Abstract
Blood and stripped hemoglobin from Nelore cattle individuals were submitted to oxygen equilibrium experiments in both gasometric and spectrophotometric methods. No difference was detected in oxygen affinity and Bohr effect among Hb A, Hb B and Hb AB in experiments with both blood and stripped hemoglobin, in the presence and absence of the chloride ion. However, n values (Hill plots) were higher in Hb B and Hb AB (>2) than in Hb A (<2).
Allohemoglobins; functional properties; Nelore cattle; cooperativity; Bohr effect; modulatators effect
Functional properties of the three hemoglobin phenotypes of Nelore cattle
A. Di Vito, A.R. Schwantes and M.L.B. Schwantes
Department of Genetics and Evolution, Universidade Federal de São Carlos, São Carlos, SP, Brazil.
Send correspondence to A. Di Vito. Caixa Postal 676, 13565-905 São Carlos, SP, Brazil. E-mail: dars@power.ufscar.br.
ABSTRACT
Blood and stripped hemoglobin from Nelore cattle individuals were submitted to oxygen equilibrium experiments in both gasometric and spectrophotometric methods. No difference was detected in oxygen affinity and Bohr effect among Hb A, Hb B and Hb AB in experiments with both blood and stripped hemoglobin, in the presence and absence of the chloride ion. However, n values (Hill plots) were higher in Hb B and Hb AB (>2) than in Hb A (<2).
Key words: Allohemoglobins, functional properties, Nelore cattle, cooperativity, Bohr effect, modulatators effect.
Received: July 3, 1998; accepted: August 17, 2000.
INTRODUCTION
Hemoglobin polymorphism in mixed European/Indian (Bos taurus taurus/B. t. indicus) Algerian cattle was first described by Cabannes & Serrain (1). This polymorphism is due to a b chain variant - bB (2) which shows a Mendelian mode of inheritance, presenting 3 phenotypes Hb A, Hb B and Hb AB. Thereafter, several papers (3,4,5) describing allele frequencies have been published. The gene for this bB chain is found mainly in tropical herds, pure Indian or mixed with European cattle. Ayala (6) claimed that this polymorphism would be maintained by natural selection. However, no direct evidence of differences in fitness among the three phenotypes has been found. On the other hand, no description of functional properties of Hb B or Hb AB has been found in the literature available.
Bunn (7) demonstrated that ruminant (including Hb A from a Holstein cow) and cat hemoglobins show intrinsically low oxygen affinity and are weakly reactive to 2,3-diphosphoglycerate (2,3-DPG), the main hemoglobin function modulator of mammals. However, Weber and coworkers (8), comparing yak hemoglobins with cow Hb A, showed a not so weak 2,3-DPG effect in the latter.
Fronticelli (9) claimed that Cl- ions would be responsible for the Hb function modulation in bovine cattle, as DPG is for human hemoglobin. However, Perutz and Fermi (10) did not confirm these results.
In order to find out if there are some differences in the functional properties of the three hemoglobin phenotypes, oxygen equilibrium experiments, including the effect of 2,3-DPG and Cl-, were carried out, using Nelore cattle.
MATERIAL AND METHODS
The Nelore cattle used here belong to the Fazenda Canchim, a unit of EMBRAPA (Empresa Brasileira de Agro-Pecuária), located in São Carlos, SP, Brazil.
Blood was obtained from the jugular vein with heparinised syringes. Red blood cells were separated from plasma by centrifugation. After washing the cells three times with isotonic saline, water was added to the packed red cells in a 2:1 ratio. The hemolysates were produced by freezing and thawing three times and were then centrifuged for 15 min, at 12,100 g, at 4 °C.
Stripped hemoglobin was obtained by passing the hemolysate through a Sephadex G-25 column using Tris HCl 0.5 M buffer and Cl--free hemolysate with Hepes-Tris 0.5 M buffer (11,12).
Electrophoreses were carried out using hydrolyzed maize starch and Tris Borate-EDTA buffers (13) to check the hemoglobin phenotypes.
Whole blood oxygen equilibrium experiments were carried out using the gasometric method (14). The spectrophotometric method was used for the hemolysates (15).
Intraerythrocytic phosphate extracts were prepared (16). The phosphates were separated in a Q Sepharose column (11 cm x 1 cm) controlled by a FPLC (Fast Protein Liquid Chromatography) system. Both the resin and the FPLC System were purchased from Pharmacia Biotechnology, Sweden. The phosphates were eluted through a potassium chloride gradient 0-1 M in a triethanolamine buffer, 20 mM, pH 8.0.
Regression lines were calculated to compare the results obtained from different phenotypes and by different methods (spectrophotometric and gasometric) and under different conditions (in both presence and absence of Cl- ions). In these regression equations (y = ax + b) a can be considered the Bohr effect.
