Abstract
Introduction
Coronavirus disease 2019 (COVID-19) has dramatically spread all over the world, crossing the borders of all countries. It is presented mainly by lower respiratory tract symptoms such as fever, cough, dyspnea, and chest tightness. However, COVID-19 causes different upper respiratory tract-related symptoms including nasal congestion, sore throat, and olfactory dysfunction.
Objective
To discuss different ear, nose and throat (ENT) manifestations in COVID-19-positive patients and their relation to other manifestations and to the severity of COVID-19.
Methods
We detected ENT manifestations in polymerase chain reaction (PCR)-confirmed positive COVID-19 patients at Zagazig Isolation Hospitals (Zagazig University hospitals, Zagazig Chest hospital, Al-Ahrar hospital, and Zagazig Fever hospital) with proportional allocation in the period from April 15 to June 15, 2020. All patients were subjected to full history taking and COVID-19 was categorized into 4 classes of severity after all patients underwent computed tomography (CT) of the chest. Afterwards, the collected data was analyzed and compared.
Results
Among the included 120 COVID-19 patients, the most frequent reported ENT manifestations were; sore throat (30%), nasal congestion (28.3%), nasal obstruction (26.7%), sneezing (26.6%), headache (25%), smell and taste dysfunction (25%), rhinorrhea (20%), upper respiratory tract infection (URTI) (15%), and tonsil enlargement (10%). The most common non-ENT manifestations were fever (88.3%), cough (63.3%), and dyspnea (45%).
Conclusion
Fever and cough are the dominant symptoms of COVID-19, but ENT manifestations for COVID-19 are common and should be a part of the suspected clinical criteria for COVID-19, particularly if the nasal examination was nonsignificant. The most common symptoms are sore throat, followed by nasal congestion and obstruction, headache, and lastly, olfactory dysfunction.
Keywords
COVID-19; coronavirus; nose; hyposmia; ENT; otorhinolaryngology
Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), first known as the 2019 novel Coronavirus (2019-nCoV), started in Wuhan, in China, in December 2019.11 Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol 2020;55(05):1169–1174 Since that moment, the new virus, also known as Coronavirus Disease 2019 (COVID-19), has dramatically spread all over the world, crossing the borders of all countries until the World Health Organization (WHO) confirmed it as a pandemic disease on March 11, 2020.22 Cucinotta D, Vanelli M.WHO declares COVID-19 a pandemic. Acta Biomed 2020;91(01):157–160
The SARS-CoV-2 is part of the species of the SARS-related coronaviruses that have led to previous epidemics over the last 2 decades, such as the SARS-CoV in 2002–2003 in China,33 Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348(20):1967–1976 and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in 2012–2013 in Saudi Arabia.44 de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016;14(08):523–534
COVID-19 is presented mainly by lower respiratory tract-related symptoms such as fever, cough, dyspnea, and chest tightness that could progress rapidly to acute respiratory distress syndrome (ARDS).55 Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al; Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19) Electronic address. https://www.lancovid.orgClinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020;34:101623
https://www.lancovid.orgClinical...
However, COVID-19 also causes different upper respiratory tract-related symptoms including nasal congestion, sore throat, and olfactory dysfunction.66 Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 2020;42(06):1252–1258
The available data on ENT manifestations of COVID-19 is sparsely published and, to the best of our knowledge, review studies on olfactory dysfunction in COVID-19-positive patients are few and lacking. Olfactory and taste dysfunctions in COVID-19 patients were sparsely mentioned in the literature and there is still a paucity of peer-reviewed literature to support a causal association between anosmia and COVID-19.77 Hopkins C, Surda P,Whitehead E, Kumar BN. Early recovery following newonsetanosmia during theCOVID-19pandemic-anobservational cohort study. J Otolaryngol Head Neck Surg 2020;49(01):26
Thus, the aim of the present study was to detect, analyze, and discuss the different ENT manifestations in COVID19-positive patients and their relation to other manifestations and to COVID-19 severity.
