Abstract
The Gram-negative bacterium Pseudomonas aeruginosa has a wide environmental and ecological distribution. It is an opportunistic pathogen that acquires resistance to multiple antimicrobial agents and can infect plants, animals and humans. We used rDNA and tDNA PCR markers to characterize the bacterial diversity of P. aeruginosa strains isolated at a Brazilian teaching hospital (Oswaldo Cruz University Hospital, Recife, Brazil) between March 2003 and February 2004. Clonal groups of P. aeruginosa clinical isolates were identified from different patients in different hospital units using either rDNA or tDNA markers, or a combination of both in a duplex PCR. These PCR-typing methods together with drug-resistance profiles were used to trace the distribution of antibiotic resistant P. aeruginosa clones and to identify cross-infection of the same patient with a different bacterial clone after being moved to a different hospital unit. The data presented here demonstrates a rapid, reliable and useful method for epidemiological surveillance that can contribute to the control of P aeruginosa infections in hospital environments.
antibiotic resistance; molecular typing; nosocomial infection; P. aeruginosa; rDNA-PCR; tDNA-PCR
GENETICS OF MICROORGANISMS
RESEARCH ARTICLES
Polymorphism of the rDNA and tDNA loci in clinical isolates of Pseudomonas aeruginosa: a perspective for molecular epidemiology surveillance
Isabel Cristina Guerra SpacovI, II; Suzileyde Alberto Marques da SilvaII; Marcos Antônio de Morais JúniorI, II; Márcia Maria Camargo de MoraisII, III
IDepartamento de Genética, Universidade Federal de Pernambuco, Recife,PE,Brazil
IILaboratório de Imunopatologia Keizo Asami, Universidade Federal de Pernambuco, Recife, PE, Brazil
IIIDepartamento de Patologia, Instituto de Ciências Biológicas, Universidade de Pernambuco, Recife, PE, Brazil
Send correspondence to Send correspondence to Márcia Maria Camargo de Morais Departamento de Patologia, Instituto de Ciências Biológicas Universidade de Pernambuco Rua Arnóbio Marques 310 50100-130 Recife, PE, Brazil E-mail: camargo@icb.upe.br
ABSTRACT
The Gram-negative bacterium Pseudomonas aeruginosa has a wide environmental and ecological distribution. It is an opportunistic pathogen that acquires resistance to multiple antimicrobial agents and can infect plants, animals and humans. We used rDNA and tDNA PCR markers to characterize the bacterial diversity of P. aeruginosa strains isolated at a Brazilian teaching hospital (Oswaldo Cruz University Hospital, Recife, Brazil) between March 2003 and February 2004. Clonal groups of P. aeruginosa clinical isolates were identified from different patients in different hospital units using either rDNA or tDNA markers, or a combination of both in a duplex PCR. These PCR-typing methods together with drug-resistance profiles were used to trace the distribution of antibiotic resistant P. aeruginosa clones and to identify cross-infection of the same patient with a different bacterial clone after being moved to a different hospital unit. The data presented here demonstrates a rapid, reliable and useful method for epidemiological surveillance that can contribute to the control of P aeruginosa infections in hospital environments.
Key words: antibiotic resistance, molecular typing, nosocomial infection, P. aeruginosa, rDNA-PCR, tDNA-PCR.
Introduction
The routine identification of hospital isolates of Pseudomonas aeruginosa is based on phenotypic analysis using characteristic biochemical reactions. However, horizontal dissemination cannot be traced properly using phenotypic methods based only on genus and specie identification. On the other hand, methods based on the polymerase chain reaction (PCR) have been widely used for the analysis of the genetic diversity of many microorganisms (Agodi et al., 2000; Lopes et al., 2005). Depending on the primers and amplification conditions employed, the results allow the discrimination of organisms at not only the genus and species level but also at the level of individual strains. By using molecular typing methods it is possible to identify genetic similarities between both phenotypically unrelated and phenotypically analogous strains. These molecular approaches provide the precise information necessary for the monitoring and control of hospital infections. Moreover, epidemiological relationships can be detected and/or confirmed and clonal groups can be delimited (Severino et al., 1999).
The fact that the complete P. aeruginosa genome sequence is known allows the identification of polymorphic regions present in different bacterial strains and the design of methods to discriminate isolates based on such polymorphisms (see The Pseudomonas Genome Project at www.pseudomonas.com).
