which were missing from our sample. The odd Y chromosome is metacentric and differs from the submetacentric Y found in C. leiolepis (Figure 2b and Figure 4) and C. nicterus. Both species present Ag-NORs on the telomeric region of the short arms of submetacentric pair 27.
Psilophthalmus paeminosus , 2n = 44 (20M+24m)
This species exhibited a 2n = 44 (20M+24m) karyotype formed by five pairs of metacentrics and submetacentrics and five pairs of acrocentric macrochromosomes. The microchromosome complement includes 12 pairs of acrocentrics and metacentrics (Figure 6a). The C-banding patterns included weakly stained small regions in some microchromosomes (Figure 7a). R-banding patterns allowed the macrochromosomes to be paired and revealed that the distal end of the long arm of pair 2 is a late replicating region (Figure 6b and c ). Ag-NORs were located on the telomeres of short arms of a microchromosome pair in 31 metaphases from eight specimens analyzed after Ag-staining (Figure 5b). Abnormal chromosomes occurred in some P. paeminosus specimens but were probably a fibroblast culture artifact. Polyploidy, chromosomal aberrations including trisomy and other kinds of rearrangements were present but do not represent the standard karyotype of that species (Figure 6b).
Tretioscincus oriximinensis , 2n = 42 (18M+24m)
In this species the diploid number is 2n = 42 (18M+24m) and the karyotype comprises seven pairs of metacentric and submetacentric (pairs 1-3, 5-8) and two pairs of subtelocentric (pairs 4 and 9) macrochromosomes and 12 pairs of acrocentric and submetacentric microchromosomes (Figure 8a). Positive C-bands at the distal end of the long arms of a few microchromosomes were observed (Figure 7b). The RBG-banding patterns allowed precise identification of the nine pairs of macrochromosomes (Figure 8b). Silver staining of 17 metaphases from three specimens revealed Ag-NORs on the short arms of a pair of microchromosomes (Figure 5c).
Discussion
The genus Calyptommatus, has four species, three of them isolated in opposite margins of the São Francisco river, with C. leiolepis occurring along the left bank of the river where it is limited to two geographically isolated dune fields while C. sinebrachiatus and C. nicterus are restricted to adjacent sandy areas of the right bank of the river. Rodrigues et al., (2001) have recently described a fourth species, Calyptommatus confusionibus, occurring in Parque Nacional da Serra das Confusões in the Brazilian state of Piauí, and although this site is not too distant from the São Francisco dunes this species seems to be isolated from the other three species because it is in a sandy area belonging to a different drainage system.
The Calyptommatus multiple sex chromosome determination X1X1X2X2:X1X2Y system described in this paper is the first report of such a sex chromosome mechanism among gymnophthalmids. Multiple sex chromosome systems usually arise as a result of rearrangements involving sex chromosomes and autosomes, either by centric fusion, reciprocal translocation between metacentric chromosomes or centric fission, although tandem fusions may also be involved in this process.
The somatic and meiotic data collected by us for the three Calyptommatus species support the occurrence of a multiple sex chromosome system with 2n = 58 in females and 2n = 57 in males. The male specific Y chromosome is easily identifiable and appears to be the result of a translocation rearrangement between different-sized chromosomes (one is the X chromosome) in C. leiolepis and C. nicterus and two similar-sized chromosomes in C. sinebrachiatus (the Y is a metacentric).
In male meiosis, the X1X2Y sex chromosomes occur in the expected trivalent configuration and probably the X1X2Y trivalent segregates in the alternated disjunction during anaphase I. Chromosomally balanced sperm nuclei are formed, with the X1 and X2 chromosomes and a complement of 28 autosomes passing to the same pole of the dividing cell while the fused Y chromosome and the same 28 autosomes are pulled to the opposite pole.
