Abstract
Amyloidosis is a disease caused by extracellular deposition of insoluble protein fibrils, that results in changes in tissue architecture and consequently modification of the organ structure. Cardiac involvement is common in amyloidosis. Two major types of systemic amyloidosis affect the myocardium - immunoglobulin light chain and transthyretin amyloidosis - each leading to different prognosis. Early detection and diagnosis of cardiac amyloidosis are the main objectives in the assessment of the disease. New techniques of magnetic resonance imaging have minimized the need for biopsies for the diagnosis. Late gadolinium enhancement technique, and more recently T1 mapping, have allowed a simplified evaluation of amyloid deposits and extracellular volume. The aim of this review was to describe basic concepts and updates of the use of magnetic resonance imaging for the diagnosis amyloidosis and evaluation of its severity.
Keywords
Amyloidosis; Myocarditis/pathology; Plaque, Amyloid/diagnostic imaging; Magnetic Resonance Imaging
Introduction
Amyloidosis is a group of diseases characterized by deposition of insoluble fibrils formed from soluble molecules that had suffered structural changes and become relatively insoluble.11 Bulluck H, White SK, Rosmini S, Bhuva A, Treibel TA, Fontana M, et al. T1 mapping and T2 mapping at 3T for quantifying the area-at-risk in reperfused STEMI patients. J Cardiovasc Magn Reson. 2015 Aug 12;17:73. Amyloid fibrils deposit either locally or systemically. Symptoms vary according to the content of amyloid deposits, which is directly related to the type of protein. Cardiac involvement is determinant for the choice of the therapy.22 Meier-Ewert HK, Sanchorawala V, Berk JL, Ruberg FL. Cardiac amyloidosis: evolving approach to diagnosis and management. Curr Treat Options Cardiovasc Med. 2011;13(6):528-42.
Immunoglobulin light chain amyloidosis is the most common form of amyloidosis in the USA, with 4,500 new cases diagnosed per year. It affects mostly older individuals, aged between 50 and 80 years, especially men (who account for one third of the cases). In the USA, wild-type transthyretin amyloidosis is more frequently diagnosed in older, African American patients, whereas patients with hereditary systemic amyloidosis are generally younger white women. Survival of transthyretin amyloidosis patients is higher than that of light-chain amyloidosis patients.33 Ritts AJ, Cornell RF, Swiger K, Singh J, Goodman S, Lenihan DJ. Current Concepts of Cardiac Amyloidosis: Diagnosis, Clinical Management, and the Need for Collaboration. Heart Fail Clin. 2017;13(2):409-16.
Several non-invasive methods have been used to predict the presence of amyloid deposits in myocardial tissue, including electrocardiogram (ECG), echocardiogram (ECHO), cardiac biomarkers, scintigraphy (SPECT) and cardiac magnetic resonance (CMR) imaging. Among these techniques, CMR has been considered the reference standard for assessment of global and regional myocardial function and for detection and quantification of fibrosis areas and expansion of myocardial extracellular volume.44 Sara L, Szarf G, Tachibana A, Shiozaki AA, Villa AV, de Oliveira AC, et al., Sociedade Brasileira de Cardiologia and Colegio Brasileiro de Radiologia. [II Guidelines on Cardiovascular Magnetic Resonance and Computed Tomography of the Brazilian Society of Cardiology and the Brazilian College of Radiology]. Arq Bras Cardiol. 2014;103(6 Suppl 3):1-86. Cardiac involvement is decisive for the prognosis and treatment of systemic amyloidosis. Although ECG, ECHO and scintigraphy are the main imaging tests used in cardiology, CMR can provide a new perspective, especially on the analysis of amyloid deposits. The use of the late gadolinium enhancement technique reveals more specific, and sometimes pathognomonic imaging features. Also, CMR enables the assessment of the extension of the cardiac area affected by amyloidosis. T1 mapping technique measures myocardial amyloid load and myocyte response to infiltration, thereby allowing monitoring and eventual change of therapy, even when cardiac function is normal. These techniques are very promising for the development of treatment and prognosis of this condition.55 Fontana M. Prognosis in cardiac amyloidosis by LGE: ready for prime time? JACC Cardiovasc Imaging. 2016;9(6):687-9.
6 Fontana M, Martinez-Naharro A, Hawkins PN. Staging cardiac amyloidosis with CMR: understanding the different phenotypes. JACC Cardiovasc Imaging. 2016;9(11):1278-9.
7 Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132(16):1570-9.-88 Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Response to letters regarding article, "Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis". Circulation. 2016;133(12):e450-1.
Based on these considerations, this study aimed to present current concepts of amyloidosis and the use of CMR in the diagnosis and follow-up of these patients. We conducted a bibliographic review on Pubmed (National Library of Medicine) database, using the terms "amyloidosis", "disease, myocardial" and "magnetic resonance imaging" for the search.
Results
A total of 135 articles were retrieved, and 60 were selected for being published in higher impact journals and consensus of the authors. We summarized current concepts of amyloidosis types, diagnostic methods, prognosis and treatment of the disease in the following text.
Discussion
Main types of amyloidosis
Two types of amyloidosis can affect the myocardial ventricle: immunoglobulin light chain amyloidosis and transthyretin amyloidosis, which, in turn, has two forms of presentation - wild type and genetically variant transthyretin amyloidosis.