Materials and Methods
The present cross-sectional study was conducted on 127 patients at Zagazig Isolation Hospitals (Zagazig university hospitals, Zagazig Chest hospital, Al-Ahrar hospital, and Zagazig Fever hospital) with proportional allocation in the period from April 15 to June 15, 2020. All enrolled subjects or their relatives signed the informed consent form after explanation of the purpose of the research. All included patients were diagnosed as having confirmed COVID-19 by polymerase chain reaction (PCR) test. Suspected and non-confirmed COVID-19 cases were excluded. The present study was conducted according to the declaration of Helsinki on Biomedical Research Involving Human Subjects, and Zagazig University' International Review Board (IRB) approval was obtained. All patients were subjected to full history taking, and COVID-19 was categorized into 4 classes of severity after all patients underwent computed tomography (CT) of the chest: (1) mild: mild respiratory symptoms without imaging features of pneumonia; (2) moderate: fever, respiratory symptoms with imaging findings of pneumonia; (3) severe: shortness of breath, systemic oxygen (O2) saturation < 93% at rest on room air, respiratory rate > 30 breaths per minute, ratio of the systemic arterial partial O2 pressure to the fraction of inspired air O2 ≤ 300 mmHg, or > 50% progress of radiologic pulmonary lesions over 24 to 48 hours; and (4) critical: demanding mechanical ventilation, extracorporeal membrane oxygenation, or other organ support therapy in the intensive care unit (ICU).88 Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395 (10223):497–506 The following data was collected from all patients: epidemiological data, age, gender, special habit (smoking), comorbidities, history of chronic ENT disease and/or surgery, COVID-19 severity, ENT manifestations, and other COVID-19 manifestations.
Statistical Methods
All analyses were performed using IBM SPSS Statistics for Windows, Version 20 (IBM Corp., Armonk, NY, USA). Quantitative data were presented with means and standard deviations (SDs). Qualitative data were presented as number and percentage. The t-test was used to compare quantitative data, while qualitative data was compared using the chi-squared test (X2). Yates correction was performed when at least 20% of the expected frequencies were < 5. Values were considered statistically significant if p< 0.05.
Results
Among the 127 studied laboratory-confirmed positive COVID-19 patients, 120 were included in the present study (7 patients were severe cases and we could not take history from them or from their relatives), 62 were females (51.7%), and 58 were males (48.3%), with a mean age of 44.35 ± 15.16 years old (range = 16–79 years old). Only 1 patient was 16 years old, and all other patients were > 20 years old, comprising 18 single and 102 married patients. A total of 94 patients were smokers, 4 were former smokers, and 22 nonsmokers. A total of 36 medical staff patients (30%) (26 physicians, 8 pharmacists, and 2 dentists) were included. In 64 patients, there were other comorbidities, including 16 with hypertension, 20 with diabetes mellitus, 10 with bronchial asthma, 10 with thyroid dysfunction (8 with hypothyroidism, 2 with hyperthyroidism), 2 patients with past history of cerebrovascular stroke, 6 with hepatic disease (all were compensated), 6 cardiac patients, 4 renal failure patients, and 2 pregnant patients with anemia.
A total of 42 patients (35%) had a history of chronic ENT diseases; 8 had obstructive sleep apnea (OSA), 10 had allergic rhinitis, 14 had sinonasal polypi, and 6 had chronic sinusitis.
The severity of COVID-19 was mild in 74 patients (61.7%), with a mean age of 37.6 ± 11.6 years old (range: 16–64 years old); moderate in 26 patients (21.7%), with a mean age of 50.8 ± 13.88 years old (range: 33–79 years old); and severe in 20 patients (16.7%), with a mean age of 61.5 ± 9.18 years old (range: 49–73 years old) (►Table 1).