The P. aeruginosa rRNA cluster (5S-23S-(ISR)-tRNAALA-(ISR)-tRNAILE -(ISR)-16S) is distributed four times throughout the genome of the sequenced P. aeruginosa strain Pa01. The intergenic spacer regions (ISRs) are subject to lower evolutionary pressure, and therefore show wider genetic variation (Gürtler and Stanisich, 1996) that is dependent on the number and type of both tRNA genes and boxA sequence between 23S and 16S sequences and by the presence of enzyme-recognition sites at the ISRs, besides the length of the ISRs themselves (García-Martínez et al., 1999). Two PCR-based methods have been proposed for identifying this cluster. Intergenic Transcribed Spacer PCR ribotyping (PCR-ribotyping) uses specific primers that amplifies sequences between the 16S and 23S gene and has been applied for molecular identification of bacteria at the species level (Jensen et al., 1993; Daffonchio et al., 1998; Agodi et al., 2000) and the discrimination of bacterial strains (Kostman et al., 1992; Clementino et al., 2001; Pereira et al., 2002). This method detects both the number of tRNA genes and the spacer length within the cluster. Another highly promising method for bacterial identification is based on the PCR length polymorphisms of the intergenic spacers between tRNA genes (tDNA-PCR) spread along the bacterial genome. The tRNA genes are highly conserved among eubacteria and occur in multiple copies throughout the bacterial genome, within and outside rDNA gene clusters. These genes are generally clustered and are separated by spacers whose length and sequences are subjected to a higher degree of variations (Welsh and McClelland, 1992). The tDNA-PCR method uses consensus primers complementary to the highly conserved edges of the flanking tRNA genes that are directed outwards (Welsh and McClelland, 1991). This method is regarded as producing species-specific banding patterns and has been applied to the differentiation of various bacterial species (Vaneechoutte et al., 1998; De Gheldre et al., 1999; Clementino et al., 2001).
A significant increase in the number of multi-drug resistant (MDR) P. aeruginosa infections has been detected by our group at the Oswaldo Cruz University Hospital, Recife, Brazil (unpublished results). However, biochemical routine identification was not able to distinguish clonal groups of multi-drug resistant strains. We tested rDNA-PCR and tDNA-PCR for their capacity to type P. aeruginosa hospital isolates, our aim being to contribute to the epidemiological control of P. aeruginosa infections. Our results indicate that rDNA/tDNA duplex PCR might be useful for P. aeruginosa typing of isolates from different hospital units. Moreover, the sequence of acquisition and/or expression of antimicrobial resistance by cells of distinct clonal groups could be identified.
Material and Methods
Bacterial strains and microbiological methods
The Pseudomonas Genome Project (Boston, USA) kindly provided Pseudomonas aeruginosa strain PA01 and The Oswaldo Cruz Foundation Microbial Collection (INCQS, Rio de Janeiro, Brazil) provided the Pseudomonas reference strains P. aeruginosa ATCC 9027, P. putida ATCC 15175 and P. fluorescens ATCC 13525.
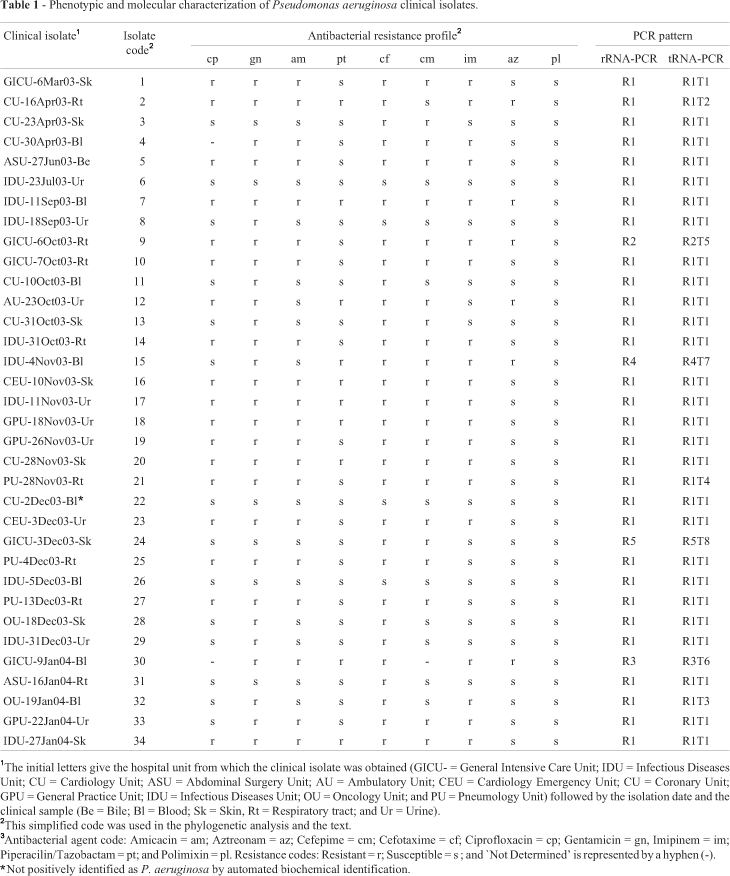