Heteromorphic sex chromosomes were not found either in the P. paeminosus or T. oriximinensis specimens described in this paper nor in the previously studied microteiids of the same radiation, G. leucomystax and G. vanzoi (Yonenaga-Yassuda et al., 1995) and P. erythrocercus, P. tetradactylus and V. rubricauda, (Yonenaga-Yassuda et al., 1996). However, a XX:XY system has been reported in M. atticolus and M. maximiliani (Yonenaga-Yassuda and Rodrigues, 1999), Nothobachia ablephara (Pellegrino et al., 1999a) and Gymnophthalmus pleei (Cole et. al., 1990).
It has been pointed out that sex chromosomes have arisen recently and independently many times in lizard lineages (Bickham, 1984). The occurrence of different sex determination mechanisms in the Gymnophthalmini clade and their absence in the putative basal genus Tretioscincus suggests that such mechanisms evolved independently at least twice in this microteiid radiation.
The multiple sex chromosome systems X1X1X2X2: X1X2Y or XXAA:XAA/Y have been reported in some species of neotropical freshwater fish (AlmeidaToledo and Foresti, 2001) and coexistence of homomorphic XY sex chromosomes and a derived Y-autosome translocation are known to occur in the amphibians Eleutherodactylus maussi (Schmid et al., 2002) and Eleutherodactylus riveroi (Schmid et al., 2003), the first examples of a such a mechanism in the class Amphibia.
Several chromosomal sex determination mechanisms (XX:XY, ZZ:ZW) involving both macrochromosomes and microchromosomes are found in lizards. The multiple sex chromosome system X1X1X2X2:X1X2Y is found in Polychrus marmoratus (2n = 29, male; 2n = 30, female) and Polychrus acustirostris (2n = 19, male; 2n = 20, female) where a large subtelocentric chromosome is involved in the translocation with the Y chromosome. After RBG banding this chromosome exhibits a heterochromatic and conspicuous late replication region, which probably bears the Y chromosome because it was only observed in males. The use of differential staining allowed the identification of the Y-autosome translocation whereas the X1 and the Y chromosomes were indistinguishable in conventional-stained metaphases (Bertolotto et al., 2001).
A sex determination mechanism involving parthenogenesis occurs among gymnophthalmids. A triploid karyotype (3n = 66, 30M+36m) was found in some Leposoma percarinatum specimens (Pellegrino et al., 2003) and in the unisexual diploid G. underwoodi (2n = 40) from Roraima, Brazil (Yonenaga-Yassuda et al., 1995) that is unequivocally very different from the hybrid-origin G. underwoodi studied by Cole et al. (1989: 1990).
Ag-NOR staining is an essential part of the characterization of a species karyotype, but although NOR chromosome distribution is considered to be species specific, the location of NORs could also result from convergent evolution. The localization of NORs studied by silver staining in the family Gymnophthalmidae showed remarkable variability.
In the present study, all species have a single pair of NOR-bearing chromosomes, although their position varies. In Calyptommatus the NORs are located on the easily identifiable small submetacentric pair 27, while in T. oriximinensis and P. paeminosus the NORs are associated with a microchromosome pair.
In the unisexual G. underwoodi (2n = 44), two Ag-NORs are located on the telomeric region of an acrocentric macrochromosome pair while in G. leucomystax (2n = 44) and G. vanzoi (2n = 44) microchromosome pairs bear multiple Ag-NORs (Yonenaga-Yassuda et. al., 1995). In P. erythrocercus and P. tetradactylus a single pair of NOR-bearing microchromosomes was described and in V. rubricauda Ag-NORs occur on one pair of microchromosomes and one pair of macrochromosomes (Yonenaga-Yassuda et. al., 1996). A remarkable variation in number and location of Ag-NORs involving small and larger chromosomes allowed the characterization of five different NOR patterns in M. maximiliani (2n = 50, 51) and M. atticolus (2n = 50, 51, 52, 53) (Yonenaga-Yassuda and Rodrigues, 1999).
Similarly, a considerable variation in number (two to six) of positive signs including a small entirely stained pair 27 was found in N. ablephara (2n = 62, 63, 64). It is possible that some of the positive Ag-stained regions are representing C-banding regions rather than NORs in this species (Pellegrino et al., 1999a).
