Abstract
The ADH (alcohol dehydrogenase) system is one of the earliest known models of molecular evolution, and is still the most studied in Drosophila. Herein, we studied this model in the genus Anastrepha (Diptera, Tephritidae). Due to the remarkable advantages it presents, it is possible to cross species with different Adh genotypes and with different phenotype traits related to ethanol tolerance. The two species studied here each have a different number of Adh gene copies, whereby crosses generate polymorphisms in gene number and in composition of the genetic background. We measured certain traits related to ethanol metabolism and tolerance. ADH specific enzyme activity presented gene by environment interactions, and the larval protein content showed an additive pattern of inheritance, whilst ADH enzyme activity per larva presented a complex behavior that may be explained by epistatic effects. Regression models suggest that there are heritable factors acting on ethanol tolerance, which may be related to enzymatic activity of the ADHs and to larval mass, although a pronounced environmental effect on ethanol tolerance was also observed. By using these data, we speculated on the mechanisms of ethanol tolerance and its inheritance as well as of associated traits.
alcohol dehydrogenase; Anastrepha; hybrids; ethanol tolerance; Tephritidae
EVOLUTIONARY GENETICS
RESEARCH ARTICLE
Alcohol dehydrogenase activities and ethanol tolerance in Anastrepha (Diptera, Tephritidae) fruit-fly species and their hybrids
Eneas CarvalhoI, II; Vera Nisaka SolferiniIII; Sergio Russo MatioliII
ILaboratório de Parasitologia, Instituto Butantan, São Paulo, SP, Brazil
IIDepartamento de Genética e Biologia Evolutiva, Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brazil
IIIDepartamento de Genética e Evolução, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, SP, Brazil
Send correspondence to Send correspondence to: Eneas Carvalho Laboratório de Parasitologia, Instituto Butantan Avenida Vital Brazil 1500 05503-900 São Paulo, SP, Brazil E-mail: eneas@butantan.gov.br
ABSTRACT
The ADH (alcohol dehydrogenase) system is one of the earliest known models of molecular evolution, and is still the most studied in Drosophila. Herein, we studied this model in the genus Anastrepha (Diptera, Tephritidae). Due to the remarkable advantages it presents, it is possible to cross species with different Adh genotypes and with different phenotype traits related to ethanol tolerance. The two species studied here each have a different number of Adh gene copies, whereby crosses generate polymorphisms in gene number and in composition of the genetic background. We measured certain traits related to ethanol metabolism and tolerance. ADH specific enzyme activity presented gene by environment interactions, and the larval protein content showed an additive pattern of inheritance, whilst ADH enzyme activity per larva presented a complex behavior that may be explained by epistatic effects. Regression models suggest that there are heritable factors acting on ethanol tolerance, which may be related to enzymatic activity of the ADHs and to larval mass, although a pronounced environmental effect on ethanol tolerance was also observed. By using these data, we speculated on the mechanisms of ethanol tolerance and its inheritance as well as of associated traits.
Key words: alcohol dehydrogenase, Anastrepha, hybrids, ethanol tolerance, Tephritidae.
Introduction
The alcohol dehydrogenase enzyme system (ADH) of Drosophila is a classical model used in understanding the question of the evolutionary relevance of enzyme polymorphism. This system permits access to several biological levels, from organismal to molecular (Chambers, 1991), and is directly related to environmental factors. As a result of this scenario, the ADH system has been one of the most studied in Drosophila (Chambers, 1991; Luque et al., 1997; Pecsenye et al., 1997). Here we used Anastrepha flies as models for ADH studies, owing to their remarkable ability to undergo viable inter-specific crosses between species that express different numbers of Adh copies and with differences in ADH related traits, a feature rarely observed in Drosophila.
The families Tephritidae and Drosophilidae are phylogenetically related (both belong to the Acalyptratae subsection of Schizophora, Yeates and Wiegmann, 1999), although tephritid larvae feed on fresh vegetal tissues whereas drosophilids feed mainly on fungi. Furthermore, Anastrepha flies are agricultural pests, remarkably jeopardizing fruit production worldwide (Aluja, 1994).
Owing to their obtaining nourishment on fruits during the larval stage, through necessity, these flies withdraw all the nutritional factors from these while ripening. Microorganisms such as yeasts attack sugary fruits and can produce high concentrations of metabolites (Janzen, 1977). One of the most common by-products through the action of such microorganisms is ethanol, which is toxic to flies when in high concentration (Parsons, 1983; Matioli et al., 1992; Chakir et al., 1993; Martel et al., 1995; Pecsenye et al., 1997). Accordingly, since the intake of these products is unavoidable, larvae must possess efficient detoxification mechanisms. An important element is the ADH system, which degrades 90% of the total ethanol in Drosophila melanogaster (Heinstra et al., 1987; Geer et al., 1993). ADH action per se is enough to convert a toxic exogenous substance (ethanol) into a common endogenous one (acetate) (Kapoun et al., 1990; Chakir et al., 1993; Geer et al., 1993).
