Abstract
Introduction
Chronic rhinosinusitis (CRS) is commonly classified based on the presence or absence of nasal polyps (NPs). Eosinophil infiltration is observed in NPs of patients in Western countries. In contrast, in East Asian countries, including Japan, CRS with NPs (CRSwNP) is subdivided based on the presence (eosinophilic CRS [ECRS]) or absence (non-eosinophilic CRS [NECRS]) of eosinophils in NPs. However, detailed analyses of other immune cells, such as lymphocytes, in NPs have not been performed. Therefore, clarification of the types of cells that infiltrate NPs is important to understand CRS pathogenesis.
Objectives
We analyzed the lymphocytes that infiltrate the paranasal sinus mucosa of ECRS and NECRS patients.
Methods
Eighteen patients with CRSwNP participated in this study, out of whom 6 were NECRS patients, and 12 were ECRS patients. The mucosa specimens, collected from patients during sinus surgeries, were subjected to collagenase treatment to prepare single cell suspensions. Then, mononuclear cells were isolated, and CD4+ T, CD8+ T, and CD20+ B-cell populations were examined using flow cytometry.
Results
In both NECRS and ECRS patients, CD8+ T-cells were dominant over CD4+ T-cells. Notably, CD4+ T-cell/B-cell ratio, but not CD8+ T-cell/B-cell or CD4+ T-cell/CD8+ T-cell ratios, was significantly higher in ECRS patients than in NECRS patients.
Conclusion
The CD4+ T-cell/B-cell ratio can be used as a potential indicator to differentiate between ECRS and NECRS.
Keywords
paranasal sinus disease; nasal mucosa; CD4-positive T-lymphocytes
Introduction
Chronic rhinosinusitis (CRS) is a frequently encountered disease in daily otorhinolaryngology practice. Patients with CRS present with nasal discharge, postnasal drip, and nasal congestion.11 Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): Adult Sinusitis Executive Summary. Otolaryngol Head Neck Surg 2015;152(04):598–609 Doi: 10.1177/0194599815574247
https://doi.org/10.1177/0194599815574247...
CRS is commonly classified based on the presence or absence of nasal polyps (NPs).22 FokkensWJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020;58(Suppl S29):1–464 Doi: 10.4193/Rhin20.600
https://doi.org/10.4193/Rhin20.600...
Patients without NPs (CRSsNP) are known to respond to treatment better than CRS patients with NPs (CRSwNP). Additionally, the prognosis of most CRSwNP patients is poor and refractory.22 FokkensWJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020;58(Suppl S29):1–464 Doi: 10.4193/Rhin20.600
https://doi.org/10.4193/Rhin20.600...
As a result, the quality of life of patients with CRSwNP is extremely low due to chronic olfaction disorder and severe nasal congestion.33 Fujieda S, Imoto Y, Kato Y, et al. Eosinophilic chronic rhinosinusitis. Allergol Int 2019;68(04):403–412 Doi: 10.1016/j.alit.2019.07.002
https://doi.org/0.1016/j.alit.2019.07.00...
,44 Bachert C, Zhang N, Hellings PW, Bousquet J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol2018;141(05):1543–1551Doi:10.1016/j.jaci.2018.03.004
https://doi.org/10.1016/j.jaci.2018.03.0...
In most cases of CRSwNP in Europe and in the United States, eosinophilic infiltration in the inflammatory regions of the nose and paranasal sinuses is observed.22 FokkensWJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020;58(Suppl S29):1–464 Doi: 10.4193/Rhin20.600
https://doi.org/10.4193/Rhin20.600...
,55 Jankowski R, Bouchoua F, Coffinet L, Vignaud JM. Clinical factors influencing the eosinophil infiltration of nasal polyps. Rhinology 2002;40(04):173–178,66 Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with andwithout nasal polyps. Allergy 2006; 61(11):1275–1279 Doi: 10.1111/j.1398-9995.2006.01132.x
https://doi.org/10.1111/j.1398-9995.2006...