In other gymnophthalmids, Ag-NORs were only identified in Leposoma species, where they were located on the telomeres of a microchromosome pair in Leposoma guianense (2n = 44) and on the telomeric region of the long arms of a large pair of submetacentric macrochromosome in Leposoma osvaldoi (2n = 44), a small pair 19 with a conspicuous secondary constriction harbored the Ag-NORs in Leposoma scincoides (2n = 52) (Pellegrino, et al., 1999b).
Although NOR localization has proved to be an important marker for lizards, very different NOR banding patterns occur among Gymnophthalmidae species and more data on NOR variability are needed to understand their role in the chromosomal evolution of this family. In some cases, Ag-NORs represent an important tool for karyotype characterization, especially when different NOR-bearing chromosomes support taxonomic relationships. Differences in the chromosomal distribution of NORs in evolutionary closely related species are attributed to karyotypic rearrangements accumulated since divergence from the common ancestor.
The chromosome data so far known for gymnophthalmids have revealed remarkable chromosome variability among these lizards with, except for Calyptommatus, all species presenting species-specific karyotypes mostly after comparative analyses of banded karyotypes (C-bands and identification of Ag-NORs).
According to the phylogenetic scheme based on 71 osteological and morphological characters suggested by Rodrigues (1995) widely congruent with phylogenetic relationships inferred from mitochondrial DNA restriction-site data (Benozzati and Rodrigues, 2003) and mitochondrial and nuclear DNA sequences (Pellegrino et al., 2001), Nothobachia is closely related to Calyptommatus. They are sister genera and considered the most derived of this eyelid-less radiation. A Robertsonian rearrangement mechanism explains the largest metacentric pair in the karyotype of Calyptommatus. These two genera are also characterized by showing the most striking adaptations to fossoriality, especially related to limb reduction. It is noteworthy that the highest chromosome numbers in this radiation are shared by Nothobachia and Calyptommatus followed by Micrablepharus, while all the remaining genera are characterized by lower diploid numbers.
The chromosomal rearrangements in the Gymnophthalmini seems to have included events of chromosome fission of a hypothetical ancestral Tretioscincus oriximinensis-like 2n = 42 (18M+24m) karyotype. A similar 2n = 42 karyotype is found in Colobosaura modesta and Iphisa elegans, two Heterodactylini genera, considered the sister group of the Gymnophthalmini (Pellegrino et al., 2001). Episodes of chromosome fusion, pericentric inversion as well as amplification or deletion of heterochromatin and shifts in Ag-NOR distribution, probably also followed speciation of these South American microteiids.
Two different types of karyotypes have been found in gymnophthalmids: 1) those with a clear distinction between macrochromosomes and microchromosomes where most species present 24 microchromosomes and a variable numbers of macrochromosomes (16, 18, 20, 22). Up to now Bachia is the only exception in this assemblage, revealing conspicuous chromosome variability with 2n = 32 (18M+14m) in Bachia dorbignyi and 2n = 46 (18M+28m) in Bachia bresslaui (Pellegrino, 1998; Pellegrino et al., 2001). 2) karyotypes with high diploid numbers without distinction in macro and microchromosomes and chromosomes decreasing gradually in size.
The presence of such distinct chromosomal complements in the same radiation, of species-specific karyotypes and chromosome markers (localization of Ag-NORs, patterns of constitutive heterochromatin and morphologies of both macro and microchromosomes) suggest that some of these characters could represent useful synapomorphies. Intrageneric chromosome variability also occurs in gymnophthalmids as demonstrated by the presence of very distinct karyotypes in closely related species of the genera Gymnophthalmus, Placosoma, Leposoma and Bachia (Yonenaga-Yassuda et al., 1995; Pellegrino, 1998; Pellegrino et al, 1999b; Pellegrino et al., 2001).