Several features of the ADH system in Drosophila are related to alcohol metabolism and tolerance (Heinstra et al., 1987; Geer et al., 1993). On the other hand, Geer et al. (1993) emphasized that many other factors may play an important role in alcohol metabolism and tolerance, such as the activity of other enzymes, the composition of the cell membrane and its susceptibility to ethanol, the intensity of signal transduction in the presence of ethanol and, finally, the physiological state of the individual larva. While ethanol tolerance is a complex trait with regard to its components, its measurement is direct and simple. What remains difficult and not totally clear is the determination of all those factors that cause the observed tolerance values.
In addition to its role as a detoxification agent, evidence from studies with Drosophila species indicate that the ADH enzyme is also involved in the regulation of fatty acid synthesis (Geer et al., 1985; Freriksen et al., 1991), and even in the use of ethanol as an energetic source, particularly at lower concentrations (Bokor and Pecsenye, 2000).
The ADH system of Anastrepha is similar to that of Drosophila in its electrophoretic patterns, the dimeric composition of the functional enzyme and the differential tissue and life stage expression of loci (Matioli et al., 1986, 1992; Nascimento and Oliveira, 1997). Their ADH enzymes seem to have evolved independently although from a common ancestral gene (Ashburner, 1998). Brogna et al. (2001) go as far as to suggest that ancestral genes of ADH from tephritid and drosophilid appeared earlier than the separation of these two families, prior to the Calyptratae/Acalyptratae divergence.
The number of Adh loci is variable in Tephritidae flies. Goulielmos et al. (2003) suggest that the Adh locus duplicated early in this family, before the emergence of various genera. Consequently, whilst some species have only one Adh locus (e.g.: Acinia fucata and Rachiptera limbata), many others have two (e.g.: Bactrocera oleae, Ceratitis capitata and A. fraterculus), and some rarely observed species (e.g.: A. obliqua) even have three. Interestingly, the group with one locus lives inside inflorescences or galls, while, on the contrary, the group with two or more loci abides inside ripening fruits (Matioli et al., 1992). As pointed out by Goulielmos et al. (2003), this observation may correlate ADH evolution with speciation through adaptation to various feeding niches. According to Eliopoulos et al. (2004), the isozymic-specific residues of ADH1 and ADH2 may be related to preferential binding of different alcohols or to interactions with other proteins.
We studied two species, A. fraterculus and A. obliqua, with two and three Adh loci, respectively (Matioli et al., 1986, 1992). Intercrossing between these two species has been described (dos Santos et al., 2001), and generates only hybrid females. These are fertile and can be backcrossed with males from both parental species. As a result, the parents, both hybrids and backcrosses, constitute groups with differences in both the number of Adh loci and their genetic background.
We considered this as an interesting model for studying the relationship between the ADH system and ethanol metabolism and tolerance. The parents, hybrids and backcrosses of A. fraterculus and A. obliqua, were studied regarding ADH activity and survival. The parameters analyzed were (1) phenotype/physiological factors (ADH activities, larval protein content and ethanol tolerance), (2) environmental factors (exposure time and ethanol concentration) and (3) genetic factors (genetic background composition).
Materials and Methods
Population rearing and crosses
Flies were reared from guavas, collected in infested orchards. A. sp 1 nr fraterculus was collected in Louveira, SP, Brazil, in February 1995, and A. obliqua in Bauru, SP, Brazil, in March 1995. Since then, the flies were being reared under laboratory conditions, with a non-fermenting artificial diet for adults and guava as a substrate for the larvae. For the crosses, the flies were separated according to sex, just after emergence. Following sexual maturity (10 days, at least), 10 virgin females of one species were placed together with 10 virgin males of the other, their number being kept constant. The cross between A. obliqua females and A. fraterculus males produced viable and fertile females. The reciprocal cross was not undertaken due to difficulties in obtaining viable offspring. The backcrosses of female hybrids with males of both parental species were also performed. Thus, we ended up with five groups to work with: A. fraterculus, A. obliqua, hybrid, backcross 1 (hybrid females backcrossed with A. fraterculus males) and backcross 2 (hybrid females backcrossed with A. obliqua males).