Immunoglobulin light chain
Light chain amyloidosis, also known as primary systemic amyloidosis, is the most common type of amyloidosis. This is a plasma cell dyscrasia characterized by deposition of amyloidogenic chains in the extracellular space, causing a lesion in this tissue affected. Cardiac dysfunction is seen in up to 50% of patients with amyloidosis,99 Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52(4):347-61. Erratum in: Prog Cardiovasc Dis. 2010;52(5):445-7. and this type of amyloidosis can cause restrictive cardiomyopathy. Congestive heart failure is quite common, caused by thickening and lack of dilatation of the left ventricle, detected by echocardiography.1010 Sher T, Gertz MA. Recent advances in the diagnosis and management of cardiac amyloidosis. Future Cardiol. 2014;10(1):131-46. Myocardial dysfunction caused by amyloidosis can be evaluated by measurement of brain natriuretic peptide (BNP) and troponin levels. Right heart failure is frequently aggravated by the concomitant presence of nephrotic syndrome, observed in 30-50% of the cases. Cardiomyopathy is the most aggressive manifestation of amyloidosis, and the main cause of poor prognosis and death. Cardiac amyloidosis has also been associated with multiple myeloma, which can also be evaluated.22 Meier-Ewert HK, Sanchorawala V, Berk JL, Ruberg FL. Cardiac amyloidosis: evolving approach to diagnosis and management. Curr Treat Options Cardiovasc Med. 2011;13(6):528-42.,99 Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52(4):347-61. Erratum in: Prog Cardiovasc Dis. 2010;52(5):445-7.,1111 Maceira AM, Prasad SK, Hawkins PN, Roughton M, Pennell DJ. Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J Cardiovasc Magn Reson. 2008 Nov 25;10:54.,1212 Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation. 2017;135(14):1357-77.
Hereditary Systemic Amyloidosis
Hereditary systemic amyloidosis is a dominant autosomal disease caused by deposition of amyloid fibrils resulting from mutations in genes that encode transthyretin and apolipoprotein A-I, A-II, fibrinogen, gelsolin, cystatin C and lysozyme. Transthyretin is mainly produced by the liver and participates in the transport of thyroxine and retinol. Hereditary systemic amyloidosis is caused by 100 different mutations in the DNA of transthyretin, generating a heterogeneity of penetrance. Clinical presentation of the disease includes cardiomyopathy, nephropathy and neuropathy. Although transthyretin-related cardiac amyloidosis is less aggressive than immunoglobulin light chain amyloidosis, hereditary systemic amyloidosis is also a cause of significant symptoms of heart failure.1313 Perfetto F, Cappelli F, Bergesio F, Ciuti G, Porciani MC, Padeletti L, et al. Cardiac amyloidosis: the heart of the matter. Intern Emerg Med. 2013;8(3):191-203.,1414 Desai HV, Aronow WS, Peterson SJ, Frishman WH. Cardiac amyloidosis: approaches to diagnosis and management. Cardiol Rev. 2010;18(1):1-11.
Senile systemic amyloidosis
Senile systemic amyloidosis, also related to transthyretin, is not hereditary and generally occurs after the seventh decade of life. This presentation of amyloidosis can be called as senile systemic or wild type amyloidosis. Similar to other types of the disease, in senile systemic amyloidosis, amyloid deposition can occur in several tissues such as cardiac, hepatic, and pancreatic tissues. Senile systemic amyloidosis is usually preceded by heart failure approximately 3-5 years before. Patients are mostly men, older than 70 years. The diagnosis of the disease had been probably underestimated; with advances in the diagnosis provided by CMR, from 2000 to 2009, the number of patients seen at amyloidosis centers in the United Kingdom increased by approximately 6.5%.55 Fontana M. Prognosis in cardiac amyloidosis by LGE: ready for prime time? JACC Cardiovasc Imaging. 2016;9(6):687-9.
6 Fontana M, Martinez-Naharro A, Hawkins PN. Staging cardiac amyloidosis with CMR: understanding the different phenotypes. JACC Cardiovasc Imaging. 2016;9(11):1278-9.
7 Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132(16):1570-9.
8 Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Response to letters regarding article, "Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis". Circulation. 2016;133(12):e450-1.-99 Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52(4):347-61. Erratum in: Prog Cardiovasc Dis. 2010;52(5):445-7.,1515 Narotsky DL, Castano A, Weinsaft JW, Bokhari S, Maurer MS. Wild-type transthyretin cardiac amyloidosis: novel insights from advanced imaging. Can J Cardiol. 2016;32(9):1166.e1-1166.e10.
Recent reports have described cases of senile amyloidosis in patients with heart failure with increased ejection fraction, highlighting the importance of clinical cardiologists considering the disease in suspected cases. Clinical suspicion is higher in cases of heart failure with increased ejection fraction associated with infiltrative cardiomyopathy revealed by cardiac imaging tests.1616 Mesquita ET, Jorge AJ, Souza CV Junior, Andrade TR. Cardiac amyloidosis and its new clinical phenotype: heart failure with preserved ejection fraction. Arq Bras Cardiol. 2017;109(1):71-80.
Other diagnostic methods
Electrocardiography
In amyloidosis patients, particularly in those with light chain amyloidosis, electrocardiography usually reveals low voltage of the QRS complexes which, together with myocardial thickening increases the suspicion of cardiac amyloidosis. One hypothesis of voltage decrease is related with amyloid infiltration, which would cause a decrease in cardiac muscle cells. Atrioventricular block, atrial flutter and ventricular tachycardia have been also reported in these patients.99 Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52(4):347-61. Erratum in: Prog Cardiovasc Dis. 2010;52(5):445-7.,1414 Desai HV, Aronow WS, Peterson SJ, Frishman WH. Cardiac amyloidosis: approaches to diagnosis and management. Cardiol Rev. 2010;18(1):1-11.,1717 Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1(2):e000364.,1818 Esplin BL, Gertz MA. Current trends in diagnosis and management of cardiac amyloidosis. Curr Probl Cardiol. 2013;38(2):53-96.
Echocardiography
When cardiac amyloidosis is suspected, echocardiography is usually the first imaging test ordered by the physician. The use of two-dimensional speckle tracking technique has been used to detect subclinical or initial changes of the disease, which may help in the treatment of the patients.1919 Rocha AM, Ferreira SG, Nacif MS, Ribeiro ML, Freitas MR, Mesquita CT. Speckle tracking and transthyretin amyloid cardiomyopathy. Arq Bras Cardiol. 2017;108(1):21-30. At initial stages, diagnosis of amyloidosis may be confounded with parietal hypertrophy, as in hypertrophic cardiomyopathy. However, characteristic findings are more frequently seen at more advanced stages of the disease and are observer-dependent. Common findings include increased ventricular parietal thickness, frequently involving the right ventricle, decreased ejection fraction2020 Patel AR, Dubrey SW, Mendes LA, Skinner M, Cupples A, Falk RH, et al. Right ventricular dilation in primary amyloidosis: an independent predictor of survival. Am J Cardiol. 1997;80(4):486-92.,2121 Porciani MC, Lilli A, Perfetto F, Cappelli F, Massimiliano Rao C, Del Pace S, et al. Tissue Doppler and strain imaging: a new tool for early detection of cardiac amyloidosis. Amyloid. 2009;16(2):63-70. and thickening of valve and interatrial septum. Echocardiography may show wall thickening, especially basal, with high birefringence in the apical area (apical sparing) of the amyloid deposits, in addition to involvement of valves and papillary muscles.1818 Esplin BL, Gertz MA. Current trends in diagnosis and management of cardiac amyloidosis. Curr Probl Cardiol. 2013;38(2):53-96.