The highest diploid numbers in the Gymnophthalmidae were found in species of Calyptommatus, Nothobachia, Micrablepharus and in Leposoma scincoides and Placosoma glabellum and are not associated with the presence of macro and microchromosomes but with chromosomes of gradually decreasing size. A clear distinction between macro and microchromosomes was found in G. leucomystax 2n = 44 (20M+24m), G. vanzoi 2n = 44 (20M+24m), in the unisexual G. underwoodi 2n = 44 (20M+24m), P. erythrocercus 2n = 40 (16M+24m), P. tetradactylus 2n = 40 (16M+24m), V. rubricauda 2n = 40 (20M+24m), L. guianense 2n = 44 (20M+24m), L. osvaldoi 2n = 44 (20M+24m), Psilophthalmus paeminosus 2n = 44 (20M+24m), T. oriximinensis 2n = 42 (18M+24m), Anotosaura vanzolinia 2n = 46 (22M+24m), B. bresslaui 2n = 46 (18M+28m), B. dorbignyi 2n = 32 (18M+14m), Cercosaura ocellata 2n = 42 (18M+24m), C. modesta 2n = 42 (18M+24m), Neusticurus bicarinatus 2n = 44 (20M+24m), Pantodactylus albostrigatus 2n = 44 (20M+24m) and Placosoma cordylinum 2n = 44 (20M+24m) with the great majority of the species presenting 24 microchromosomes.
We have demonstrated in this paper that an extensive karyotype differentiation characterizes the monophyletic radiation of the Gymnophthalmini and the other gymnophthalmids. We are aware that comparative R-banding patterns must reveal putative homologies among chromosome segments and that this is crucial to the better understanding of the mechanisms of chromosomal evolution in this group of South America microteiids.
Acknowledgments
The authors would like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) for financial support. We are also very grateful to P.L.B. Rocha, J.M. Martins, G. Skuk, V. Xavier, and R. Moraes for help in fieldwork and to Dr. Tien Hsi Chu, Mrs. Miriam Romeo and Cyntia Esteves de Lima for technical assistance.
Received: September 27, 2004; Accepted: March 23, 2005.
Associate Editor: Sérgio Furtado dos Reis
- Almeida Toledo LF and Foresti F (2001) Morphologically differentiated sex chromosomes in neotropical freshwater fish. Genetica 111:91-100.
- Benozzati ML and Rodrigues MT (2003) Mitochondrial restriction-site characterization of a Brazilian group of eyelid-less Gymnophthalmidae lizards. J Herpetology 37:161-168.
- Bertolotto CEV, Rodrigues MT and Yonenaga-Yassuda Y (2001) Banding patterns, multiple sex chromosome system and localization of telomeric (TTAGGG)n sequences by FISH on two species of Polychrus (Squamata, Polychrotidae). Caryologia 54:214-226.
- Bickham JW (1984) Patterns and modes of chromosomal evolution in reptiles. In: Sharma AK and Sharma A (eds) Chromosome in Evolution of Eukaryotic groups. V. I. CRPress, Boca Raton, pp 13-69.
- Castoe TA, Doan TM and Parkinson CL (2004) Data partitions and complex models in bayesian analysis: The phylogeny of gymnophthalmid lizards. Syst Biol 53:448-469.
- Cole CJ, Townsend CR, Dessauer HC and Hardy LM (1989) A lizard foretold. Nat Hist 5:12-17.
- Cole CJ, Dessauer HC, Townsend CR and Arnold MG (1990) Unisexual lizards of the genus Gymnophthalmus (Reptilia, Teiidae) in the neotropics: Genetics, origin, and systematics. Am Mus Novitates 2994:1-29
- Pellegrino KCM (1998) Diversidade cariotípica e evolução cromossômica em lagartos Gymnophthalmidae e Gekkonidae (Squamata): Evidências baseadas em coloração diferencial e hibridação in situ fluorescente (FISH). PhD Thesis, Universidade de São Paulo, São Paulo.
- Pellegrino KCM, Rodrigues MT and Yonenaga-Yassuda Y (1999a) Chromosomal polymorphisms due to supernumerary chromosomes and pericentric inversions in the eyelid-less microteiid lizard Nothobachia ablephara (Squamata, Gymnophthalmidae). Chrom Res 7:247-254.
- Pellegrino KCM, Rodrigues MT and Yonenaga-Yassuda Y (1999b) Chromosomal evolution in Brazilian lizards of genus Leposoma (Squamata, Gymnophthalmidae) from Amazon and Atlantic forests: Banding patterns and FISH of telomeric sequences. Hereditas 131:15-21.