It was found that the mean age was significantly higher with increasing severity of the disease (F = 37.227; p< 0.0001).
The severity of COVID-19 in females was mild in 40 patients, moderate in 18 patients, and severe in 4 patients, while the severity in males was mild in 34 patients, moderate in 8 patients, and severe in 16 patients, with a significant difference (p= 0.0033); more severe cases were observed in male patients ►Fig. 1.
The severity of COVID-19 in smokers was mild in 12 patients, moderate in 2 patients, and severe in 8 patients. In nonsmoker patients, the severity was mild in 62 patients, moderate in 20 patients, and severe in 12 patients. In former smokers, 4 cases were severe.
Severe COVID-19 cases were reported more significantly in former smokers and in smokers (p= 0.0001; X2= 23.336), and after Yates correction (Yates p-value= 0.0027; Yates X2 = 16.23).
Most severe COVID-19 cases were reported in patients with comorbidities; COVID-19 was severe in 14 patients, moderate in 16 patients, and mild in 34 patients; while in patients without comorbidities, COVID-19 was severe in 6 patients, moderate in 10 patients, and mild in 40 patients. However, this difference was found to be statistically nonsignificant (p= 0.102).
However, when comparing COVID-19 severity in patients with multiple comorbidities with those with a single comorbidity, it was detected that multiple comorbidities were associated with a statically significantly higher incidence of severe disease than single comorbidities (p< 0.0001); the difference in the incidence of severe COVID-19 from patients without comorbidities was also statistically significantly (X2 = 28.211; p< 0.0001) (►Table 1).
The severity of COVID-19 in the medical staff was mild in 26 patients, moderate in 8 patients, and severe in 2 patients; while in nonmedical patients, 48 cases were mild, 18 cases were moderate, and 18 cases were severe, without significant difference between medical and nonmedical patients (p= 0.09).
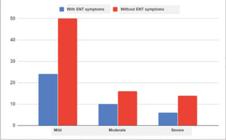
The most frequently reported ENT manifestations were sore throat in 36 patients (30%), nasal congestion in 34 patients (28.3%), nasal obstruction in 32 patients (26.7%), sneezing in 32 patients (26.6%), headache in 30 patients (25%), olfactory and taste dysfunction in 30 patients (25%), runny nose or rhinorrhea in 24 patients (20%), upper respiratory tract infection (URTI) in 18 patients (15%), and tonsil enlargement in 12 patients (10%). When we revised the severity of COVID-19 disease in patients who had ENT symptoms, we found that 24 patients (20%) were mild cases, 10 patients (8.3%) were moderate cases, and 6 patients (5%) were severe cases, without a significant difference in COVID-19 severity between patients who had ENT manifestations and those who did not (p= 0.8) (►Table 1). ►Fig. 2.
When we revised the history of chronic ENT disease, we found that 40 patients had an ENT disease history (33.3%), and 80 patients did not have an ENT disease history (66.6%). No reported epistaxis, postnasal discharge, facial edema or tenderness, reduction of hearing, vertigo, hoarseness, or stridor.
The non-ENT manifestations in those patients were fever in 106 patients (88.3%), cough in 76 patients (63.3%), dyspnea in 54 patients (45%), asthenia/fatigue in 54 patients (45%), arthralgia/myalgia in 30 patients (25%), diarrhea in 28 patients (23.3%), expectoration in 18 patients (15%), chest pain in 10 patients (8.3%), nausea/vomiting in 12 patients (10%), hemoptysis in 6 patients (5%), acute respiratory failure (ARF) in 6 patients (5%), and constipation in 4 patients (3.3%).
All severe cases were admitted to the ICU (20 patients; 16.7%). Ten patients (2 females, 8 males) died (50% of the ICU patients and 8.3% of all the COVID-19 patients admitted to the hospital). A total of 60% of the patients who died had other comorbidities and were smokers. A total of 60% of the patients that died were within the age range between 50 and 55 years old, 20% were within the age range between 59 and 60 years old, and 20% were > 70 years old. None of the patients who died complained of anosmia, and only 10% of them suffered from sinusitis as an early manifestation.