We collected 34 P. aeruginosa isolates from nosocomial infections of patients hospitalized in different units of the Oswaldo Cruz University Hospital (Recife, Brazil) between March 2003 and February 2004, the isolates being obtained from a variety of clinical samples (Table 1). The isolates were identified by colony pigmentation, grape-like odor, motility and biochemical tests (carbohydrate fermentation (-), citrate assimilation (+), lysine decarboxylase (-), indol (-), oxidase (+), beta-hemolysis on blood-agar (+), DNAse (-). Identifications were confirmed using the ID32 Mini-Api automatic system (BioMerieux, France). Antibiogram data were registered by disk diffusion method according to the criteria of National Committee for Clinical Laboratory Standards (NCCLS M100). Strains were maintained on nutrient agar (pH 7.4) containing (gL-1) beef extract, 10; tryptone, 5; NaCl, 5; bacteriological agar 12 g or by freezing in 15% (v/v) glycerol at -80 °C. All reagents were of at least analytical purity, and beef extract, tryptone and bacteriological agar were supplied by Oxoid (Oxoid Brasil Ltda, São Paulo, Brazil).
Of the 34 P. aeruginosa hospital isolates collected, 33 were confirmed by automated identification except for isolate 22 (Table 1) which gave an unacceptable profile for P. aeruginosa. All the isolates, including isolate 22, were submitted to antibiogram assays with nine antibacterial agents suitable for Pseudomonas (Pellegrino et al., 2002). Isolates 6, 22 and 26 were susceptible to all drugs tested while the other isolates displayed different individual susceptibility profiles, with some showing multiple drug resistance (MDR) to up to eight different antibacterial agents. No correlation was observed between bacterial antibacterial susceptibility profiles and site of infection or hospital unit (Table 1). Genomic DNA from each isolate was extracted for further molecular analysis.
Genotyping
Genomic DNA was extracted according to Ausubel et al (1989), separated on 0.8% (w/v) agarose gel, stained using ethidium bromide and visualized under UV light.
We carried out rDNA-PCR by amplification of ISRs regions between the rRNA16S-23S genes with the 03 primer (5'-TTGTACACACCGCCCGTCA-3') complementary to the rRNA 23S conserved region and the 04 primer (5'-GGTACCTTAGATTGTTTCAGTTC-3'), complementary to the rRNA 16S conserved region according to Kostman et al. (1992). The PCR reactions were prepared containing 50 ng of genomic DNA, 20 pmol of each primer, 4 pmols of dNTP, 37.5 pmols of MgCl2, 1U of Taq DNA polymerase and 1x reaction buffer (Invitrogen) in a final volume of 25 µL and the amplifications carried out in a Hybaid Touchdown PCR thermocycler (Thermo Electron Corp., Waltham, MA) using an initial denaturing step of 2 min at 94 °C, followed by 30 cycles of 1 min at 94 °C, 1 min at 55 °C and 1 min at 72 °C with a final 7 min extension at 72 °C. Amplification products were separated on 1.6% (w/v) agarose gel in 0.5x TBE buffer at 10 V.cm-1 and stained with ethidium bromide.
The tDNA-PCR consisted of amplification of ISRs regions between the tDNA genes using the conserved primers T5A (5'-AGT CCG GTG CTC TAA CCA ACT GAC-3') and T3B (5'-AGG TCG CGG GTT CGA ATC C-3') described by Welsh and McClelland (1991). The reaction mix was prepared as described for the rDNA-PCR. Amplification conditions consisted of denaturation for 2 min at 94 °C followed by 30 cycles of 30 s at 94 °C, 30 s at 55 °C and 2 min at 72 °C with a final 10 min extension at 72 °C. Amplification products were separated and visualized as described above.