Experimental design
The parental and hybrid groups have a characteristic genetic background and number of Adh gene copies: A. obliqua has a 100% A. obliqua genetic background and six copies of Adh genes; A. fraterculus has a 100% A. fraterculus genetic background and four copies of Adh genes; the hybrid has a 50% A. fraterculus genetic background and a 50% A. obliqua genetic background, and five copies of Adh genes, since these genes seem to have an autosomal inheritance (S. R. Matioli, unpublished data). As a consequence of the recombination and chromosomal segregation in hybrid meiosis, the Adh gene copies and the genetic background of the backcrosses cannot be precisely deduced. However, as a group and on an average, backcross 1 had a 75% A. fraterculus genetic background and a 25% A. obliqua one, whereas backcross 2 had the reverse, a 25% A. fraterculus genetic background and a 75% A. obliqua one. The average Adh gene copies in the backcrosses cannot even be estimated, since these genes were not detected in the genome and its segregation is not as yet understood.
Ethanol exposure
Third instar larvae were exposed to ethanol. The exposure was carried out in Petri dishes sealed with PVC film, each with a cellulose sponge soaked in a solution containing ethanol at different concentrations, 0.15 M NaCl (to maintain the osmotic equilibrium) and 1% glucose (to minimize the use of ethanol as a source of energy or carbon). This experiment was carried out at 25 °C in the absence of light. In each exposure experiment and after the first 12 h of exposure, the larvae were transferred to new Petri dishes with fresh solutions at the same ethanol concentration.
Two protocols of exposure to ethanol were employed:
(1) Exposure to 8% ethanol for 28 h. One hundred larvae of each group were treated and then frozen in liquid nitrogen.
(2) Exposure to 0%, 8%, 12%, 16% and 20% ethanol. One hundred larvae per group were exposed to the five concentrations, twenty to each. They were examined every four hours over a period of 28 h, whereupon immobilized and stretched larvae were considered as dead. These were then removed and frozen in liquid nitrogen, for later measurement of ADH activities and protein contents. The remaining larvae, whether dead or alive, were frozen. Contracted specimens or those in the pupal stage were considered as alive.
Lethal concentration 50 (LC 50) determination
The concentration required to kill half of the larvae exposed during a given time was called the Lethal Concentration 50 (LC 50), and was calculated for all exposure-times (4, 8, 12, 16, 20, 24 and 28 h) for those exposed, according to protocol 2.
LC 50 was calculated by using "EPA PROBIT ANALYSIS" software, from the Ecological Monitoring Research Division Environmental Monitoring Systems Laboratory U. S. Environmental Protection Agency Cincinnati, Ohio 45268, available in their website. When either the model requirements or the heterogeneity test (from EPA software) based on the Chi-square distribution were not satisfied, the calculation was either not carried out, or if so, did not have a measurable error.
Specific enzymatic activity and determination of enzymatic activity per larva
ADH enzymatic activity was determined for the exposed larvae of both protocols 1 and 2. We measured the specific enzymatic activity, which is the enzymatic activity of ADH per protein content (unit: mMol NADH x min-1 x mg total protein-1), as well as the enzymatic activity per larva (unit: mMol NADH x min-1), which is the enzymatic activity of ADH for each individual.
The larvae were removed from the liquid nitrogen and immediately ground up in 50 µL of a pre-cooled homogenization buffer (0.15 M Tris-HCl pH 8.5, EDTA 1 mM, 0.05% β-mercaptoethanol), to be then kept on ice. After homogenization, the samples were centrifuged at RCF = 20800 g for 20 min, and maintained at 4°. Ten microliters of the aqueous phase were mixed with one milliliter of the reacting solution (30 mM isopropanol and 3 mM NAD+ in a 0.15 M Tris-HCl pH 8.5 buffer) pre-heated to 30 °C. NADH formation in this solution was determined every 15 s during a period of 5 min, through spectrophotometry at 340 nm. The temperature was kept at 30 °C and enzymatic activities were calculated from data collected in the first 165 s, so as to avoid substrate limitation. ADH activity was estimated by linear regression. In order to reach the Vmax of ADH, the concentrations of isopropanol and NAD+ in the reacting solution were at least ten times higher than the Km calculated for A. fraterculus ADH (S. R. Matioli, unpublished data).
Protein content
Protein content was determined by using the Bradford (1976) method.
Effects of the developmental environment on alcohol tolerance
To verify the effects of the developmental environment on ethanol tolerance, we reared larvae of both parental species on guava, mango or papaya, the fruits being placed in cages with adult populations. Three distinct samples (20 larvae each) of A. fraterculus and two distinct samples (20 larvae each) of A. obliqua were used. Two samples of A. fraterculus and one of A. obliqua were reared on guava, one of A. fraterculus on papaya and one of A. obliqua on mango. Third instar larvae were collected from the decaying fruits and exposed to ethanol, as previously described in protocol 2. The mortality-data thus obtained was used to calculate LC50.
Statistical analysis
General procedures: Statistical analyses were carried out with JMP software (SAS Institute Inc., Release 5.1.2). Enzymatic activity data were transformed into natural logs, so as to assure normal distribution. For larvae exposed to ethanol according to protocol 1 (no environmental variation), we carried out ANOVA for comparison of sample means, and Student's t test as well as the Tukey-Kramer HSD test for pair-wise comparison of means. In all statistical tests, we considered the significance level at 0.05.