Cardiac biomarkers
Immunoglobulin light chain amyloidosis is commonly associated with increased levels of troponin and BNP and N-terminal pro-B-type natriuretic peptide (NT-proBNP), which is not observed in transthyretin amyloidosis. This is important for patients with some renal dysfunction, since natriuretic peptides require renal excretion. Increased NT-proBNP in patients with systemic amyloidosis is predictive of cardiac disease.2222 Wechalekar AD, Gillmore JD, Wassef N, Lachmann HJ, Whelan C, Hawkins PN. Abnormal N-terminal fragment of brain natriuretic peptide in patients with light chain amyloidosis without cardiac involvement at presentation is a risk factor for development of cardiac amyloidosis. Haematologica. 2011;96(7):1079-80. More recently, high-sensitivity troponin has been used as a marker of morbidity and mortality.2323 Dispenzieri A, Kyle RA, Gertz MA, Therneau TM, Miller WL, Chandrasekaran K, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet. 2003;361(9371):1787-9. At diagnosis, measurements of natriuretic peptide and troponin are used for risk stratification of patients.
Scintigraphy
Scintigraphy is performed with intravenous injection of iodine-123 (123I) for locating amyloid deposit in different organs, including liver, kidney, spleen, adrenal glands, and bones. Thus, this is not an adequate technique for cardiac evaluation in amyloidosis.2424 Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990;323(8):508-13. On the other hand, the use of other markers - 99m Technetium labelled 3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) - has been suggested to specifically locate cardiac amyloid in transthyretin amyloidosis and considered the gold standard for the diagnosis of this condition.2525 de Miguel C, Llorente L, de Haro-Del Moral FJ, Garcia-Pavia P, Gonzalez-Lopez E, Segovia J, et al. Myocardial uptake of (99m)Tc-DPD in patients with AL amyloidosis. Amyloid. 2017;24(supl1):48-9.
26 Hutt DF, Fontana M, Burniston M, Quigley AM, Petrie A, Ross JC, et al. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur Heart J Cardiovasc Imaging. 2017;18(12):1344-50.
27 Moore PT, Burrage MK, Mackenzie E, Law WP, Korczyk D, Mollee P. The utility of (99m)Tc-DPD scintigraphy in the diagnosis of cardiac amyloidosis: an Australian experience. Heart Lung Circ. 2017;26(11):1183-90.-2828 Sachchithanantham S, Hutt DF, Quigley AM, Hawkins P, Wechalekar AD. Role of (99m) Tc-DPD scintigraphy in imaging extra-cardiac light chain (AL) amyloidosis. Br J Haematol. 2017 Oct 30. [Epub ahead of print].
Cardiac magnetic resonance
CMR has been widely used as the gold standard for the assessment of myocardial function and characterization of myocardial tissue. The technique is more precise than echocardiography, allowing the detection of earlier changes.44 Sara L, Szarf G, Tachibana A, Shiozaki AA, Villa AV, de Oliveira AC, et al., Sociedade Brasileira de Cardiologia and Colegio Brasileiro de Radiologia. [II Guidelines on Cardiovascular Magnetic Resonance and Computed Tomography of the Brazilian Society of Cardiology and the Brazilian College of Radiology]. Arq Bras Cardiol. 2014;103(6 Suppl 3):1-86.
CMR without contrast can also be used to provide a precise quantification of amyloid infiltration associated with morphological changes (left ventricular hypertrophy, decreased chamber size and atrial dilation). The method can be used for patients with contraindication to contrast, such as patients with renal failure, using the T1 mapping technique.1515 Narotsky DL, Castano A, Weinsaft JW, Bokhari S, Maurer MS. Wild-type transthyretin cardiac amyloidosis: novel insights from advanced imaging. Can J Cardiol. 2016;32(9):1166.e1-1166.e10.,2929 Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6(4):488-97.
A CMR test for evaluation of diseases characterized by abnormal protein deposit, such as amyloidosis, should have an average duration of 35 minutes. Although routine sequences are cine and delayed enhancement, we believe that, when T1 mapping technique is available, it should be chosen for routine use. Other techniques such as anatomy, perfusion and flow techniques may be included or not in amyloidosis protocol depending on patients' clinical status and information obtained from the addition of these sequences to the test.
Cine MR
In the analysis of ventricular, segmental and total activity, gradient-recalled echo (GRE) and balanced steady-state free precession (SSFP) imaging is the most used sequence. The sequence is added to the electrocardiographic tracings by segmented K-space technique, eliminating motion artifacts.3030 Bluemke DA, Boxerman JL, Mosher T, Lima JA. Segmented K-space cine breath-hold cardiovascular MR imaging: Part 2. Evaluation of aortic vasculopathy. AJR Am J Roentgenol. 1997;169(2):401-7.
Segmented K-space GRE sequences allow acquisition of many frames during the cardiac cycle in a dissection plane. Presentation of the frames in sequence allows dynamic, motion visualization of the heart during cardiac cycle, due to real-time interpolation of the R-R interval in the ECG. Strengths of the technique include optimal temporal resolution, the clear definition of endocardial and epicardial borders and acquisition of bright-blood cine images. Also, images can be obtained in any geometric plan.3131 Bluemke DA, Boxerman JL, Atalar E, McVeigh ER. Segmented K-space cine breath-hold cardiovascular MR imaging: Part 1. Principles and technique. AJR Am J Roentgenol. 1997;169(2):395-400.