However, infiltration of neutrophils and lymphocytes without eosinophils is commonly observed in the NPs of CRSwNP patients in East Asian countries, such as Korea and Japan.77 Ikeda K, Shiozawa A, Ono N, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope 2013;123(11):E1–E9 Doi: 10.1002/lary.24154
https://doi.org/0.1002/lary.24154...
,88 WenW, LiuW, Zhang L, et al; Nasal Health Group, China (NHGC). Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol 2012;129(06): 1522–8.e5 Doi: 10.1016/j.jaci.2012.01.079
https://doi.org/10.1016/j.jaci.2012.01.0...
Since eosinophils, in combination with T-helper 2 (Th2) cells, play a role in allergic immune response,99 Jacobsen EA, Ochkur SI, Pero RS, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T-cells. J Exp Med 2008;205(03):699–710 Doi: 10.1084/jem.20071840
https://doi.org/10.1084/jem.20071840...
the presence of eosinophilic infiltration, but not NPs, appears to be one of the exacerbation factors of CRS. Therefore, distinction of CRSwNP based on the presence or absence of eosinophils in the NPs has been clinically considered important, especially in East Asian countries.
Accordingly, a disease category, eosinophilic CRS (ECRS) has been introduced to represent CRSwNP patients with eosinophilic infiltration in the NPs. Clinically, ECRS and non-ECRS (NECRS) can be classified based on the guidelines of the Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis (JESREC), according to which assessment of computed tomography findings, eosinophil ratio in the peripheral blood, and the presence or absence of NPs can be addressed.1010 Tokunaga T, SakashitaM, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 2015;70(08):995–1003 Doi: 10.1111/all.12644
https://doi.org/10.1111/all.12644...
However, information on the infiltrated immune cells, other than eosinophils, in the NPs of ECRS patients is scant, maybe because the main focus has always been on the presence of eosinophils in the NPs.1111 Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol 1997;158(08): 3902–3908
The epithelia of the nasal and paranasal sinuses not only are the important first barriers in the body, but also respond to external stimuli and induce diverse immune responses.1212 Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int 2015;64(02):121–130 Doi: 10.1016/j.alit.2014.12.006
https://doi.org/0.1016/j.alit.2014.12.00...
However, the immune status, especially the involvement of acquired immunity in the paranasal sinus mucosa in ECRS patients, has not been well documented. To clarify the different types of lymphocytes infiltrated in NPs between ECRS and NECRS, we isolated the infiltrating cells from the surgically removed polyps in the paranasal sinus mucosa of CRSwNP patients and found that the CD4+ T-cell/B-cell ratio was significantly higher in ECRS patients than in NECRS patients. Therefore, the CD4+ T-cell/B-cell ratio could be an informative indicator for ECRS.
Materials and Methods
Patients
All patients included in the study signed informed consent forms. The study was approved by the ethics committees of Toho University School of Medicine (27028 and A18086_27028). The present study included 18 patients with CRSwNP who were operated at Toho University Omori Medical Center. We divided CRSwNP patients into two subgroups, ECRS (≥ 11 points) and NECRS (≤ 10 points) based on the JESREC scoring system1010 Tokunaga T, SakashitaM, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 2015;70(08):995–1003 Doi: 10.1111/all.12644
https://doi.org/10.1111/all.12644...
before surgery. We histologically confirmed the presence of eosinophils in the NPs of the ECRS patients.
Preparation of Nasal Sinus Mononuclear Cells
The uncinate processes of anterior ethmoid sinus of patients who underwent endoscopic sinus surgery were obtained. The mucosa specimens from each patient were minced and incubated with Hanks' balanced salt solution (HBSS) containing 5% fetal calf serum, 0.5 mg/ml collagenase A (Roche, Basel, Switzerland), 0.2 mg/ml DNase I (Sigma-Aldrich, St. Louis, MO, USA), and 10 mM hydroxyethyl piperazine ethanesulfonic acid (HEPES) at 37°C for 30 minutes. After incubation, 100 mM ethylenediaminetetraacetic acid (EDTA) was added to stop the enzyme reaction, and the specimens were passed through a 70-µm filter (BD Bioscience, Franklin Lakes, NJ, USA). After centrifugation, the cells were resuspended in 10 ml of 40% (v/v) Percoll (GE Healthcare Bioscience, Uppsala, Sweden) and overlaid with 2 ml of 80% (v/v) Percoll. Subsequently, the suspension was continuously centrifuged at 2,400 rpm for 20 minutes at 25°C. Mononuclear cells were collected from the interface.