Groups of dead and live larvae were also compared to validate measurements (enzymatic activities and protein content) carried out on dead larva, as well as to verify whether there was any detectable ADH or protein degradation that could take place in the period of four hours after death. This was carried out by comparison of means (t test) with the residues saved after multiple regression analysis, in order to eliminate effects of other variables.
Simple regression when environmental conditions were constant - Exploring the inheritance of the traits: Larvae submitted to protocol 1 were exposed to the same ethanol concentration (8%) over a constant time (28 h), whereby environmental conditions were maintained fixed. Thus, any variation observed in enzymatic activities and protein content could be analyzed only in terms of the average composition of the genetic background. We carried out simple regressions (linear and polynomial) of genetic variation against (1) protein content (2) specific enzymatic activity and (3) enzymatic activity per larva. The best-fit-curve among the different degrees was chosen according to its F value.
Multiple regression when all variables were varying - Estimating the level of the effect of each variable on traits: When all the larvae exposed in protocols 1 and 2 were analyzed together, there were variations in environmental (time of exposure and ethanol concentration), genetic (composition of the genetic background) and phenotype/physiological (enzymatic activities and protein content) factors. To model some of these variables in terms of the remainder, we performed multiple regressions so as to discover the role played by each of these variables in the determination of that variable of interest. The modeled variables were specific enzymatic activity and time of resistance to ethanol (time elapsed until death). Nevertheless, in the latter case (multiple regression for time of resistance to ethanol), only data from larvae exposed to protocol 2 and that were considered as dead, were utilized. The relative importance of each regressor in affecting the modeled variable was inferred by its standardized partial angular coefficient, this being the angular coefficient found for each regressor multiplied by the ratio of its standard deviation and divided by the standard deviation of the modeled variable (Zar, 1999).
In order to choose the independent variables employed in each model, we used a factorial combination among all possible variables. Following this, we used a stepwise selection in these regressors, to keep the most informative ones. Stepwise regression was performed in the backward direction, regarding variable hierarchy, for the presence of significant composite variables, the variables that compose it cannot be withdrawn from the model, even though they are non-significant.
Results
General procedures
For all the variables analyzed data from protocol 1 - the ANOVA test was significant (p < 0.001), which implies that sample means were significantly different from one another. Thus we analyzed them by pair-wise comparison for a more detailed view (data not shown).
The use of dead larvae is plausible
In protocol 2, we described a new methodology of ethanol exposure in which larvae are collected after death. This new methodology allows for directly co-relating data on individual phenotypes and physiological state up to the time of death, which is not possible otherwise, thereby making this a highly potential process. Thus, we were able to create a model of mortality regarding variables that were measured in the individual larva. For validation of the use of dead larvae, we compared the mean values (with a t test) for all measured variables (specific enzymatic activity, enzymatic activity per larva and protein content) between the groups of both dead and live larvae. To eliminate the effects of environmental and genetic variation, we built a regression model (p < 0.001) and saved the residual values before mean comparison. The result was that both groups were statistically indistinguishable for all measured variables, hence validating the use of dead larvae (for the t test, p = 0.66 for protein content, p = 0.87 for enzymatic activity per larva and p = 0.58 for specific enzymatic activity).
Lethal concentration 50 (LC 50) data shows apparent heterosis
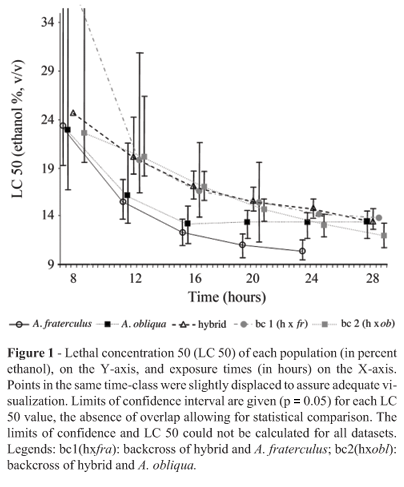
Figure 1 profers a summary of the results for LC 50. In most cases, the 95% confidence interval overlapped, thus the greater part of LC50 values could not be confidently distinguished. Even so, backcrosses appeared to be more tolerant than parental. A. fraterculus also presented many significantly lower values than those of the other groups. Thus the A. fraterculus sample showed significantly less tolerance to ethanol than the remainder.
Exploring the inheritance of the traits Simple regressions when environmental conditions were constant
All the regressions obtained were significant (p < 0.001). For (a) - protein content we obtained a first degree function, with a positive slope, for (b) - specific enzymatic activity we also obtained a first degree function with a negative slope, and for (c) - enzymatic activity per larva we obtained a third degree polynomial. First degree functions are characteristic of additively inherited traits, while a third degree polynomial is not clearly related to any particular inheritance pattern.