Similarly, fast-GRE images, previously used for functional evaluation, are more sensitive to turbulent flow, and are valuable for assessment of stenosis and valve insufficiency. Nevertheless, the method produces worse specification between the myocardium and blood in cardiac cavities when compared with SSFP.3232 Barkhausen J, Ruehm SG, Goyen M, Buck T, Laub G, Debatin JF. MR evaluation of ventricular function: true fast imaging with steady-state precession versus fast low-angle shot cine MR imaging: feasibility study. Radiology. 2001;219(1):264-9.,3333 Tyler DJ, Hudsmith LE, Petersen SE, Francis JM, Weale P, Neubauer S, et al. Cardiac cine MR-imaging at 3T: FLASH vs SSFP. J Cardiovasc Magn Reson. 2006;8(5):709-15. A new sequencing technique, known as "real time" sequencing has high diagnostic quality and can be used for patients with arrhythmias and patients with inability to sustain apnea.44 Sara L, Szarf G, Tachibana A, Shiozaki AA, Villa AV, de Oliveira AC, et al., Sociedade Brasileira de Cardiologia and Colegio Brasileiro de Radiologia. [II Guidelines on Cardiovascular Magnetic Resonance and Computed Tomography of the Brazilian Society of Cardiology and the Brazilian College of Radiology]. Arq Bras Cardiol. 2014;103(6 Suppl 3):1-86.
In the assessment of cardiac amyloidosis, characterization of morphology and function of the disease by cine-MR (Figure 1) is crucial, especially in established disease. This is a widely used technique and is used in all protocols of cardiac study by MR.
Evaluation of systolic mass and systolic function by three-dimensional and Simpson's technique time-volume curve. (A) Left ventricular time-volume curve; (B) cine-magnetic resonance imaging during diastole. Left ventricular mass (blue) and final diastolic volume (green).
Tagging
Myocardial tagging by CMR provides a noninvasive, powerful method to quantify segmental and diastolic functions. The development of the technology, particularly of the sequences, type of devices and analysis software have facilitated the use of this technique. This will be very useful to assess patients' conditions and course of the disease.
Myocardial tagging uses a fast gradient echo sequence with saturation lines, creating a grid on the images that moves with cardiac motion, allowing an objective quantification of myocardial contraction during cardiac cycle (myocardial strain).3434 Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171(3):841-5.
35 Biglands JD, Radjenovic A, Ridgway JP. Cardiovascular magnetic resonance physics for clinicians: Part II. J Cardiovasc Magn Reson. 2012 Sep 20;14:66.-3636 Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988;169(1):59-63.
Myocardial tagging (Figure 2) should be used for the diagnosis of initial or subclinical amyloidosis. In addition, the technique may detect longitudinal or circumferential functional changes in individuals with mutations but without a clear phenotype determined by other techniques.3737 Kuetting DL, Homsi R, Sprinkart AM, Luetkens J, Thomas DK, Schild HH, et al. Quantitative assessment of systolic and diastolic function in patients with LGE negative systemic amyloidosis using CMR. Int J Cardiol. 2017 Apr 1;232:336-41.,3838 Oda S, Utsunomiya D, Nakaura T, Yuki H, Kidoh M, Morita K, et al. Identification and assessment of cardiac amyloidosis by myocardial strain analysis of cardiac magnetic resonance imaging. Circ J. 2017;81(7):1014-21. We understand that one limitation for the use of myocardial tagging in many centers is the lack of specific softwares for analysis, but we believe in the benefits of the implementation of the technology.
Myocardial tagging for quantification of left ventricular systolic and diastolic function; (A) Circumferential strain curves over a cardiac cycle; (B) subendocardial, mesocardial and epicardial tracings for quantification of myocardial deformation using cine-tagging.
Anatomy
In the analysis of the anatomy the heart and large vessels, double-inversion fast spin-echo is the most used sequence. It is based on the acquisition of fast spin-echo combined with double inversion preparation pulse - the first applied to the whole tissue (nonselective) and the second, slice-selective. This technique has high spatial resolution (selective); blood is dark in the images (null signal) and, because of that, the technique is known as "black-blood" imaging. This occurs because fast moving tissues, similarly to blood, when moving outside the slice of interest, do not produce any signal, while stable tissues or slow-moving tissues, like the myocardium, produce a high signal. However, endocardial borders (between the blood and the myocardium) are well defined. This sequence has a segmented acquisition, obtained from final expiratory apnea, synchronized with the electrocardiogram (ECG). An acceptable change, known as triple inversion recovery, is the addition of a third saturation pulse, to suppress the signal from the adipose tissue (fat saturation), which may help in the diagnosis in certain situations, such as characterization of cardiac tumors.3030 Bluemke DA, Boxerman JL, Mosher T, Lima JA. Segmented K-space cine breath-hold cardiovascular MR imaging: Part 2. Evaluation of aortic vasculopathy. AJR Am J Roentgenol. 1997;169(2):401-7.,3131 Bluemke DA, Boxerman JL, Atalar E, McVeigh ER. Segmented K-space cine breath-hold cardiovascular MR imaging: Part 1. Principles and technique. AJR Am J Roentgenol. 1997;169(2):395-400.,3434 Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171(3):841-5.
These techniques add little to the assessment of amyloidosis, and often are not included in the protocols to optimize the time of test execution. Nevertheless, in some situations, differential diagnosis is important, and situations that require evaluation of the pericardium, better tissue or even morphological characterization can be added to the basic protocol.