Discussion
In December 2019, a novel coronavirus (CoV) epidemic produced by the severe acute respiratory syndrome coronavirus–2 (SARS-CoV-2) emerged from China.99 Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med 2020;382:1708–1720. Doi: 10.1056/NEJMoa2002032
https://doi.org/10.1056/NEJMoa2002032...
On February 11, 2020, the WHO named the disease caused by this new virus as COVID-19. The widespread infectivity and distribution of COVID-19 make it an important pathogen with a limitless health threat.1010 Hassan SA, Sheikh FN, Jamal S, Ezeh JK, Akhtar A. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus 2020;12(03):e7355–7362
COVID-19 manifests with a wide-ranging clinical spectrum, ranging from no symptoms to septic shock and multiple organs dysfunctions.1111 Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus. 2020 Oct 4. In: StatPearls [Internet]Treasure Island (FL): StatPearls Publishing; 2020 Jan–. PMID: 32150360 Despite its rapid spread worldwide, the clinical characteristics of COVID-19 remain to a large extent imprecise.99 Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med 2020;382:1708–1720. Doi: 10.1056/NEJMoa2002032
https://doi.org/10.1056/NEJMoa2002032...
The nasal, nasopharyngeal and/or the oropharyngeal tissue are one of the main harbor sites of COVID-19 infection, being the main sites for collecting the sample for testing and a main source of transmission of infection. However, most published COVID-19 research focused on the manifestations and sequelae of the lower respiratory tract due to their life-threatening nature.
However, the literature on the ENT manifestations of COVID-19 is still scarce. Hence, it is worth studying the ENT manifestations of this novel virus and there is a demand to identify precisely the defining ENT epidemiological and clinical characteristics of COVID-19. Thus, in the present study, we studied, reported, and analyzed the different ENT manifestations in confirmed COVID19 patients and analyzed their relation to other manifestations and to the severity of COVID-19.
The results of the present study agree with previous reports1212 Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 2020;92(06):568–576,1313 Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020;92(06):577–583 that fever (reported in 88.3% of the patients) and cough (reported in 63.3% of the patients) are the dominant symptoms of COVID-19. We reported gastrointestinal (GIT) symptoms; mainly diarrhea (23%), nausea and vomiting (10%,) and constipation (3%), which is in line with other studies.1313 Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020;92(06):577–583
All the reported ENT manifestations in COVID-19 patients are nonspecific and, therefore, could be easily missed, and no emergency ENT symptoms, such as nosebleed, throat bleeding, or stridor were reported in COVID-19 cases.
In our study, 98 patients (81.6%) had at least 1 ENT manifestation. The most frequent reported ENT symptoms were: sore throat (30%), nasal congestion and nasal obstruction (28.3 and 26.7%, respectively), sneezing in 32 patients (26.6%), while headache, anosmia and taste affection had the same percentage (25%). Rhinorrhea was reported in 24 patients (20%), and tonsil enlargement in 12 patients (10%). Speth et al.1414 Speth MM, Singer-Cornelius T, Obere M, et al. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19:Prevalence. Otolaryngol Head Neck Surg 2020 Jul;163(01):114–120 reported nasal obstruction in 49.5% of the patients, runny nose in 35%, and olfactory dysfunction in 61%.
In the present study, 40 patients (33.3%) had a history of chronic ENT diseases; 8 cases of OSA, 10 of allergic rhinitis, 14 of sinonasal polyps, and 8 of chronic sinusitis.