We plotted these results on a single graph (Figure 2), through standardizing the magnitude of each variable by subtracting the mean for each value and dividing it by the standard deviation. Thus, the Y axis presents the variation of the variables in standard deviations.
Estimating the level of effect of each variable on traits Multiple regressions when all variables were varied
Specific enzymatic activity: We carried out a multiple regression for specific enzymatic activity as the dependent variable. The independent variables were time of ethanol exposure, ethanol concentration, composition of average genetic background and a factorial combination of all these variables. Table 1 shows the fit of the model, its variance analysis and the relative effect of each variable on specific enzymatic activity.
Survival time: The time elapsed until death may be considered as a measure of ethanol tolerance. Based on this, we used survival time as a dependent variable. As independent variables, we used ethanol concentration, protein content, specific enzymatic activity, genetic factors, composition of the average genetic background and a factorial combination of all. Table 2 shows the fit of the model, its variance analysis and the relative effect of each variable on survival time.
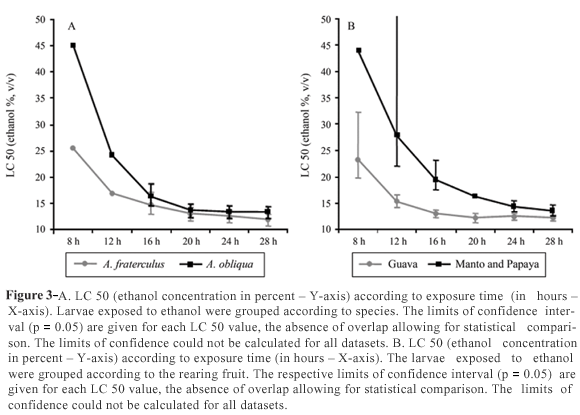
Effects of the developmental environment on alcohol tolerance
The species A. obliqua (one group reared on guava and another on mango) was the most tolerant to ethanol, whereas A. fraterculus (two groups reared on guava and one on papaya) was the most sensitive, although with only a very slight difference (Figure 3A). However, when larvae reared on guava (two groups of A. fraterculus and one of A. obliqua) were analyzed together as a single group, and the larvae reared on papaya and mango (one group each of A. fraterculus and A. obliqua) were also analyzed together as another separate group, we observed a significant difference in ethanol tolerance between these groups (Figure 3B), the guava being more sensitive and both the papaya and mango more tolerant.
Discussion
The methodology applied in exposure protocol 1 minimizes the effects of environmental variation, so that sample-response can be mostly attributed to genetic effects. Under these conditions, we detected an additive inheritance pattern in both protein content and specific enzymatic activity (Figure 2). Protein content in the samples was directly proportional to the genetic background of A. obliqua. On the other hand, specific enzymatic activity was inversely related to the genetic background of A. obliqua, signifying less enzymatic activity per tissue as the genetic background of this species increases. Similarly, dos Santos et al. (2001) also reported several intermediate phenotypes between hybrids of A. fraterculus and A. obliqua, as expected for additively inherited traits. However, we obtained different results for specific enzymatic activity when we analyzed data from protocols 1 and 2 together. Based on this finding, it may be suggested that specific enzymatic activity is a more complex inheritable trait (more detailed discussion below).
On the other hand, enzymatic activity per larva could not be fitted into either the additive or dominant models of inheritance (Figure 2). Its pattern could be best explained as the result of epistatic effects. Epistasis seems to be almost universally found in complex genetic systems as well as in apparently simple Mendelian traits (Matioli and Templeton, 1999; Templeton, 2000). Moreover, it has been shown that the Drosophila ADH system is subject to the influence of several epistatic effects (McKechnie and Geer, 1998; Pecsenye and Saura, 1998; Leal and Barbancho, 1992; Laurie and Stam, 1994; Stam and Laurie, 1996). A. fraterculus, A. obliqua and the hybrid disclosed a similar medium value for enzymatic activity. However, backcrossing of the hybrid with A. fraterculus resulted in a decrease in enzymatic activity whereas backcrossing with A. obliqua resulted in an increase. Therefore, the genetic background of the parental species is the factor responsible for enhancing or reducting the enzymatic activity (Figure 2). Both effects can be observed in hybrids. However, according to current evidence, hybrid inferiority is more frequent, while hybrid superiority (heterosis) is rarer (Burke and Arnold, 2001). We also observed a positive correlation (p < 0.001) between protein content and enzymatic activity per larva for all the samples, except for backcross 2 (data not shown). The size of those organs in which ADH is expressed may be a determinant of ADH expression level, or the control of ADH expression may even be influenced by the same factors that regulate determination of the size of developing larvae. As such, and independent of the causes of such enzymatic activity-size association, its breakdown could be related to the heterosis observed in backcross 2.