- Pellegrino KCM, Rodrigues MT, Yonenaga-Yassuda Y and Sites JWJr (2001) A molecular perspective on the evolution of microteiid lizards (Squamata, Gymnophthalmidae), and a new classification for the family. Biol J of the Linnean Society 74:315-338.
- Pellegrino KCM, Rodrigues MT and Yonenaga-Yassuda Y (2003) Triploid karyotype of Leposoma percarinatum (Squamata, Gymnophthalmidae). J Herpetology 37:197-199.
- Rodrigues MT (1991a) Herpetofauna das dunas interiores do Rio São Francisco, Bahia, Brasil. I. Introdução à área e descrição de um novo gênero de microteídeos (Calyptommatus) com notas sobre sua ecologia, distribuição e especiação (Sauria, Teiidae). Pap Av Zool S Paulo 37:285-320.
- Rodrigues MT (1991b) Herpetofauna das dunas interiores do Rio São Francisco, Bahia, Brasil. II Psilophthalmus: Um novo gênero de microteídeos sem pálpebra (Sauria, Teiidae). Pap Av Zool S Paulo 37:321-327.
- Rodrigues MT (1995) Filogenia e história geográfica de uma radiação de lagartos microteídeos (Sauria, Teiioidea, Gymnophthalmidae). Unpublishes Thesis. Universidade de São Paulo, Brazil.
- Rodrigues MT (1996) Lizards, snakes, and amphisbaenians from the quaternary sand dunes of the middle Rio São Francisco, Bahia, Brazil. J Herpetology 30:513-523.
- Rodrigues, MT (2003) Herpetofauna da Caatinga. In: Tabarelli M and Silva JMC (eds) Biodiversidade, Ecologia e Conservação da Caatinga. Ed. Recife, Recife, 804 pp.
- Rodrigues MT, Zaher H and Curcio F (2001) A new species of lizard, genus Calyptommatus, from the caatingas of the state of Piauí, Northeastern Brazil (Squamata, Gymnophthalmidae). Pap Av Zool S Paulo 41:529-546.
- Schmid M, Feichtinger W, Steinlein C, Haaf T, Schartl M, Visbal-Garcia R, Manzanilla-Pupo J and Fernández Badillo A (2002) Chromosome banding in Amphibia. XXVI. Coexistence of homomorphic XY sex chromosomes and a derived Y-autosome translocation in Eleutherodactylus maussi (Anura, Leptodactylidae). Cytogenet Genome Res 99:330-343.
- Schmid M, Feichtinger W, Steinlein C, Visbal-Garcia R and Fernández Badillo A (2003) Chromosome banding in Amphibia. XXVIII. Homomorphic XY sex chromosomes and a derived Y-autosome translocation in Eleutherodactylus riveroi (Anura, Leptodactylidae). Cytogenet Genome Res 101:62-73.
- Yonenaga-Yassuda Y, Vanzolini PE, Rodrigues MT and Carvalho CM (1995) Chromosome banding patterns in the unisexual microteiid Gymnophthalmus underwoodi and in two related sibling species (Gymnophthalmidae, Sauria). Cytogenet Cell Genet 70:29-34.
- Yonenaga-Yassuda Y, Mori L, Chu TH and Rodrigues MT (1996) Chromosomal banding patterns in the eyelid-less microteiid radiation: Procellosaurinus and Vanzosaura (Squamata, Gymnophthalmidae). Cytogenet Cell Genet 74:203-210.
- Yonenaga-Yassuda Y and Rodrigues MT (1999) Supernumerary chromosome variation, heteromorphic sex chromosomes and banding patterns in microteiid lizards of the genus Micrablepharus (Squamata, Gymnophthalmidae). Chrom Res 6:21-29.
Send correspondence to
Publication Dates
-
Publication in this collection
03 Feb 2006 -
Date of issue
Dec 2005
History
-
Accepted
23 Mar 2005 -
Received
27 Sept 2004