Duplex PCR used both sets of primer pairs, rDNA and tDNA markers, and the reaction was conducted according to the rDNA-PCR protocol described above, with minor modifications (2 min for the cyclic extensions at 72 °C and 10 min final extension). Amplification products were separated on 1% (w/v) agarose gel and stained as described above.
Computational analysis
The bacterial isolates were analyzed using the NetWork program version 4.1.0.8 (Fluxus Technology Ltd, www.fluxus-engineering.com) designed for phylogeny analysis and showing the geographic distribution of haplotypes. Binary data, grouped according median analysis, consisted of susceptibility (0) and resistance (1) to the antibiotics tested.
Results
Genetic typing
For the rDNA marker the reference strains P. aeruginosa PA01 and ATCC 9027 showed the same amplification profile (banding pattern) and a characteristic 800 base pair (bp) band, which was not shown by P. putida ATCC 15175 and P. fluorescens ATCC 13525 (Figure 1A). We included P. putida because it had been reported in cases of bacteremia in intensive care units for newborn babies (Bouallegue, et al., 2004) while P. fluorescens was selected due to reports of its presence in oncology units (Hsueh, et al., 1998). We found that isolate 22 showed an rDNA marker banding pattern similar to the two P. aeruginosa reference strains (Figure 1B), suggesting that was indeed a P. aeruginosa isolate. The rDNA marker banding patterns for the remaining 33 isolates were classified in five distinct rDNA-PCR banding patterns (profiles R1 to R5, Figure 1B) of which the R1 pattern was observed in 88% of the isolates (34/30) and the two P. aeruginosa reference strains. The other four patterns were observed for only one isolate each. The rDNA marker showed 15% of genetic variability among the isolates (5/34).
For the tDNA marker the two P. aeruginosa reference strains again presented the same banding pattern, including four common bands (365, 250, 170 and 125 bp). As was seen for the rDNA marker banding patterns, the tDNA marker banding patterns for the P. putida and P. fluorescens reference strains also differed from those observed for the P. aeruginosa reference strains (Figure 2A). The tDNA marker banding pattern of isolate 22 was similar to the reference strain P. aeruginosa ATCC 9027 (Figure 2B), as also observed for the rDNA-PCR marker. Analysis of the other 33 isolates revealed eight different tDNA-PCR banding patterns (profiles T1 to T8, Figure 2C) of which the T1 pattern was seen in 79% of our bacterial isolates (27/34) as well as the two P. aeruginosa reference strains. The tDNA marker showed 23% of genetic variability among the isolates (8/34).
Using the R1 rDNA profile and four of the tDNA profiles together resulted in four additional tDNA profiles (profiles R1T1, R1T2, R1T3 and R1T4), with R1T1 representing the most common pattern found among the isolates. Although the polymorphism occurring for both primers cannot be considered species-specific for P. aeruginosa it can, however, be concluded that any bacterial isolate presenting the R1T1 profile can be identified as belonging to this species, even, as the case of isolate 22, with ambiguous identification by the biochemical tests.
The results showed the discrimination power of both markers, which can be used as an epidemiological tool for detection of strain dispersion. In this sense, the results of PCR typing and the resistance profiles showed that bacterial isolates 16, 17, 118, 20 and 34 may correspond to a unique P. aeruginosa strain which was isolated from four different hospital units and infected at least five different patients during the period of study (Table 1). Moreover, in one case two genotypically different isolates were collected from the same patient. Isolate 21 ( genotype R1T4) was isolated first but five days after the patient had been transferred to another hospital unit isolate 23 (genotype R1T1) was recovered from the same patient (Figure 3), suggesting that isolate 23 may represent a second nosocomial infection episode.
Duplex-PCR typing
The results above suggested the possibility of combining both markers in a duplex PCR for use in typing and screening of P. aeruginosa clinical isolates. Duplex PCR of the two P. aeruginosa reference strains and the P. putida and P. fluorescens reference strains generated the expected profiles resulted from the combination of the both markers (data not shown). When duplex PCR was used for typing the 34 isolates, the profiles observed corresponded to the eight profiles detected, showing the same percentage of genetic variability observed for the tDNA marker (data not shown). Duplex rDNA/tDNA-PCR may thus be used as a reliable approach to follow the spread of different bacterial clonal lineages across different hospital units.