It is remarkable that 6.6% of the COVID-19 patients had OSA; although the direct association between OSA and COVID-19 has not been reported yet, untreated OSA patients suffer from chronic intermittent hypoxia that leads to hypertension, heart failure, cerebrovascular disease and obesity; those are risk factors for higher severity of COVID-19. Angiotensin converting enzyme 2 (ACE2) is the entry receptor of COVID-19;1515 South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 2020;318 (05):H1084–H1090 in OSA patients, dysregulation of the renin angiotensin system occurs.1616 Barceló A, ElorzaMA, Barbé F, Santos C, Mayoralas LR, Agustí AGN. Angiotensin converting enzyme in patients with sleep apnoea syndrome: plasma activity and gene polymorphisms. Eur Respir J 2001;17(04):728–732 Therefore, we agree with a previous study1717 Pazarli AC, Ekiz T, ilik Faik. Coronavirus disease 2019 and obstructive sleep apnea syndrome. Sleep Breath; 2020;Apr 28: 1(letter to editor). that we need to keep in mind the effect of disturbed sleep on the immune system and that we may protect OSA patients from COVID-19 if we treat them. In addition, we need to keep in mind that patients who recovered from severe COVID-19 cases may be under risk of OSA due to lung fibrosis.1818 Ye Z, Zhang Y,Wang Y,Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol 2020;30(08):4381–4389
A total of 26.6% of our patients (32 cases) were suffering from allergic rhinitis and sinusitis with or without nasal polyps, which is similar to what has been reported by in the study by Speth et al.,1414 Speth MM, Singer-Cornelius T, Obere M, et al. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19:Prevalence. Otolaryngol Head Neck Surg 2020 Jul;163(01):114–120 in which 36% of the patients were allergic. In a study from China,1919 Wu Z,McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239–1242 asthma and respiratory allergy were not included as risk factors for severe COVID-19; many studies2020 Jia HP, Look DC, Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 2005;79(23): 14614–14621,2121 Daniel JJackson, William WBusse, Leonard BBacharier, Meyer Kattan, George TO’Connor, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2receptor ACE2. J Allergy Clin Immunol. july 2020; Volume 146, NUMBER 1(litter to editor). tried to investigate this point and found that ACE2 expression is markedly decreased in the nasal and respiratory mucosa in allergic patients. COVID -19 uses ACE2 enzyme to enter the cell; so, to some extent, allergic patients are protected from the severity of COVID-19. Actually, we did not report any severe cases among our allergic patients; all of them were mild and moderate cases.
The clinical presentation of COVID-19 might be confused with allergic rhinitis (AR); sudden and complete anosmia may be an early sign of COVID-19 infection, differentiating it from AR;2222 Scadding GK, Hellings PW, Bachert C, et al. Allergic respiratory disease care in the COVID-19 era: A EUFOREA statement. World Allergy Organ J 2020;13(05):100124–100128 in our study, 80% of AR patients (8 out of 10) complained of sudden complete anosmia.
The mortality rate in ICU patients was of ∼ 50%, which is similar to that of Wang, in Wuhan, China (62.5%), and to that in Washington, USA (50%),2323 Quah P, Li A, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care 2020;24(01):285–289 considering that the included patients were only patients who were admitted to hospitals. The fatality rate of all COVID-19 patients in our study was of 8.3%; this is quite similar to a Chinese metanalysis, which reported a fatality rate of 7%.1313 Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020;92(06):577–583 Another study in the USA reported a fatality rate of 21%;2424 Richardson S, Hirsch JS, Narasimhan M, et al;the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020;323(20): 2052–2059 this discrepancy can be attributed to difference in demographic characteristics, race and comorbidities between Egypt and other countries.