Flow Cytometry
The following antibodies were used for cell-surface staining: anti-CD4 (OKT4) antibodies labeled with fluorescein isothiocyanate (FITC), and CD8 (RPA-T8) antibodies conjugated with APC-Cy7 from TONBO Bioscience (San Diego, CA, USA). Anti-CD20 (2H7) antibodies with PE-Cy7 were purchased from Bio Legend (San Diego, CA, USA). Cells prepared as described above were stained with the mixture of labeled antibodies on ice for 20 minutes. After washing, cells were fixed with paraformaldehyde and analyzed with FACS Canto II flow cytometer (BD Bioscience). The data were analyzed with Flow Jo software (Tree Star, Ashland, OR, USA).
Statistical Analysis
Statistical analysis was performed using the IBM SPSS Statistics Version 25.0. software (IBM Corp., Armonk, NY, USA). Comparison between 2 groups was performed using the Mann-Whitney U test. P-values < 0.05 were considered to be statistically significant.
Results
ECRS is a subtype of CRSwNP with eosinophil infiltration in the NPs. But it had not been clearly clarified types of lymphocytes infiltrated in NPs of ECRS patients. To address this issue, we isolated mononuclear cells in NPs from NECRS and ECRS patients and analyzed cell types by a flow cytometry. In this study, a total of 18 patients with CRSwNP (12 male, 6 female), with a mean age of 56.61 ± 6.79 years old and a mean blood eosinophil count of 8.49 ± 5.82%, were included. Of the 18 patients, 6 had NECRS and 12 had ECRS.
First, the lymphocytes infiltrating the paranasal sinus mucosa were analyzed in NECRS and ECRS groups. Mononuclear cells were isolated from the paranasal sinus mucosa, were stained with anti-CD4, anti-CD8, and anti-CD20 antibodies, and were analyzed on FACS. As shown in ►Fig. 1, CD8+ T-cells were dominant over CD4+ T-cells in both NECRS and ECRS. This is different from the secondary lymphoid organs, in which more CD4+ T-cells are present than CD8+ T-cells.1313 Amadori A, Zamarchi R, De Silvestro G, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med 1995;1(12):1279–1283 Doi: 10.1038/nm1295-1279
https://doi.org/0.1038/nm1295-1279...
Based on the percentage of each cell population, it is possible to calculate absolute cell numbers and standardize the numbers according to the weight and/or size of the collected tissues. But it must be very difficult because recovery efficiency of the infiltrating cells from the tissue might be different in each preparation and/or sample. Therefore, we utilized the percentage of cell populations in the FACS analysis and looked for the combination of populations whose ratio between NECRS and ECRS would be significant.
Representative fluorescence-activated cell sorter (FACS) plots of infiltrating cells in the paranasal sinus mucosa. Infiltrating cells in the paranasal sinus mucosa of NECRS (a) and ECRS (b) patients were collected and were analyzed using FACS. The number shown in figures is the percentage of cells in each gate indicated.
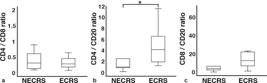
As shown in ►Fig. 2, no significant differences were observed in the CD8+ T-cell/B-cell or CD4+ T-cell/CD8+ T-cell ratios between ECRS and NECRS patients; however, the CD4+ T-cell/B-cell ratio was significantly higher in ECRS patients than in NECRS patients (mean value = NECRS 0.95 vs ECRS 4.15, Mann-Whitney U test for independent samples, p= 0.032) (►Fig. 2b). Thus, the relative increase in the counts of CD4+ T-cells infiltrating the paranasal sinus mucosa in ECRS patients reflected the diagnostic criteria for ECRS, which is associated with a high recurrence rate and a high proportion of refractory cases.