Evolution of the resistance to antibiotics
The molecular typing results suggested that some strains were disseminated to different units in the hospital. In addition, such strains may suffer distinct selective pressures at the different units, resulting in the acquisition and/or expression of differential resistance to antibiotics. In such a context, the isolates of the clonal lineage R1T1 were submitted to computational analysis that revealed a haplotypic distribution among its isolates and the emergence of antibiotic resistance (Figure 4). In this analysis, isolates 6, 22 and 26 (clonal group 6) were susceptible to all the antibiotics tested (Table 1) and could be the basal isolates from which isolate 8 (gentamicin resistant) and 31 (cefotaxime resistant) evolved by distinct events of acquiring or expressing resistance. The expression of resistance to cefotaxime by isolate 8 or to gentamicin by isolate 31 could have generated clonal group 11 (isolates 11, 28 and 29) and, likewise, expression of resistance to cefepime could have generated isolate 3. The expression of resistance to cefepime by group 11 isolates or to gentamicin by isolate 3 could have generated the isolate 13 and so on. Our analysis suggests that isolate 7 emerged from group 6 after sequential expression of resistance to eight different antibiotics. Moreover, this in silico analysis suggests that at least one representative of the R1T1 genotype has not been isolated among our bacterial samples (Figure 4). This clone, showing resistance to four antibiotics, could have been the basal strain for clonal groups 4 and 25 and may be present, but occult, in one of the hospital units. Obviously, this analysis is an attempt to establish the relatedness among the observed clonal lineages, which does not excluded the possibility that new unrelated R1T1 clones could enter the hospital.
Discussion
At 6.3 Mb the P. aeruginosa genome is the largest bacterial genome so far sequenced, and this size, coupled with the high number of regulatory genes and subsequent complex metabolism, is consistent with its versatility in adapting to different environments (Stover et al., 2000). Based on this genomic versatility we used two PCR markers that were sufficiently polymorphic to discriminate bacterial isolates from different clonal origins, our results being reproducible and allowed the establishment of common amplification patterns detected for the type strains and for the majority of hospital isolates. However, because of the polymorphism presented by both markers, we cannot suggest their use for routine bacterial identification, except, as in the case of the isolate 22, when bacterial isolates present the common pattern generated by type-strains.
Many techniques have been described for typing P. aeruginosa, such as arbitrarily primed PCR (Kersulyte et al., 1995; Bennekov et al., 1996), pulsed field gel electrophoresis (Kersulyte et al., 1995; Agodi et al., 2000; Nakamura et al., 2001), traditional ribotyping (Bennekov et al., 1996; Agodi et al., 2000) and more recently multi-locus sequence typing (MLST; Curran et al, 2004) and enterobacterial repetitive intergenic consensus-based PCR (ERIC-PCR; Yang et al., 2005). One of these methods, rDNA-PCR typing, has been reported as a rapid and accurate method for typing P. aeruginosa (Liu et al, 1996; Agodi et al., 2000) and Burkholderia (Pseudomonas) cepacia (Dasen et al. 1994; Kostman at al., 1992). In our present work the rDNA marker revealed four distinct clonal groups among our P. aeruginosa clinical isolates. Our technique was based on the amplification of ITS loci at rDNA gene clusters and any polymorphism detected may have been due to significant genetic variation such as nucleotide deletions or insertions of tRNA genes or ISRs. Therefore, two bacterial isolates presenting the same amplification pattern could be considered as originating from the same clonal origin. The use of ITS locus restriction analysis and RFLP-ribotyping has been proposed for strain discrimination and clustering by evaluating genetic differences caused by point mutations that create or remove restriction sites (Gruner et al., 1993).
Several authors have used tDNA-PCR typing to identify bacterial genera and species, e.g. Lactobacillus (Baele et al., 2002) and other bacteria (Welsh and McClelland, 1991) but our results suggest that this approach is more appropriated for intraspecific discrimination and we have found similar results for clinical isolates of Klebsiella pneumoniae (manuscript in preparation). Our work is the first report of the application of tDNA to P. aeruginosa typing, which increased the discriminatory power of the rDNA marker and the combination of both markers in a duplex PCR proved to be a reliable tool with high discriminatory power for typing P. aeruginosa isolates. The combination of both markers generated amplification patterns that varied from three to ten bands, which may be enough for strain discrimination.