Perfusion
Myocardial perfusion imaging using CMR is obtained by the first pass of contrast (gadolinium) into ventricular cavities and then into the myocardium. One of the most frequently used methods constitutes a hybrid of fast gradient-echo and ultra-fast echo- planar imaging preceded by a saturation pulse from the tissue signal. This allows the acquisition of images in several planes every one-two heartbeats, repeatedly over a 60-second time period, along with the contrast passage. Myocardial perfusion can be used at rest or with pharmacological stress using adenosine, dipyridamole or regadenoson. The method is considered adequate to identify myocardial ischemia and has also been widely used in the identification of cardiac tumors. Progress has been made in the velocity of data acquisition, yielding perfusion images with high spatial and temporal resolution, as well as motion correction methods that positively contribute to the quality of the data.3535 Biglands JD, Radjenovic A, Ridgway JP. Cardiovascular magnetic resonance physics for clinicians: Part II. J Cardiovasc Magn Reson. 2012 Sep 20;14:66.,3939 Diesbourg LD, Prato FS, Wisenberg G, Drost DJ, Marshall TP, Carroll SE, et al. Quantification of myocardial blood flow and extracellular volumes using a bolus injection of Gd-DTPA: kinetic modeling in canine ischemic disease. Magn Reson Med. 1992;23(2):239-53.
40 Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94(12):3318-26.-4141 Saeed M, Wendland MF, Masui T, Higgins CB. Reperfused myocardial infarctions on T1- and susceptibility-enhanced MRI: evidence for loss of compartmentalization of contrast media. Magn Reson Med. 1994;31(1):31-9. In a protocol for cardiac amyloidosis investigation, this sequence will be probably unnecessary, since a suspicion of myocardial ischemia is not considered for differential diagnosis in almost all cases.
Myocardial delayed enhancement
Myocardial delayed enhancement is based on a T1-weighted fast gradient echo sequence, with an inversion recovery pre-pulse and inversion time (TI) adjusted to null the normal myocardial signal after infusion of gadolinium-based contrast (0.02 - 0.04 mmol/kg). Gadolinium does not penetrate intact cell membranes and hence is distributed in the extracellular space; in case of myocyte membrane rupture (e.g., infarction), gadolinium shows a larger volume of distribution.4141 Saeed M, Wendland MF, Masui T, Higgins CB. Reperfused myocardial infarctions on T1- and susceptibility-enhanced MRI: evidence for loss of compartmentalization of contrast media. Magn Reson Med. 1994;31(1):31-9.,4242 Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218(1):215-23. In addition, kinetics of the contrast distribution is altered, with a slower washout.4040 Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94(12):3318-26.
Consequently, gadolinium concentrations are much higher in regions of greater extracellular volume, and areas of membrane rupture and communication between intra and extracellular space as compared with normal myocardial tissue approximately 10 minutes after the contrast administration.3939 Diesbourg LD, Prato FS, Wisenberg G, Drost DJ, Marshall TP, Carroll SE, et al. Quantification of myocardial blood flow and extracellular volumes using a bolus injection of Gd-DTPA: kinetic modeling in canine ischemic disease. Magn Reson Med. 1992;23(2):239-53. These areas are white in delayed enhancement images (hypersignal), whereas normal myocardium appears black (low signal - null).
Recent technological progresses have allowed acquisition of three-dimensional late gadolinium enhancement, with respiratory navigator during free breathing, one respiratory pause, in real time and without manual adjustment of TI (self-viability or phase sensitivity inversion recovery - PSIR - technique).77 Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132(16):1570-9.,3535 Biglands JD, Radjenovic A, Ridgway JP. Cardiovascular magnetic resonance physics for clinicians: Part II. J Cardiovasc Magn Reson. 2012 Sep 20;14:66.
Therefore, in cardiac amyloidosis, tissue definition after contrast administration is obtained from gadolinium deposition and accumulation in areas with increased myocardial extracellular volume. Such increase in extracellular space results from expansion of amyloid deposits to the extracellular space. Several studies using the delayed enhancement technique have enabled the classification of amyloid deposition patterns - subendocardial (Figure 3), mesocardial (Figure 4) and transmural (Figure 5).1515 Narotsky DL, Castano A, Weinsaft JW, Bokhari S, Maurer MS. Wild-type transthyretin cardiac amyloidosis: novel insights from advanced imaging. Can J Cardiol. 2016;32(9):1166.e1-1166.e10. The most common patterns of amyloid distribution in immunoglobulin light chain amyloidosis and transthyretin amyloidosis are subendocardial and transmural, respectively.4343 Raina S, Lensing SY, Nairooz RS, Pothineni NV, Hakeem A, Bhatti S, et al. Prognostic value of late gadolinium enhancement CMR in systemic amyloidosis. JACC Cardiovasc Imaging. 2016;9(11):1267-77. The treatment of amyloidosis depends on the disease subtype; the use of delayed enhancement CMR is hence paramount as it serves as a screening test that lead to other test, such as biopsy, for establishment of disease subtype and definition of therapy.