RESULTS
Electrophoreses showed one slow band for Hb A, a fast one for Hb B and two bands for the heterozygous Hb AB.
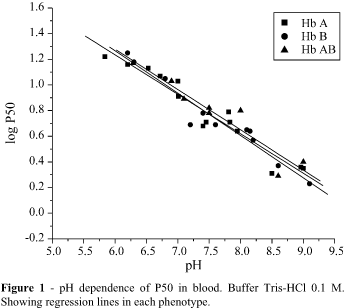
Figures 1, 2 and 3 show the results obtained with oxygen equilibrium experiments on the three phenotypes - Hb A, Hb AB and Hb B, using blood and stripped hemoglobin in the presence of the chloride ion, respectively.
The cooperativity showed by stripped Hb A, in the presence of the chloride ion, was different from those of Hb B and Hb AB. The Student t tests showed that n values were independent of pH, in the three hemoglobin phenotypes: regression lines drawn were parallel to the abscissa axis. Therefore, the differences in n values for Hb A, represented by b in the regression lines, varied significantly from those of Hb B and Hb AB, at the 0.05 level. No significant difference between n values of Hb B and Hb AB was detcted. Hb A presented n values below 2 while Hb B and Hb AB presented values greater than 2 (Figure 3).
Figure 5 shows the results obtained in oxygen equilibrium experiments on the three phenotypes - Hb A, Hb B and Hb AB, using stripped hemoglobin in the absence of the the chloride ion.
No differences were observed in oxygen affinity and Bohr effect among Hb A, Hb B and Hb AB in experiments with stripped hemoglobin in the absence of the chloride ion. However, these results are different from those using blood and stripped hemoglobin in the presence of the chloride ion.
Table I shows Bohr effect values obtained under various conditions.
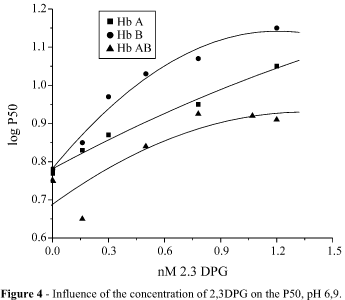
Plotting log P50 against 2,3-diphosphoglycerate (2,3-DPG) concentrations confirmed its weak effect on cattle hemoglobin. (7). However 2,3-DPG seemed to be more effective on Hb B than on Hb A and Hb AB, in molar ratios Hb:DPG of 1:20 (5 mM Hb: 100 mM of 2,3-DPG) or higher (Figure 4).
The concentration of 2,3-DPG in erythrocytes was 1.27 mM/mL packed red cells for Hb A, 1.3 mM/mL for Hb B and 1.01 mM/mL for Hb AB.
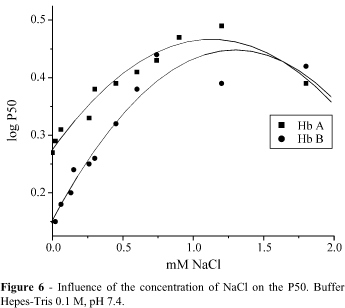
O2 affinity experiments using Hb A and Hb B stripped of the chloride ion showed different sodium chloride titration curves (Figure 6).
DISCUSSION
The absence of difference in oxygen affinity among Hb A, B and AB phenotypes was expected. This had been shown in previous results from experiments with Canchim cattle (17) comparing O2 affinities of Hb A and Hb B at 5 different pHs . However, the P50 obtained for the latter was around 3.2 mmHg at pH 7.4 for the 3 phenotypes, while that presented in this paper was 4.3. In our laboratory a blood and hemolysate sample of dairy cattle (Holstein Friesan) showed a P50 = 6.3 at the same pH. In hemoglobin samples of adult Belgian White Blue cattle a P50 = 25 mmHg, at pH 7.4, was found (18). These differences may be due to different Cl- concentrations.
A less likely hypothesis would be the following: cattle hemoglobin separated by liquid chromatography on rigid resin Mono Q, besides the main peak or peaks (Hb A, Hb B or both) showed two to three small peaks (Vito, Schwantes and Schwantes, in preparation). On the other hand most of the Artiodactylae, especially bovine, showed one to six minor hemoglobin components in isoelectric focusing on polyacrylamide gels (19). Although Bos taurus is not included in the latter paper, its hemoglobin heterogeneity might be expected. The differences in the P50 values mentioned above could be due to variations in relative concentrations of these minor peaks. Differences in amino acid sequences, not detected by the usual electrophoretic methods, could also explain variations found in the stripped hemoglobin P50 in diverse cattle populations.