Strain discrimination and clustering can also be obtained using RAPD analysis (Matar et al., 2005), which generates more bands than tDNA-PCR typing. However, RAPD analysis has been criticized because of its low reproducibility and reliability and the fact that amplification can be influenced by factors other than the genetic variation between bacterial isolates. Therefore, it can be difficult to reproduce RAPD results between different laboratories, which is a great disadvantage for epidemiological surveillance studies. On the other hand, the specific primer annealing which occurs with both rDNA-PCR and tDNA-PCR ensures reproducibility wherever these techniques are used.
Gencer et al. (2002) have pointed out that P. aeruginosa strains are the third most prevalent pathogen isolated from cases of hospital infections, while Pellegrino et al. (2002) reported that in some hospitals it is the main bacterial species isolated. The Intensive Care Unit (ICU) at the hospital where our isolates were collected has shown an increase in the number of cases of P. aeruginosa infections over the last five years as well as in the antibiotic resistance (data not shown) and diversity of the clonal groups of the P. aeruginosa isolates recovered (Table 1). Similar genetic variability in ICU P. aeruginosa clinical isolates has been reported by Sarwari et al. (2004) and Stover et al. (2000) have noted that the genome complexity of P. aeruginosa seems to be responsible for the versatility, environmental adaptation and intrinsic drug resistance exhibited by this organism.
Nosocomial infections related to P. aeruginosa are frequently caused by multi-drug resistant (MDR) strains (Sarwari et al., 2004), which we also detected in our study (Table 1). However, no correlations between clonal groups and susceptibility profiles were detected. The same finding was observed when the different units of the hospital or the sites of infection were compared to the genotype profiles (Table 1). Nevertheless, data from susceptibility tests and amplification profiles could be used to follow the spread of clonal groups through the hospital units. In addition, such data could be useful for analysis of the evolution of the phenotype of resistance to antibiotics, which may represent the expression of intrinsic mechanisms or the acquisition of resistance mechanisms by horizontal genetic transmission (Poole and Srikumar, 2001). Analysis of the distribution of antibiotic resistance could help in developing an effective control of nosocomial infections by identifying the origin of contamination. By assuming that all the isolates in the R1T1 group were genetically similar we were able to follow the spread of members of this group (and of the other seven groups) to the various hospital units and infection sites. This was the case for clonal group 16 (isolates 16, 17, 18, 20 and 34), members of which were detected in five different patients in distinct units of the hospital, indicating that members of this group were dispersed in different points within the hospital. On the other hand, some isolates seemed to be confined to a particular unit (Table 1). Our results support those of Gales et al. (2001) and suggest that informative molecular markers can be a powerful method for following the dispersion of clonal groups and to detect the occurrence of cross-infections (Blanc et al., 1997; Ruiz et al., 2004). Another example of informative data produced by our analysis is the identification of the two clones which were collected from the same patient at different times and in different hospital units, these clones showing the same antibiotic resistance phenotype but distinct tDNA profiles. These results indicate that phenotypic convergence for antibiotics resistance can occur in different bacterial strains submitted to similar selective pressure.
In conclusion, our results indicate that the use of rDNA and tDNA markers for P. aeruginosa typing was sufficiently discriminatory to generate very informative data regarding the clonal dispersion of nosocomial hospital strains of P. aeruginosa. Moreover, results from the duplex-PCR associated to antibiotic susceptibility analysis may be used as an epidemiological approach to prevent P. aeruginosa outbreaks.
Acknowledgments
The authors thank the following: Oswaldo Cruz University Hospital; University of Pernambuco Bacteriology Laboratory; and the Keizo Asami Immunopathology Laboratory of the Federal University of Pernambuco, Recife, Pernambuco, Brazil. We also thank INCQS/FIOCRUZ for the reference strains and the Pseudomonas Genome Project for the sequenced strain and the public information about the bacterial genome. This work was supported by grants from the Brazilian agencies CAPES, CNPq and FACEPE.
Received: July 4, 2005; Accepted: April 4, 2006.
Associate Editor: Sérgio Olavo Pinto da Costa
- Agodi A, Sciacca A, Campanile F, Messina C, Barchitta M, Sciacca S and Stefani S (2000). Molecular epidemiology of Pseudomonas aeruginosa from cystic fibrosis in Sicily: Genome macrorestriction analysis and rapid PCR-ribotyping. New Microbiol 23:319-327.