When comparing our Anastrepha data with published data on D. melanogaster, broadly speaking, it seems that the specific enzymatic activity (Stam and Laurie, 1996) and ethanol tolerance (Chakir et al., 1996) of D. melanogaster are higher than the ones observed in Anastrepha. This may be attributable to different evolutionary pathways followed by the two. Drosophila is a saprophytic organism, feeding on the micro-flora that develops on senescent fruits (Parsons and Stanley, 1981), whereas Anastrepha larvae feed on fruits at an earlier stage, from the period of unripe fruits up to the beginning of the decomposition process (Zucoloto, 2000). Once ethanol concentration increases at the time of ripening, on an average, Drosophila will live in environments with a higher concentration than Anastrepha during its life cycle. Thus, these different environmental conditions can have lead to the different adaptations in each genus.
The effects-model (Table 1) indicates that specific enzymatic activity is increased by a longer ethanol exposure time and concentration. This may reflect the induction of Adh, similar to that reported by several authors regarding the same process in Drosophila (Kapoun et al., 1990; Martel et al., 1995; Pecsenye et al., 1997; Pecsenye and Saura, 1998).
The effects-model also suggests that specific enzymatic activity increases with the A. obliqua genetic background (p < 0.0001 - Table 1). Hence, we observed two opposite effects for the A. obliqua genetic background in specific enzymatic activity, for in a simple regression (Figure 2), there was a negative effect, whereas in a multiple regression (Table 1), the effect was positive. As in the simple regression data were only obtained in the scant conditions of 8% ethanol, it appears that under these conditions, a more ample A. fraterculus background leads to higher efficiency in ethanol degradation. On the other hand, for multiple regression analyses data on larvae exposed to ethanol concentrations higher or equal to 8% was used. Under these conditions, the more ample the A. obliqua background, the higher the efficiency in ethanol degradation. We can hypothesize that A. fraterculus is more efficient in using ethanol as an energy resource (at lower concentrations, above 8%) and A. obliqua was more efficient in degrading ethanol to avoid toxic effects, which is in agreement with the data from LC 50 and the regression model for time of survival. Both sets of data analysis suggest that A. obliqua is more resistant to ethanol than A. fraterculus.
The survival of larvae exposed to ethanol (whose toxicity was placed in evidence by our model) was dependent on several factors. Regarding the effects of the genetic background, data from LC analysis (Figure 1) suggest that there was hybrid superiority, thus characterizing a heterosis effect, although there was no clear statistical significance for this statement. The multiple regression model for time of survival, both in greater detail and with statistical significance (p < 0,001), points to the A. obliqua genetic background as being the most important variable for larval survival (Table 2). This could not be detected in LC analysis. Nevertheless, LC 50 data (Figure 1) could significantly show that the less tolerant sample is A. fraterculus, in agreement with the model. We also observed that protein content was the second most important variable in the increase in survival-time. The third most relevant variable was specific enzymatic activity. Thus, since the increase in the A. obliqua genetic background increases the value of these two variables (Figure 1 and Table 1), we can say that the A. obliqua genetic background is decisive to enhancing larval survival in the presence of high ethanol concentrations. As can be seen, ethanol tolerance is a very complex trait, which is not explained only by ADH activity, although ADH is necessary in the overall model.
Notwithstanding, environmental factors seem to be the key to ethanol resistance. Data from diverse Anastrepha species reared on different fruits showed that ethanol resistance is more related to the fruit in which the larva has been reared than to the population itself and even more so than the species (Figure 3). When larvae were grouped according to the fruit, there were greater differences in ethanol tolerance than when grouped according to species. Larvae reared on papayas or mangoes were more tolerant than those reared on guavas. Geer et al. (1993) pointed out that diet can influence stress-tolerance, and that levels of vitamins or nutrients can affect tolerance under alcoholic stress. If we consider fruit as a complex environment, it is difficult to say what affects ethanol tolerance. Larger fruits such as mango and papaya, can, however, provide the larval population infesting it with higher quantities of nutrients than smaller ones such as guava, which could result in an increase in larval mass. Since protein content seems to be one of the most important factors in ethanol tolerance, then larger fruits may indirectly influence larvae to be more tolerant to ethanol than smaller ones. This may explain our data.