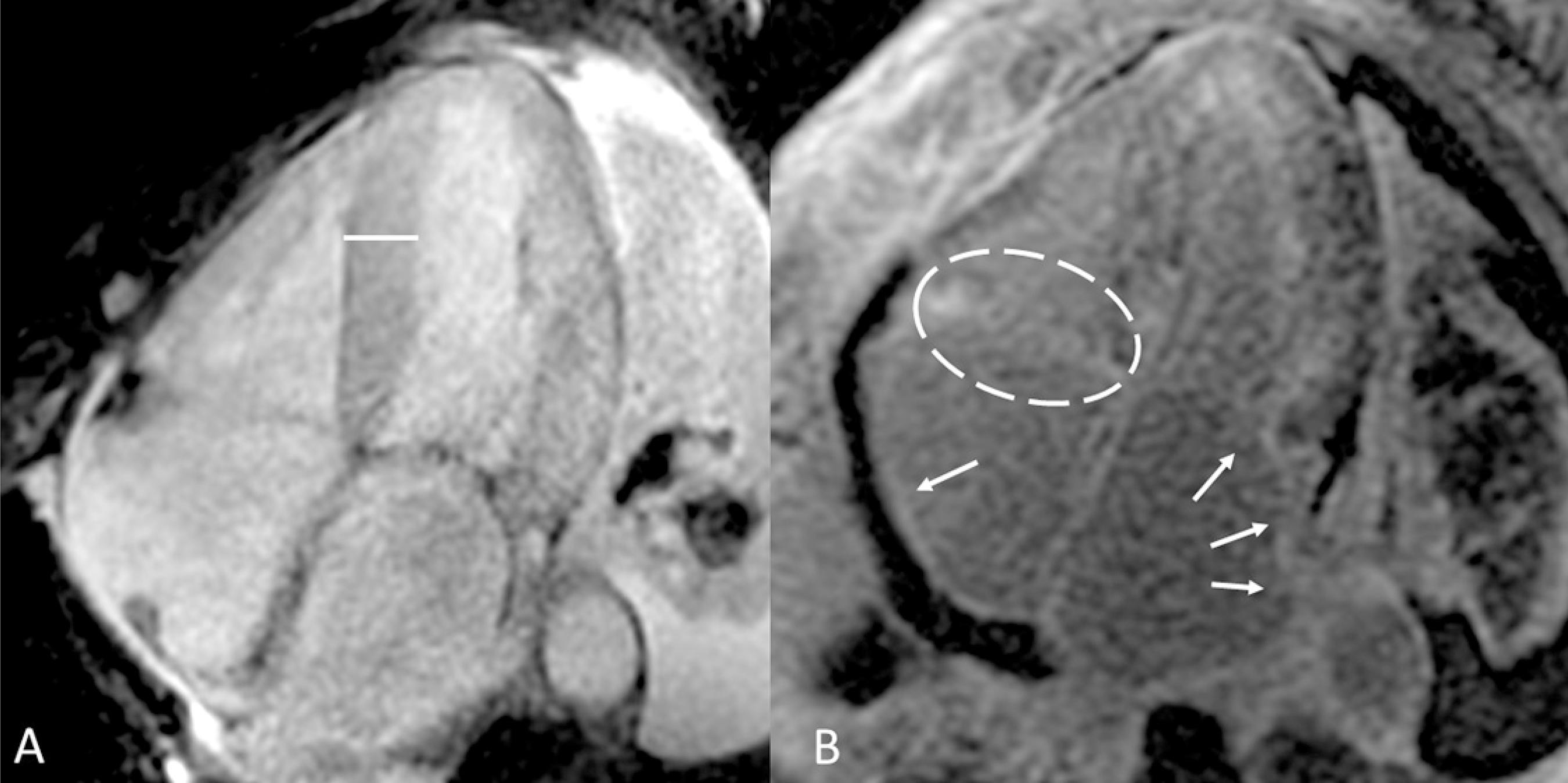
Cardiac magnetic resonance from the four-chamber axis view showing (A) increased interventricular septal thickness. (B) Delayed myocardial enhancement in right atrium (white arrow), left atrium (white arrows) and tricuspid valve system (white dashed elliptical circle).
Cardiac magnetic resonance from the four-chamber axis view showing (A) left ventricular hypertrophy. (B) Delayed myocardial enhancement in interatrial septum (white dashed elliptical circle) and mesocardial interventricular septum (white arrow).
Cardiac magnetic resonance from the long axis two-chamber (A) and three-chamber (B) view showing mid-apical transmural delayed myocardial enhancement (white arrows).
Thus, it is important to reinforce that CMR with late enhancement can be used not only for the diagnosis of cardiac amyloidosis, but also can influence the type of therapy and follow-up of patients, and hence be decisive for the prognosis of these individuals.
T1 mapping and extracellular volume (ECV)
An important advance in cardiac amyloidosis treatment is the increasing use of quantitative analysis by magnetic resonance, including measurements of the T2 (edema) and T2* (iron deposition) and T1 mapping, focusing on non-invasive quantification of diffuse myocardial fibrosis. The most used sequence for T1 mapping quantification is the modified lock-locker inversion recovery (MOLLI) (Figure 6).4444 Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, et al. T1 mapping of the myocardium: intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson. 2012 May 1;14:27.
45 Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, et al. T1 mapping of the myocardium: intra-individual assessment of post-contrast T1 time evolution and extracellular volume fraction at 3T for Gd-DTPA and Gd-BOPTA. J Cardiovasc Magn Reson. 2012 Apr 28;14:26.
46 Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, et al. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011 Nov 28;13:75.
47 Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;62(14):1280-7.
48 Liu S, Han J, Nacif MS, Jones J, Kawel N, Kellman P, et al. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: sample size considerations for clinical trials. J Cardiovasc Magn Reson. 2012 Dec 28;14:90.
49 Nacif MS, Raman FS, Gai N, Jones J, van der Geest RJ, T Sibley C, et al. Myocardial T1 mapping and determination of partition coefficients at 3 tesla: comparison between gadobenate dimeglumine and gadofosveset trisodium. Radiol Bras. 2018;51(1):13-9.
50 Nacif MS, Turkbey EB, Gai N, Nazarian S, van der Geest RJ, Noureldin RA, et al. Myocardial T1 mapping with MRI: comparison of look-locker and MOLLI sequences. J Magn Reson Imaging. 2011;34(6):1367-73.-5151 Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, et al. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265(3):724-32.
(A) Modified lock-locker inversion recovery (MOLLI) T1 mapping on the short axis showing the myocardium (green) and the blood (blue). (B) T1 relaxation curve (482 ms for the myocardium).
With technological advances in the context of amyloidosis or even infiltrative diseases, the use of ECV expansion and T1 mapping has been consolidated in the diagnosis and follow-up of patients with myocardial interstitial disease caused by increased amyloid deposition or fibrosis. T1 mapping transforms a physical principle of magnetic resonance into a quantifiable number (expressed in milliseconds) in an image. T1 relaxation time is the measure of how quickly the longitudinal magnetization component recovers to its equilibrium state. In the T1 mapping technique, measurements of T1 relaxation with and without contrast can be obtained by a simple software that directly defines a region of interest within the myocardium, generating pre- and post-contrast administration values in milliseconds. Amyloid deposition increases T1 in the pre-contrast phase and reduces T1 in the post-contrast phase due to increased extracellular space by the amyloid infiltration.4444 Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, et al. T1 mapping of the myocardium: intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson. 2012 May 1;14:27.
45 Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, et al. T1 mapping of the myocardium: intra-individual assessment of post-contrast T1 time evolution and extracellular volume fraction at 3T for Gd-DTPA and Gd-BOPTA. J Cardiovasc Magn Reson. 2012 Apr 28;14:26.