- Ausubel FM, Brent R, Kingston RE, Moore DD and Smith JA (1989) Current Protocols in Molecular Biology. v 1 and 2. Jonh Willey & Sons, Inc. New York, 650 pp.
- Baele M, Vaneechoutte M, Verhelst R, Vancanneyt M, Devriese LA and Haesebrouck F (2002) Identification of Lactobacillus species using tRNA-PCR. J Microbiol Methods 50:263-271.
- Benekov T, Colding H, Ojeniyi B, Bentzon MW and Hoiby N (1996) Comparison of ribotyping and genome fingerprinting of Pseudomonas aeruginosa isolates from cystic fibrosis patients. J Clin Microbiol 34:202-204.
- Blanc DS, Parret T, Janin B, Raselli P and Francioli P (1997) Nosocomial infections and pseudoinfections from contaminated bronchoscopes: Two-year follow up using molecular markers. Infect Control Hosp Epidemiol 18:134-136.
- Bouallegue O, Mzoughi R, Weill FX, Mahdhaoui N, Ben Salem Y, Sboui H, Grimont F and Grimont PA (2004) Outbreak of Pseudomonas putida bacteraemia in a neonatal intensive care unit. J Hosp Infect 57:88-91.
- Clementino MM, Filippis I, Nascimento CR, Branquinho R, Rocha CL and Martins O (2001) PCR analysis of tRNA intergenic spacer, 16S-23S internal transcribed spacer, and randomly amplified polymorphic DNA reveal inter- and intraspecific relationships of Enterobacter cloacae strains. J Clin Microbiol 39:3865-3870.
- Curran B, Jonas D, Grundmann H, Pitt T and Dowson CG (2004) Development of a multilocus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa J Clin Microbiol 42:5644-5649.
- Daffonchio D, Borin S, Giuseppe F, Manachini PL and Sorlini C (1998) PCR fingerprinting of whole genome: The spacers between the 16S and 23S rRNA genes and of intergenic tRNA gene regions reveal a different intraspecific genomic variability of Bacillus cereus and Bacillus licheniformis Int J Syst Bacteriol 48:107-116.
- Dasen SE, LiPuma JJ, Kostman JR and Stull TL (1994) Characterization of PCR-ribotyping for Burkholderia (Pseudomonas) cepacia J Clin Microbiol 32:2422-2424.
- De Gheldre Y, Vandamme P, Gooses H and Struelens MJ (1999) Identification of clinically relevant viridans streptococci by analysis of transfer DNA intergenic spacer length polymorphism. Int J Syst Bacteriol 49:1591-1598.
- Gales AC, Jones RN, Turnidge J, Rennie R and Ramphal R. (2001) Characterization of Pseudomonas aeruginosa isolates: Occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY antimicrobial surveillance program, 1997-1999. Clin Infect Dis 15:146-155.
- García-Martínez J, Acinas SG, Antón AI and Rodríguez-Valera F (1999). Use of the 16S-23S ribosomal genes spacer region in studies of prokaryotic diversity. J Microbiol Meth 36:55-64.
- Gencer S, Ak O, Benzonana N, Batirel A and Ozer S (2002) Susceptibility patterns and cross resistances of antibiotics against Pseudomonas aeruginosa in a teaching hospital of Turkey. Ann Clin Microbiol Antimicrob 9:2.
- Gürtler V and Stanisich A (1996) New approaches to typing and identification of bacteria using the 16S-23S rRNA spacer region. Microbiology 142:3-6.
- Gruner E, Kropec A, Huebner J, Altwegg M and Daschner F (1993) Ribotyping of Pseudomonas aeruginosa strains isolated from surgical intensive care patients. J Infect Dis 167:1216-20.
- Hsueh PR, Teng LJ, Pan HJ, Chen YC, Sun CC, Ho SW and Luh KT (1998) Outbreak of Pseudomonas fluorescens bacteremia among oncology patients. J Clin Microbiol 36:2914-2917.
- Jensen MA, Webster JA and Straus N (1993) Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl Environ Microbiol 59:945-952.