We hypothesize that the enzymatic activity of larvae exposed to ethanol can reach a physiological maximum. The concentration used here (> 8%) was much higher than that normally observed in fruits infested by Anastrepha (~ < 1%, Matioli et al., 1992). Consequently, larvae were kept in an extreme situation and could reach their maximum ADH enzymatic activity, in which all available ADH enzymes were fully dehydrogenating ethanol. In this situation, the maximum potential of the ADH system in helping to avoid ethanol toxicity could be reached, and subsequent increases in ethanol concentration would lead to the more preeminent effect of protein content in enhancing survival. As a point of discussion, under conditions of lower ethanol concentrations and longer exposure periods (closer to the natural environmental conditions of larvae), and when ethanol is predominantly used as an energy source, specific enzymatic activity would have greater importance in larval survival. A high concentration was used here since there was almost no mortality with lower ones. In Drosophila and in concentrations lower than 7.5%, ethanol is used as an energy source with no toxic effects (Sanches-Canete et al., 1986). The same could occur with Anastrepha. Results from Bokor and Pecsenye (2000) indicate that ADH are important in ethanol utilization when used as nutrients, but when ethanol concentration becomes toxic, survival (as related to ethanol tolerance) is not associated with the Adh genotypes, but to other unknown genetic factors. In our case, if the ethanol metabolism of Anastrepha is similar to Drosophila, a candidate factor for this other variable could be the protein content of larvae, an indicative of body mass. Ethanol tolerance seems to be mainly mediated by the capacity to metabolize the product, decrease its concentration in hemolymph and thus protect the nervous system (David, 1988). Selective pressure for an increase in body mass can lead to an increase in the amount of ethanol that can be ingested before reaching toxic internal concentration in hemolymph, thus theoretically allowing for an increase in ethanol consumption.
Through this rare and informative model, in which the crossing of species with differences in genetic constitution, as in phenotypic traits, is made possible, we demonstrated that there are genetic factors acting on the enzymatic activity of ADH and on ethanol tolerance as well, which also seem to be largely affected by environmental conditions. Furthermore, we suggest the mechanisms involved in the determination of these traits.
Acknowledgments
This work was supported by grants from the Brazilian agencies Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Internet Resources
Received: December 12, 2007; Accepted: June 3, 2008.
Associate Editor: Fábio de Melo Sene
- Aluja M (1994) Bionomics and management of Anastrepha Annu Rev Entomol 39:155-178.
- Ashburner M (1998) Speculations on the subject of alcohol dehydrogenase and its properties in Drosophila and other flies. Bioessays 20:949-954.
- Bokor K and Pecsenye K (2000) Differences in the effect of ethanol on fertility and viability components among laboratory strains of Drosophila melanogaster. Hereditas 132:215-227.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248-254.
- Brogna S, Benos PV, Gasperi G and Savakis C (2001) The Drosophila alcohol dehydrogenase gene may have evolved independently of the functionally homologous medfly, olive fly, and flesh fly genes. Mol Biol Evol 18:322-329.
- Burke JM and Arnold ML (2001) Genetics and the fitness of hybrids. Annu Rev Genet 35:31-52.
- Chakir M, Peridy O, Capy P, Pla E and David JR (1993) Adaptation to alcoholic fermentation in Drosophila: A parallel selection imposed by environmental ethanol and acetic acid. Proc Natl Acad Sci USA 90:3621-3625.
- Chakir M, Capy P, Genermont J, Pla E and David JR (1996) Adaptation to fermenting resources in Drosophila melanogaster: Ethanol and acetic acid tolerances share a common genetic basis. Evolution 50:767-776.
- Chambers KG (1991) Gene expression, adaptation and evolution in higher organisms. Evidence from studies of Drosophila alcohol dehydrogenases. Comp Biochem Physiol 99:723-730.
- David JR (1988) Ethanol adaptation and alcohol dehydrogenase polymorphism in Drosophila: From phenotypic functions to genetic structures. In: de Jong G (ed) Population Genetics and Evolution. Springer-Verlag, Berlin, pp 163-172.
- dos Santos P, Uramoto K and Matioli SR (2001) Experimental hybridization among Anastrepha species (Diptera, Tephritidae): Production and morphological characterization of F1 hybrids. Ann Entomol Soc Am 94:717-725.
- Eliopoulos E, Goulielmos GN and Loukas M (2004) Functional constraints of alcohol dehydrogenase (ADH) of tephritidae and relationships with other Dipteran species. J Mol Evol 58:493-505.
- Freriksen A, Seykens D, Scharloo W and Heinstra PW (1991) Alcohol dehydrogenase controls the flux from ethanol into lipids in Drosophila larvae. A 13C NMR study. J Biol Chem 266:21399-21403.
- Geer BW, Langevin ML and McKechnie SW (1985) Dietary ethanol and lipid synthesis in Drosophila melanogaster Biochem Genet 23:607-622.
- Geer BW, Heinstra PWH and McKechnie SW (1993) The biological basis of ethanol tolerance in Drosophila Comp Biochem Physiol B 105:203-209.
- Goulielmos GN, Loukas M, Bondinas G and Zouros E (2003) Exploring the evolutionary history of the alcohol dehydrogenase gene (Adh) duplication in species of the family tephritidae. J Mol Evol 57:170-80.