A total of 60% of the patients who died had other comorbidities (diabetes, hypertension, renal failure, cardiac), which is in line with Liu K. et al.,2525 Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(09):1025–1031,2626 Wang XF, Shi GC, Wan HY, et al. Clinical features of three avian influenza H7N9 virus-infected patients in Shanghai. Clin Respir J 2014;8(04):410–416 who found that the fatality rate of patients with viral pneumonia increased when they had another comorbidity, a result similar to that of our study. The mortality of males in our study was higher than that of females (60% male), which is in line with a recent meta-analysis that showed that male sex is a risk factor for death from COVID-19;2727 Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020;11(01):6317 we noticed that all males who died were heavy smokers (100% smokers) and had comorbidities. It is known that smoking is a risk factor for developing acute respiratory distress syndrome (ARDS),2828 Wacharasint P, Fuengfoo P, Rangsin R, Morakul S, Chittawattanarat K, Chaiwat O. Hazards and Intensive Care Unit Economic Burden of Cigarette Smoking on Critically Ill Surgical Patients: Analysis of the THAI-SICU Study. J Med Assoc Thai 2016;99(Suppl 6):S38–S46 but a recent meta-analysis showed that smoking doubles the mortality risk of COVID-19.2929 Salah HM, Sharma T, Mehta J. Smoking Doubles theMortality Risk in COVID-19: A Meta-Analysis of Recent Reports and Potential Mechanisms. Cureus 2020;12(10):e10837–10843
The age of the patients who died ranged from 50 to 72 years old. The mortality of COVID-19 increases with age, since older patients have other comorbidities and may develop more severe complications than younger patients.3030 Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID-19 and Older Adults: What We Know. J Am Geriatr Soc 2020;68(05): 926–929
None of the dead patients suffered from anosmia; olfactory dysfunction was detected in 25% of the included patients, with no significant difference between males and females (16 female and 14 male patients). In < 5% of the patients with olfactory dysfunction, COVID-19 was severe or critical. This may reflect no association between disease severity and olfactory dysfunction, but it may be due to more orientation to respiratory and critical manifestations in severe COVID-19 patient with missed asking about olfactory dysfunction. This may also be due to the fact that, in critical condition, mostly oxygen therapy and/or intubation are used. Our results were similar to those of other studies that stated that the prevalence of olfactory dysfunction in association with COVID-19 ranges from 19.4 to 85.6%.3131 Giacomelli A, Pezzati L, Conti F, et al. Self-reported Olfactory and Taste Disorders in Patients With Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin Infect Dis 2020; 71(15):889–890,3232 Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol International Forum of Allergy & Rhinology; 2020; Vol. 10, No. 7. Mao et al.3333 Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77(06):683–690 detected anosmia in 5.1% of their studied cases, and El-Anwar et al.3434 El-Anwar MW, Elzayat S, Fouad YA. ENT manifestation in COVID- 19 patients. Auris Nasus Larynx 2020;47(04):559–564 detected an incidence of 6%. Most COVID-19 studies did not mention olfactory dysfunction, particularly the early and primary reports, and most COVID-19 patients (66%) reported a complete recovery of their chemosensitive functions during the course of the disease.3535 Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC III. COVID-19 Anosmia Reporting Tool: Initial Findings. Otolaryngol Head Neck Surg 2020;163(01):132–134
Olfactory dysfunctions in COVID-19 patients were sporadically mentioned in the literature, with a lack of the peer-reviewed literature to support a causal association between anosmia and COVID-19.77 Hopkins C, Surda P,Whitehead E, Kumar BN. Early recovery following newonsetanosmia during theCOVID-19pandemic-anobservational cohort study. J Otolaryngol Head Neck Surg 2020;49(01):26 In addition, most studies on smell in COVID-19 patients did not entirely define the clinical manifestations of the patients. This reflects the importance of the present study.
Menni et al.3636 Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of selfreported symptoms to predict potential COVID-19. Nat Med 2020;26:1037–1040 suggested that a combination of loss of smell and taste, fever, persistent cough, fatigue, and GIT symptoms is a predictive of COVID-19 positive testing. However, they did not study anosmia as a part of other manifestations. Vroegop et al.3737 Vroegop AV, Eeckels AS, Van Rompaey V, et al. COVID-19 and olfactory dysfunction – an ENT perspective to the current COVID- 19 pandemic. B-ENT 2020;16(01):81–85 recommend COVID-19 suspicion and initiating testing or self-isolation for patients with anosmia without nasal obstruction or runny nose.