- Kersulyte D, Struelens MJ, Deplano A and Berg DE (1995) Comparison of arbitrarily primed PCR and macrorestriction (pulsed field gel electrophoresis) typing of Pseudomonas aeruginosa strains from cystic fibrosis patients. J Clin Microbiol 33:2216-2219.
- Kostman JR, Edlind TD, Lipuma JJ and Stull TL (1992) Molecular epidemiology of Pseudomonas cepacia determined by polymerase chain reation. J Clin Microbiol 30:2084-2087.
- Liu Y, Davin-Regli A, Bosi C, Charrel RN and Bollet C (1996) Epidemiological investigation of Pseudomonas aeruginosa nosocomial bacteraemia isolates by PCR-based DNA fingerprinting analysis. J Med Microbiol 45:359-365.
- Lopes ACS, Rodrigues JF and Morais Jr MA (2005) Molecular typing of Klebsiella pneumoniae isolates from public hospitals in Recife, Brazil. Microbiol Res 160:37-46.
- Matar GM, Chaar MH, Araj GF, Srour Z, Jamaleddine G and Hadi U (2005) Detection of a highly prevalent and potentially virulent strain of Pseudomonas aeruginosa from nosocomial infections in a medical center. BMC Microbiol 20:29.
- Nakamura ALC, Novales MGM, Miranda BL, Gómez LP, Chavez AH, Rendón JÁ and Benavides AS (2001) Epidemiologic study of Pseudomonas aeruginosa in critical patients and reservoirs. Arch Med Res 32:238-242.
- Pellegrino FL, Teixeira LM, Carvalho M da G, Aranha NS, Pinto OM, Mello SJL, D'Avila FA, Ferreira AL, Amorim EL, Riley LW and Moreira BM (2002) Occurrence of a multidrug-resistant Pseudomonas aeruginosa clone in different hospitals in Rio de Janeiro, Brazil. J Clin Microbiol 40:2420-4.
- Pereira MSV, Leal NC, Leal TCA, Sobreira M, Almeida AMP, Siqueira-Júnior JP and Campos-Takaki GM (2002) Typing of human and bovine Staphylococcus aureus by RAPD-PCR and ribotyping-PCR. Lett Appl Microbiol 35:32-36.
- Poole K and Srikumar R (2001) Multidrug efflux in Pseudomonas aeruginosa: Components, mechanisms and clinical significance. Curr Top Med Chem 1:59-71.
- Ruiz L, Dominguez MA, Ruiz N and Vinas M (2004) Relationship between clinical and environmental isolates of Pseudomonas aeruginosa in a hospital setting. Arch Med Res 35:251-257.
- Sarwari A, Hasan R, Lim CB, Ng Y, Ng C and Zaman S (2004) PCR identification and automated ribotyping of Pseudomonas aeruginosa clinical isolates from intensive care patients. Scand J Infect Dis 36:342-349.
- Severino P, Darini AL and Magalhães VD (1999) The discriminatory power of ribo-PCR compared to conventional ribotyping for epidemiological purposes. APMIS 107:107910-84.
- Stover KC, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FSL, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK-S, Wu Z, Paulsen I, Reizer J, Saier MH, Hancock REW, Lory S and Olson MV (2000). Complete genome sequence of Pseudomonas aeruginosa PAO1: An opportunistic pathogen. Nature 406:959-964.
- Vaneechoutte M, Boerlin P, Tichy HV, Bannerman E, Jäger B and Bille J (1998) Comparison of PCR-based DNA fingerprinting techniques for the identification of Listeria species and their use for atypical Listeria isolates. Int J Syst Bacteriol 48:127-139.
- Yang W, Shi L, Jia WX, Yin X, Su JY, Kou Y, Yi X, Shinoda S and Miyoshi S (2005) Evaluation of the biofilm-forming ability and genetic typing for clinical isolates of Pseudomonas aeruginosa by enterobacterial repetitive intergenic consensus-based PCR. Microbiol Immunol 49:1057-1061.
- Welsh J and McClelland M (1991) Genomic fingerprints produced by PCR with consensus tRNA gene primers. Nucleic Acids Res 19:861-866.
- Welsh J and McClelland M (1992) PCR-amplified length polymorphisms in tRNA intergenic spacers for categorizing staphylococci. Mol Microbiol 6:1673-168.
Send correspondence to
Publication Dates
-
Publication in this collection
21 Nov 2006 -
Date of issue
2006
History
-
Accepted
04 Apr 2006 -
Received
04 July 2005