The CD4+ T-cell/B-cell ratio was significantly different between ECRS and NECRS patients. CD4+ T-cell/CD8+ T-cell ratio (a), CD4+ T-cell/B-cell ratio (b), and CD8+ T-cell/B-cell ratio (c) in the paranasal sinus mucosa of NECRS and ECRS patients were determined. Each ratio was compared between NECRS and ECRS (*: p< 0.05). All data expressed as median interquartile range.
Discussion
Moderate and severe ECRS, defined based on the JESREC scoring system, are commonly concomitant with bronchial asthma; however, the pathogenic mechanism of ECRS remains unclear.1010 Tokunaga T, SakashitaM, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 2015;70(08):995–1003 Doi: 10.1111/all.12644
https://doi.org/10.1111/all.12644...
Therefore, it is important to understand the clinical features of ECRS to establish a better treatment strategy for ECRS. Since CRSwNP in Europe and the United States is generally associated with poor prognosis,22 FokkensWJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020;58(Suppl S29):1–464 Doi: 10.4193/Rhin20.600
https://doi.org/10.4193/Rhin20.600...
the pathogenesis of CRSwNP has been extensively investigated. Immunostaining of NPs in patients with CRSwNP showed infiltration of eosinophils, CD4+ T-cells, and CD68+ macrophages, and these cells produced interleukin 17A, which might be one of the factors that causes inflammation.1414 Saitoh T, Kusunoki T, Yao T, et al. Role of interleukin-17A in the eosinophil accumulation and mucosal remodeling in chronic rhinosinusitis with nasal polyps associated with asthma. Int Arch Allergy Immunol 2010;151(01):8–16 Doi: 10.1159/000232566
https://doi.org/10.1159/000232566...
,1515 Makihara S, Okano M, Fujiwara T, et al. Regulation and characterization of IL-17A expression in patients with chronic rhinosinusitis and its relationship with eosinophilic inflammation. J Allergy Clin Immunol 2010;126(02):397–400, 400.e1–400.e11 Doi: 10.1016/j.jaci.2010.05.014
https://doi.org/10.1016/j.jaci.2010.05.0...
Additionally, compared with normal inferior turbinate mucosa, NPs in patients with CRSwNP showed infiltration of significantly higher counts of CD4+ T-cells.1616 Shi LL, Song J, Xiong P, et al. Disease-specific T-helper cell polarizing function of lesional dendritic cells in different types of chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med 2014;190(06):628–638 Doi: 10.1164/rccm.201402-0234OC
https://doi.org/10.1164/rccm.201402-0234...
These results suggested that CD4+ T-cells in NPs were strongly involved in the pathogenesis of CRSwNP. In contrast, we and others1717 Ma J, Shi LL, Deng YK, et al. CD8(þ) T-cells with distinct cytokineproducing features and low cytotoxic activity in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Clin Exp Allergy 2016;46(09):1162–1175 Doi: 10.1111/cea.12758
https://doi.org/10.1111/cea.12758...
,1818 Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol 2009;124(03):478–484, 484.e1–484. e2 Doi: 10.1016/j.jaci.2009.05.017
https://doi.org/10.1016/j.jaci.2009.05.0...
demonstrated that the CD8+ T-cell count was higher than the CD4+ T-cell count in the paranasal sinus mucosa of patients with CRSwNP, compared with that of patients with CRSsNP (►Fig. 1). Although it has been suggested that CD8+ T-cells that infiltrated the paranasal sinus mucosa in patients with CRSwNP are largely effector-memory phenotype cells,1717 Ma J, Shi LL, Deng YK, et al. CD8(þ) T-cells with distinct cytokineproducing features and low cytotoxic activity in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Clin Exp Allergy 2016;46(09):1162–1175 Doi: 10.1111/cea.12758
https://doi.org/10.1111/cea.12758...
1919 Pant H, Hughes A, Schembri M, Miljkovic D, Krumbiegel D. CD4þ and CD8þ regulatory T-cells in chronic rhinosinusitis mucosa. Am J Rhinol Allergy 2014;28(02):83–89 Doi: 10.2500/ ajra.2013.28.4014
https://doi.org/10.2500/ajra.2013.28.401...
the reason for high counts of CD8+ T-cells in the paranasal sinus mucosa of patients with CRSwNP and the functions of these cells remain unclear. This CD8+ T-cell dominance was observed in NPs irrespective of the presence of eosinophilic infiltration (►Fig. 1).