46 Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, et al. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011 Nov 28;13:75.
47 Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;62(14):1280-7.
48 Liu S, Han J, Nacif MS, Jones J, Kawel N, Kellman P, et al. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: sample size considerations for clinical trials. J Cardiovasc Magn Reson. 2012 Dec 28;14:90.
49 Nacif MS, Raman FS, Gai N, Jones J, van der Geest RJ, T Sibley C, et al. Myocardial T1 mapping and determination of partition coefficients at 3 tesla: comparison between gadobenate dimeglumine and gadofosveset trisodium. Radiol Bras. 2018;51(1):13-9.
50 Nacif MS, Turkbey EB, Gai N, Nazarian S, van der Geest RJ, Noureldin RA, et al. Myocardial T1 mapping with MRI: comparison of look-locker and MOLLI sequences. J Magn Reson Imaging. 2011;34(6):1367-73.-5151 Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, et al. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265(3):724-32.
It has demonstrated that T1 mapping without contrast can identify amyloid deposits with a T1 of approximately 1,140 ± 61 ms.2929 Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6(4):488-97. In post-contrast studies, the cut-off value demonstrated to have a worse prognosis was 565 ms when combined with a pre-contrast value greater than 1,044 ms.5252 Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36(4):244-51.
In addition, the use of pre- and post-contrast T1 mapping enables the calculation of the ECV fraction by the relationship between relaxation fractions of the cardiac muscle and the blood, corrected by hematocrit:
Normal ECV in healthy volunteers is 27 ± 3%.4646 Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, et al. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011 Nov 28;13:75.,4949 Nacif MS, Raman FS, Gai N, Jones J, van der Geest RJ, T Sibley C, et al. Myocardial T1 mapping and determination of partition coefficients at 3 tesla: comparison between gadobenate dimeglumine and gadofosveset trisodium. Radiol Bras. 2018;51(1):13-9.
These made the assessment of interstitial fibrosis or subclinical amyloid deposition by T1 mapping possible, even in situations of negative delayed enhancement magnetic resonance imaging. T1 time is decreased in the presence of fibrosis (Figure 7), making this map and powerful tool for quantification of ECV expansion and fibrosis. T1 mapping has been validated by endomyocardial biopsies in patients with non-ischemic heart diseases, referred for cardiac transplantation.5151 Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, et al. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265(3):724-32. The measurement of the ECV fraction determines, in percentage, areas of possible fibrosis not yet detected by delayed enhancement technique.5252 Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36(4):244-51.
53 Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010 Nov 19;12:69.-5454 Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, et al. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011 Mar 4;13:16.
Postcontrast T1 mapping short axis, showing the myocardium with high T1 value (approximately 500 ms) in green (*). Inferoseptal, anteroseptal and anterior subendocardial fibrosis, (approximately 307 ms) in blue (***).
Prognosis
In 2008, Maceira et al.,1111 Maceira AM, Prasad SK, Hawkins PN, Roughton M, Pennell DJ. Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J Cardiovasc Magn Reson. 2008 Nov 25;10:54. have evaluated the capacity of CMR in characterizing mortality, survival with therapeutic response and development of diastolic function.
In 2015, the study by Fontana et al.,77 Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132(16):1570-9. made clear the importance of detecting delayed enhancement in immunoglobulin light chain and transthyretin amyloidosis and differentiating subendocardial from transmural pattern. Regardless of the type of amyloidosis, transmural delayed enhancement is an important indicator of mortality, which seems to occur earlier in immunoglobulin light chain amyloidosis.
In 2016, Boynton et al.,5555 Boynton SJ, Geske JB, Dispenzieri A, Syed IS, Hanson TJ, Grogan M, et al. LGE immunoglobulin light chain. JACC Cardiovasc Imaging. 2016;9(6):680-6. have demonstrated that delayed enhancement technique provides additional prognostic information to serum biomarkers in patients with cardiac amyloidosis. An important finding was the definition of disease severity by the method in case of inability to null the myocardium by delayed enhancement or in case of diffuse or transmural enhancement. This study had a long editorial highlighting the importance of the delayed enhancement technique in patients with amyloidosis,55 Fontana M. Prognosis in cardiac amyloidosis by LGE: ready for prime time? JACC Cardiovasc Imaging. 2016;9(6):687-9. corroborated by the study by Raina et al.4343 Raina S, Lensing SY, Nairooz RS, Pothineni NV, Hakeem A, Bhatti S, et al. Prognostic value of late gadolinium enhancement CMR in systemic amyloidosis. JACC Cardiovasc Imaging. 2016;9(11):1267-77.
CMR can also help in the characterization of severe patients by assessment of left ventricular function, as proposed by Mohty et al.5656 Mohty D, Boulogne C, Magne J, Varroud-Vial N, Martin S, Ettaif H, et al. Prognostic value of left atrial function in systemic light-chain amyloidosis: a cardiac magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2016;17(9):961-9.
Recently, Martinez-Naharro et al.,5757 Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, et al. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol. 2017;70(4):466-77. in a study published in JACC in 2017, demonstrated the role of the ECV as an independent factor of survival in amyloidosis patients. We believe that, in combination with technological updates of imaging systems, ECV can provide incremental prognostic information in patients with amyloidosis over myocardial delayed enhancement.
Treatment
In general, cardiac amyloidosis has a poor prognosis, depending on the type of amyloidosis, therapy availability and response to treatment. The treatment can be classified as - supportive therapy (specific treatment for heart failure, including pacemakers and automated defibrillators); therapies to suppress the synthesis of amyloid precursor protein (e.g. chemotherapy in immunoglobulin light chain amyloidosis); and new strategies to inhibit the formation of amyloid fibrils, as those targeting amyloid deposits or those including promising medications (such as tafamidis and diflunisal) to stabilize amyloid precursor protein. Despite its low availability, heart transplantation can be a very successful approach in carefully selected patients.1717 Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1(2):e000364.