Acute smell loss is 13.2 times more likely in COVID-19 patients than in normal individuals, and acute smell loss is 4.5 times more likely in COVID -19 patients than in than influenza patients.3838 Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, et al. Acute-onset smell and taste disorders in the context of COVID- 19: a pilot multicentre polymerase chain reaction based casecontrol study. Eur J Neurol 2020;27(09):1738–1741
Therefore, if we select ENT manifestations to be added to the definition of suspected cases or to the covid-19 triage checklist, we will select sore throat and nasal congestion and/or obstruction, followed by olfactory dysfunction. Therefore, COVID-19 triage checklists need to be revised.
In the present study, we tried to overcome the limitations that are usually met during studies3434 El-Anwar MW, Elzayat S, Fouad YA. ENT manifestation in COVID- 19 patients. Auris Nasus Larynx 2020;47(04):559–564 on the novel COVID-19 by using a precise definition of the clinical manifestations during data collection, with complete documentation, using a universal questionnaire for all the patients, and by studying only confirmed COVID-19 cases based on RT-PCR testing and chest CT. To the best of our knowledge, this is the first study that reported all the ENT manifestations with a clear description and definition of the COVID-19 manifestation and that analyzed the relations between these manifestations and the severity of the disease. The limitation of the present study is missed asymptomatic or mild cases managed at home that were not included.
We hope that the present article will contribute to the understanding of the epidemiology of COVID-19, establishing a standard universal questionnaire for well-defined COVID-19 manifestations to make the COVID-19 data well defined, complete and homogenous, and guide infection control measures, while also delivering insights on the diagnostic topographies of the ENT manifestations. Otolaryngologists, physicians, patients, and the community should be mindful of the ENT manifestations of COVID-19 patients so as not to delay the diagnosis of COVID-19.
Conclusion
Fever and cough are the dominant symptoms of COVID-19, but ENT manifestations of COVID-19 are common and should be a part of the suspected clinical criteria for COVID-19, particularly if the nasal examination was nonsignificant. The most common symptoms are sore throat, nasal congestion and obstruction, headache, and, lastly, olfactory dysfunction. A universal questionnaire via well-defined COVID-19 manifestations is required to make the COVID-19 data accurately defined, homogenous and complete.
-
Financial SupportThe authors declare no financial support or interest regarding the present study.
References
-
1Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol 2020;55(05):1169–1174
-
2Cucinotta D, Vanelli M.WHO declares COVID-19 a pandemic. Acta Biomed 2020;91(01):157–160
-
3Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348(20):1967–1976
-
4de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016;14(08):523–534
-
5Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al; Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19) Electronic address. https://www.lancovid.orgClinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020;34:101623
» https://www.lancovid.orgClinical -
6Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 2020;42(06):1252–1258
-
7Hopkins C, Surda P,Whitehead E, Kumar BN. Early recovery following newonsetanosmia during theCOVID-19pandemic-anobservational cohort study. J Otolaryngol Head Neck Surg 2020;49(01):26
-
8Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395 (10223):497–506
-
9Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med 2020;382:1708–1720. Doi: 10.1056/NEJMoa2002032
» https://doi.org/10.1056/NEJMoa2002032 -
10Hassan SA, Sheikh FN, Jamal S, Ezeh JK, Akhtar A. Coronavirus (COVID-19): a review of clinical features, diagnosis, and treatment. Cureus 2020;12(03):e7355–7362
-
11Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus. 2020 Oct 4. In: StatPearls [Internet]Treasure Island (FL): StatPearls Publishing; 2020 Jan–. PMID: 32150360
-
12Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 2020;92(06):568–576
-
13Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020;92(06):577–583
-
14Speth MM, Singer-Cornelius T, Obere M, et al. Olfactory Dysfunction and Sinonasal Symptomatology in COVID-19:Prevalence. Otolaryngol Head Neck Surg 2020 Jul;163(01):114–120
-
15South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 2020;318 (05):H1084–H1090
-
16Barceló A, ElorzaMA, Barbé F, Santos C, Mayoralas LR, Agustí AGN. Angiotensin converting enzyme in patients with sleep apnoea syndrome: plasma activity and gene polymorphisms. Eur Respir J 2001;17(04):728–732
-
17Pazarli AC, Ekiz T, ilik Faik. Coronavirus disease 2019 and obstructive sleep apnea syndrome. Sleep Breath; 2020;Apr 28: 1(letter to editor).