In contrast, the CD4+ T-cell/B-cell ratio in the infiltrated cells in the paranasal sinus mucosa of CRSwNP patients was significantly higher in ECRS than in NECRS, indicating that the proportion of cell population infiltrating the paranasal sinus mucosa was highly correlated with the CRS classification developed by JESREC. In the past, the activation of CD4+ T-cells and dysfunction of the regulatory T-cells have been shown to play important roles in the pathogenesis of CRS.2020 Van Bruaene N, Pérez-Novo CA, Basinski TM, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol 2008;121(06):1435–1441, 1441.e1–1441.e3 Doi: 10.1016/j. jaci.2008.02.018
https://doi.org/10.1016/j.jaci.2008.02.0...
2121 Zhang N, Van Zele T, Perez-Novo C, et al. Different types of Teffector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol 2008;122(05):961–968 Doi: 10.1016/j.jaci.2008.07.008
https://doi.org/0.1016/j.jaci.2008.07.00...
Particularly, local CD4+ T-cells have been found to exhibit different phenotypes of CRS by producing different types of cytokines.1616 Shi LL, Song J, Xiong P, et al. Disease-specific T-helper cell polarizing function of lesional dendritic cells in different types of chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med 2014;190(06):628–638 Doi: 10.1164/rccm.201402-0234OC
https://doi.org/10.1164/rccm.201402-0234...
ECRS has a higher rate of recurrence (more than double) and intractable percentage (more than triple) than NECRS.1010 Tokunaga T, SakashitaM, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 2015;70(08):995–1003 Doi: 10.1111/all.12644
https://doi.org/10.1111/all.12644...
Thus, CD4+ T-cells might be more involved in the pathogenesis of ECRS than in that of NECRS. In the present study, we could not investigate the functionality of CD4+ T-cells due to the limitation of samples. Further studies must be conducted to obtain insights into the pathogenesis of ECRS.
Conclusion
In the present study, we found that the CD4+ T-cell/B-cell ratio in the paranasal sinus mucosa was significantly higher in ECRS patients than in NECRS patients. Hence, the CD4+ T-cell/B-cell ratio can be used as a potential indicator for differentiating between ECRS and NECRS. Further studies are necessary to clarify the role of mucosal CD4+ T-cells in the pathogenesis of ECRS.
-
Funding InformationJSPS KAKENHI (Grant/Award Number: ’JP17K11367’ ’JP26462588’), Toho University School of Medicine Yanase Takeshi Scholarship Fund. The Research Promotion Grant fromToho University Graduate School ofMedicine (Grant/Award Number: ’19-02’), Toho University School of Medicine Project Research Funding (Grant/Award Number: ’28-22’), Toho University Grant for Research Initiative Program (Grant/Award Number: 2020).