- Heinstra PWH, Scharloo W and Thörig EW (1987) Physiological significance of the alcohol dehydrogenase polymorphism in larvae of Drosophila Genetics 117:75-84.
- Janzen DH (1977) Why fruits rot, seeds mold, and meat spoils Am Nat 111:619-713.
- Kapoun AM, Geer BW, Heinstra PWH, Corbin V and McKechnie SW (1990) Molecular control of the induction of alcohol dehydrogenase by ethanol in Drosophila melanogaster larvae. Genetics 124:881-888.
- Laurie CC and Stam LF (1994) The effect of an intronic polymorphism on alcohol dehydrogenase expression in Drosophila melanogaster Genetics 138:379-385.
- Leal JFM and Barbancho M (1992) Acetaldehyde detoxification mechanisms in Drosophila melanogaster adults involving aldehyde dehydrogenase (ALDH) and alcohol dehydrogenase (ADH) enzymes. Insect Biochem Mol Biol 22:885-892.
- Luque T, Marfany G and Duarte RG (1997) Characterization and molecular analysis of Adh retrosequences in species of the Drosophila obscura group. Mol Biol Evol 14:1316-1325.
- Martel ML, Baumgardner C, Linda KD and Geer BW (1995) The toxicities of short-chain primary alcohols and the accumulation of storage bodies in the larval fat body of Drosophila melanogaster Comp Biochem Physiol C 111:99-108.
- Matioli SR and Templeton AR (1999) Coadapted gene complexes for morphological traits in Drosophila mercatorum Two-loci interactions. Heredity 83:54-61.
- Matioli SR, Morgante JS and Malavasi A (1986) Genetical and biochemical comparation of alcohol dehydrogenase isozymes from A. fraterculus and A. obliqua (Diptera, Tephritidae): Evidence for gene duplication. Biochem Genet 24:13-25.
- Matioli SR, Morgante JS, Solferini VN and Frias LD (1992) Evolutionary trends of alcohol dehydrogenase isozymes in some species of Tephritidae flies. Rev Brasil Genet 15:33-50.
- McKechnie SW and Geer BW (1998) The epistasis of Adh and Gpdh allozymes and variation in the ethanol tolerance of Drosophila melanogaster larvae. Genet Res 52:179-184.
- Nascimento JC and Oliveira AK (1997) Ontogenetic development of Anastrepha fraterculus (Diptera, Tephritidae): Isoenzyme patterns of isocitrate and alcohol dehydrogenases. Comp Biochem Physiol 118:847-854.
- Parsons PA (1983) The Evolutionary Biology of Colonizing Species. Cambridge University Press, Cambridge, 284 pp.
- Parsons PA and Stanley SM (1981) Domesticated and widespread species. In: Ashburner M, Carson HL, Thompson Jr. JN (eds) The Genetics and Biology of Drosophila, v. 3a. Academic Press, London, pp 349-393.
- Pecsenye K and Saura A (1998) Interactions between the Adh and Odh loci in response to ethanol in Drosophila melanogaster Biochem Genet 36:147-170.
- Pecsenye K, Bokor K, Lefkovitch LP, Giles BE and Saura A (1997) Enzymatic responses of Drosophila melanogaster to long- and short-term exposures to ethanol. Mol Gen Genet 255:258-268.
- Sanches-Canete FJS, Dorado G and Barbancho M (1986) Ethanol and isopropanol detoxification associated with the Adh locus of Drosophila melanogaster Heredity 56:167-175.
- Stam LF and Laurie CC (1996) Molecular dissection of a major gene effect on a quantitative trait: The level of alcohol dehydrogenase expression in Drosophila melanogaster. Genetics 144:1559-1564.
- Templeton AR (2000) Epistasis and complex traits. In: Wolf JB, Brodie III ED and Wade MJ (eds) Epistasis and the Evolutionary Process. Oxford University Press, New York, pp 41-57.
- Yeates DK and Wiegmann BM. (1999) Congruence and controversy: Toward a higher-level phylogeny of Diptera. Annu Rev Entomol 44:397-428.
- Zar JH (1999) Biostatistical Analysis, 4th edition. Prentice-Hall, New Jersey, 930 pp.
- Zucoloto FS (2000) Alimentação e nutrição de moscas-das-frutas. In: Malavasi A and Zucchi RA (eds) Moscas-das-Frutas de Importância Econômica no Brasil - Conhecimento Básico e Aplicado. Editora Holos, Ribeirão Preto, pp 67-80.
- EPA Probit Analysis Program, http://www.epa.gov/nerleerd/stat2.htm
Publication Dates
-
Publication in this collection
16 Jan 2009 -
Date of issue
2009
History
-
Received
12 Dec 2007 -
Accepted
03 June 2008