-
18Ye Z, Zhang Y,Wang Y,Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol 2020;30(08):4381–4389
-
19Wu Z,McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239–1242
-
20Jia HP, Look DC, Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 2005;79(23): 14614–14621
-
21Daniel JJackson, William WBusse, Leonard BBacharier, Meyer Kattan, George TO’Connor, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2receptor ACE2. J Allergy Clin Immunol. july 2020; Volume 146, NUMBER 1(litter to editor).
-
22Scadding GK, Hellings PW, Bachert C, et al. Allergic respiratory disease care in the COVID-19 era: A EUFOREA statement. World Allergy Organ J 2020;13(05):100124–100128
-
23Quah P, Li A, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care 2020;24(01):285–289
-
24Richardson S, Hirsch JS, Narasimhan M, et al;the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020;323(20): 2052–2059
-
25Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(09):1025–1031
-
26Wang XF, Shi GC, Wan HY, et al. Clinical features of three avian influenza H7N9 virus-infected patients in Shanghai. Clin Respir J 2014;8(04):410–416
-
27Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020;11(01):6317
-
28Wacharasint P, Fuengfoo P, Rangsin R, Morakul S, Chittawattanarat K, Chaiwat O. Hazards and Intensive Care Unit Economic Burden of Cigarette Smoking on Critically Ill Surgical Patients: Analysis of the THAI-SICU Study. J Med Assoc Thai 2016;99(Suppl 6):S38–S46
-
29Salah HM, Sharma T, Mehta J. Smoking Doubles theMortality Risk in COVID-19: A Meta-Analysis of Recent Reports and Potential Mechanisms. Cureus 2020;12(10):e10837–10843
-
30Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID-19 and Older Adults: What We Know. J Am Geriatr Soc 2020;68(05): 926–929
-
31Giacomelli A, Pezzati L, Conti F, et al. Self-reported Olfactory and Taste Disorders in Patients With Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin Infect Dis 2020; 71(15):889–890
-
32Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol International Forum of Allergy & Rhinology; 2020; Vol. 10, No. 7.
-
33Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77(06):683–690
-
34El-Anwar MW, Elzayat S, Fouad YA. ENT manifestation in COVID- 19 patients. Auris Nasus Larynx 2020;47(04):559–564
-
35Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC III. COVID-19 Anosmia Reporting Tool: Initial Findings. Otolaryngol Head Neck Surg 2020;163(01):132–134
-
36Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of selfreported symptoms to predict potential COVID-19. Nat Med 2020;26:1037–1040
-
37Vroegop AV, Eeckels AS, Van Rompaey V, et al. COVID-19 and olfactory dysfunction – an ENT perspective to the current COVID- 19 pandemic. B-ENT 2020;16(01):81–85
-
38Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, et al. Acute-onset smell and taste disorders in the context of COVID- 19: a pilot multicentre polymerase chain reaction based casecontrol study. Eur J Neurol 2020;27(09):1738–1741
Publication Dates
-
Publication in this collection
13 Sept 2021 -
Date of issue
Jul-Sep 2021
History
-
Received
25 Sept 2020 -
Accepted
14 Feb 2021