References
-
1Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): Adult Sinusitis Executive Summary. Otolaryngol Head Neck Surg 2015;152(04):598–609 Doi: 10.1177/0194599815574247
» https://doi.org/10.1177/0194599815574247 -
2FokkensWJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020;58(Suppl S29):1–464 Doi: 10.4193/Rhin20.600
» https://doi.org/10.4193/Rhin20.600 -
3Fujieda S, Imoto Y, Kato Y, et al. Eosinophilic chronic rhinosinusitis. Allergol Int 2019;68(04):403–412 Doi: 10.1016/j.alit.2019.07.002
» https://doi.org/0.1016/j.alit.2019.07.002 -
4Bachert C, Zhang N, Hellings PW, Bousquet J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol2018;141(05):1543–1551Doi:10.1016/j.jaci.2018.03.004
» https://doi.org/10.1016/j.jaci.2018.03.004 -
5Jankowski R, Bouchoua F, Coffinet L, Vignaud JM. Clinical factors influencing the eosinophil infiltration of nasal polyps. Rhinology 2002;40(04):173–178
-
6Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with andwithout nasal polyps. Allergy 2006; 61(11):1275–1279 Doi: 10.1111/j.1398-9995.2006.01132.x
» https://doi.org/10.1111/j.1398-9995.2006.01132.x -
7Ikeda K, Shiozawa A, Ono N, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope 2013;123(11):E1–E9 Doi: 10.1002/lary.24154
» https://doi.org/0.1002/lary.24154 -
8WenW, LiuW, Zhang L, et al; Nasal Health Group, China (NHGC). Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol 2012;129(06): 1522–8.e5 Doi: 10.1016/j.jaci.2012.01.079
» https://doi.org/10.1016/j.jaci.2012.01.079 -
9Jacobsen EA, Ochkur SI, Pero RS, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T-cells. J Exp Med 2008;205(03):699–710 Doi: 10.1084/jem.20071840
» https://doi.org/10.1084/jem.20071840 -
10Tokunaga T, SakashitaM, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 2015;70(08):995–1003 Doi: 10.1111/all.12644
» https://doi.org/10.1111/all.12644 -
11Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol 1997;158(08): 3902–3908
-
12Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int 2015;64(02):121–130 Doi: 10.1016/j.alit.2014.12.006
» https://doi.org/0.1016/j.alit.2014.12.006 -
13Amadori A, Zamarchi R, De Silvestro G, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med 1995;1(12):1279–1283 Doi: 10.1038/nm1295-1279
» https://doi.org/0.1038/nm1295-1279 -
14Saitoh T, Kusunoki T, Yao T, et al. Role of interleukin-17A in the eosinophil accumulation and mucosal remodeling in chronic rhinosinusitis with nasal polyps associated with asthma. Int Arch Allergy Immunol 2010;151(01):8–16 Doi: 10.1159/000232566
» https://doi.org/10.1159/000232566 -
15Makihara S, Okano M, Fujiwara T, et al. Regulation and characterization of IL-17A expression in patients with chronic rhinosinusitis and its relationship with eosinophilic inflammation. J Allergy Clin Immunol 2010;126(02):397–400, 400.e1–400.e11 Doi: 10.1016/j.jaci.2010.05.014
» https://doi.org/10.1016/j.jaci.2010.05.014 -
16Shi LL, Song J, Xiong P, et al. Disease-specific T-helper cell polarizing function of lesional dendritic cells in different types of chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med 2014;190(06):628–638 Doi: 10.1164/rccm.201402-0234OC
» https://doi.org/10.1164/rccm.201402-0234OC -
17Ma J, Shi LL, Deng YK, et al. CD8(þ) T-cells with distinct cytokineproducing features and low cytotoxic activity in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Clin Exp Allergy 2016;46(09):1162–1175 Doi: 10.1111/cea.12758
» https://doi.org/10.1111/cea.12758 -
18Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol 2009;124(03):478–484, 484.e1–484. e2 Doi: 10.1016/j.jaci.2009.05.017
» https://doi.org/10.1016/j.jaci.2009.05.017 -
19Pant H, Hughes A, Schembri M, Miljkovic D, Krumbiegel D. CD4þ and CD8þ regulatory T-cells in chronic rhinosinusitis mucosa. Am J Rhinol Allergy 2014;28(02):83–89 Doi: 10.2500/ ajra.2013.28.4014
» https://doi.org/10.2500/ajra.2013.28.4014 -
20Van Bruaene N, Pérez-Novo CA, Basinski TM, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol 2008;121(06):1435–1441, 1441.e1–1441.e3 Doi: 10.1016/j. jaci.2008.02.018
» https://doi.org/10.1016/j.jaci.2008.02.018 -
21Zhang N, Van Zele T, Perez-Novo C, et al. Different types of Teffector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol 2008;122(05):961–968 Doi: 10.1016/j.jaci.2008.07.008
» https://doi.org/0.1016/j.jaci.2008.07.008
Publication Dates
-
Publication in this collection
13 Sept 2021 -
Date of issue
Jul-Sep 2021
History
-
Received
24 Apr 2020 -
Accepted
05 July 2020