The n values here obtained with stripped hemoglobin in the presence of the chloride ion indicate cooperativity in Hb B and Hb AB, but very low, if any, in Hb A. These results are shown in experiments with stripped hemoglobin in the presence of the chloride ion. Since in tropical countries, due to high environmental temperatures, and consequently greater energy requirements than those in temperate areas, the cooperativity of Hb B carriers could facilitate oxygen uptake and release. Thus, an advantage over Hb A homozygotes could be supposed. However, it is unlikely that cooperativity of Hb B and AB is the only factor and further studies, especially on comparative respiration physiology of individuals showing different Hb phenotypes, should be performed to find out the real contribution of the Hb bB gene to the apparent adaptation of their carriers in the tropics. On the other hand, no differences in n values were obtained in these experiments with stripped hemoglobin in the absence of the chloride ion. No differences in cooperativity among the three phenotypes were found in the Cl- titration experiments. However, the dimerization effect of NaCl (used as the Cl- source) on cooperativity cannot be ruled out. The P50 values decreased above 1.2 mM NaCl. These data suggest that the reverse of the slopes obtained at high ionic strength would be consistent with salt dissociation induction of tetramers into dimers (20).
No differences among oxygen affinity and the Bohr effect of Hb A, Hb AB and Hb B were obtained here when compared in blood or stripped hemoglobin, in the presence or absence of the Cl- ion. The lack of differences between blood and stripped hemoglobin in the presence of Cl- as well as the higher O2 affinity of samples in the absence of this ion were confirmed. Apparently, the sensitivity to chloride ion is greater for Hb A than for Hb B in NaCl concentrations less than 0.75 mM. However, when concentration of this salt in the experimental solution was higher than 0.75 mM, this difference disappeared.
The apparent effectiveness of high 2,3-DPG: Hb molar ratios does not seem to be important for bovine Hb adaptation, since the concentration of this organic phosphate never reaches the necessary concentration to play any role in its respiration. It is interesting to point out, that in terms of cooperativy the Hb bB is dominant over the Hb bA gene, while in terms of DPG biding, Hb bA is the dominant gene.
ACKNOWLEDGMENTS
This work could not have been done without the help of several researchers and technicians form EMBRAPA-UEPAE São Carlos (Fazenda Canchim) who provided the samples for this study. Alfredo Di Vito Neto has held fellowships from FAPESP, CAPES and CNPq during different phases of the present work. This work was supported by CNPq, FAPESP and CAPES. The authors, wish to thank Dr. Paula Ann Matvienko-Sikar, who critically reviewed this manuscript.
- Ayala FJ (1982) Population and Evolutionary Genetics, The Benjamin Cummings Publishing Company, Inc. Menlo Park, California.
- Bartlett GR (1959) J. Biol. Chem. 234:466.
- Bunn HF (1971) Science 172:1049.
- Butcher PD and Hawkey CM (1971) Comp. Biochem. Physiol. 56B:335.
- Cabannes R and Serain CH (1995) Comptes Rendus Soc. Biol. 149:5.
- Fronticelli CA (1990) Biophysical Chemistry 37:141.
- Giri KV, Pillai NC (1965) Nature 178:1057.
- Gustin P, Clerbaux Th, Willems E, Lekeux P, Lomba F and Frans (1988) Comp. Biochem. Physiol. 89:553.
- Johansen L, Mangun CP, and Lykkeboe G (1978) Can. J. Zool. 56:898.
- Kellett GL (1977) J. Mol. Biol. 59:401.
- Lehmann H, Rossi JG. Man, 61:81, 1961.
- Masina P, Iannelli D, Iorio M, Ramunno L (1977) Anim. Blood Grps Biochem. Genet. 8:65.
- Perutz MF and Fermi G (1993) J. Mol. Biol. 233:536.
- Petersen CG, Schwantes R, and Schwantes MLB (1989) Comp. Biochem. Physiol. 94B:823.
- Riggs , and Wolbach R (1955) J. Gen. Physiol. 39:585.
- Schroeder WA, Shelton JR, Robberson B and Babin DR (1967) Archs Biochem. Biophys. 120:124.
- Schwantes R, Schwantes MLB, Bonaventura C, Sullivan B and Bonaventura J (1976) Comp. Biochem. Physiol. 54B:447.
- Teixeira U Estudos do polimorfismo de hemoglobina em gado Canchim, Master Dissertation, São Carlos, 1980.
- Val L, Schwantes R, Schwantes MLB and De Luca PH (1981) Ciência e Cultura 33:982.
- Weber RE, Lalthantluanga R and Braunitzer G (1988) Archs. Biochem. Biophys. 263:199.
Publication Dates
-
Publication in this collection
11 Sept 2002 -
Date of issue
2002
History
-
Received
03 July 1998 -
Accepted
17 Aug 2000