Standard supportive therapy for heart failure is of limited benefit and occasionally deleterious to cardiac amyloidosis patients. Since cardiac amyloidosis leads to a classical phenotype of restrictive cardiomyopathy, cardiac output can be dependent on heart rate. In this case, patients can be intolerant to beta-blockers, in contrast to heart failure patients with reduced or preserved ejection fraction. There is scarce evidence for the use of angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs) in patients with myocardial amyloid deposition, and in dysautonomic patients, these drugs can even aggravate the symptoms of delayed orthostatic hypotension. Medications such as calcium channel blockers and digitalis should be avoided as they can selectively concentrate in myocardial tissue with amyloid deposition, causing increased toxicity. The use of anticoagulants should be considered in atrial fibrillation or intracavitary thrombus, which is not infrequent in these patients, even in sinus rhythm.1616 Mesquita ET, Jorge AJ, Souza CV Junior, Andrade TR. Cardiac amyloidosis and its new clinical phenotype: heart failure with preserved ejection fraction. Arq Bras Cardiol. 2017;109(1):71-80.,1717 Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1(2):e000364.
Preservation of adequate filling pressures is vital because of the restrictive physiology of this heart disease, to achieve the balance between the treatment of peripheral edema and the development of prerenal kidney failure caused by water-salt depletion and careful use of diuretics. In contrast to other types of heart failure, maintenance of blood pressure, often requiring the use of alpha-agonists such as Midodrine, can make the use of high doses of diuretics possible, especially in patients with autonomic neuropathy.33 Ritts AJ, Cornell RF, Swiger K, Singh J, Goodman S, Lenihan DJ. Current Concepts of Cardiac Amyloidosis: Diagnosis, Clinical Management, and the Need for Collaboration. Heart Fail Clin. 2017;13(2):409-16.,1717 Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1(2):e000364.,5858 Tuzovic M, Yang EH, Baas AS, Depasquale EC, Deng MC, Cruz D, et al. Cardiac amyloidosis: diagnosis and treatment strategies. Curr Oncol Rep. 2017;19(7):46.
Pacemakers and implantable defibrillators may not prevent sudden death, since it is known that the mechanism of death in these patients involves electromechanical dissociation. Given the lack of robust evidence, indication of these devices is similar to that in other heart diseases. High defibrillation threshold may be present in cardiac amyloidosis patients and the benefit of these devices remain uncertain.3333 Tyler DJ, Hudsmith LE, Petersen SE, Francis JM, Weale P, Neubauer S, et al. Cardiac cine MR-imaging at 3T: FLASH vs SSFP. J Cardiovasc Magn Reson. 2006;8(5):709-15. Biventricular pacemakers seem to be the ideal therapeutic option to prevent worsening of cardiac function resulting from desynchrony caused by excessive stimulation of the right ventricle.5959 Bellavia D, Pellikka PA, Abraham TP, Al-Zahrani GB, Dispenzieri A, Oh JK, et al. 'Hypersynchronisation' by tissue velocity imaging in patients with cardiac amyloidosis. Heart. 2009;95(3):234-40.
Currently, amyloidosis treatment is based on reducing the provision of amyloid precursor protein. In immunoglobulin light chain amyloidosis, therapy is focused on plasma cell clones, and combines chemotherapy with autologous bone marrow transplantation. Most chemotherapeutic regimens include dexamethasone combined with an alkylating agent (melphalan or others), which, although they are very useful in the treatment of immunoglobulin light chain amyloidosis, they should be used with caution by patients with cardiac disease due to the high risk of volume overload. New therapeutic strategies include bortezomib, a proteasome inhibitor, and novel immunomodulators (lenalidomide and pomalidomide).
Regarding transthyretin amyloidosis, based on the fact that transthyretin is a transport protein mostly produced in the liver, scientists have developed studies on RNA interference therapies, two recently investigated in phase III trials,6161 Adams D, Gonzalez-Duarte A, O,Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an a RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11-21.,6262 Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22-31. which showed that small interfering RNA (patisiran) or "antisense" (inotersen) constructs, can reduce levels of TTR messenger RNA, the amount of transthyretin synthesized, the serum concentrations of transthyretin, and the amount of misfolded monomer available to aggregate and form deposits. In both trials, the patients who received the active drug had a lower mean rate of progression of the manifestations of the neuropathy (as determined by the modified Neuropathy Impairment Score+7 [NIS+7]) than did the patients who received placebo.33 Ritts AJ, Cornell RF, Swiger K, Singh J, Goodman S, Lenihan DJ. Current Concepts of Cardiac Amyloidosis: Diagnosis, Clinical Management, and the Need for Collaboration. Heart Fail Clin. 2017;13(2):409-16.,1313 Perfetto F, Cappelli F, Bergesio F, Ciuti G, Porciani MC, Padeletti L, et al. Cardiac amyloidosis: the heart of the matter. Intern Emerg Med. 2013;8(3):191-203.,1717 Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1(2):e000364.
Liver transplantation has been used in some cases as a strategy to eliminate transthyretin variants from the circulation. However, although the therapy has been shown to be effective for the amyloidogenic variant Val30Met, disappointing results have been reported with other genetic variants, which frequently involves the heart, since cardiac disease continues to progress with continuous deposition of wild-type transthyretin amyloid.5858 Tuzovic M, Yang EH, Baas AS, Depasquale EC, Deng MC, Cruz D, et al. Cardiac amyloidosis: diagnosis and treatment strategies. Curr Oncol Rep. 2017;19(7):46.
Strategies for the inhibition of amyloid fibril formation assume a massive change of precursor protein into a completely different form. The key stage of transthyretin amyloid fibril formation is the transthyretin tetramer dissociation into monomers prone to self-aggregation. Diflunisal, a nonsteroidal anti-inflammatory drug rarely used nowadays, binds to plasma transthyretin, increasing the structural stability of this soluble protein. Studies involving diflunisal are in progress. Tafamidis, a novel drug with no analgesic or anti-inflammatory properties but with similar mechanisms of action, can selectively bind to thyroxine binding sites, and is currently the most promising drug for amyloidosis treatment. The drug was approved for use by ANVISA in November 2016 for the treatment of transthyretin familial amyloidotic polyneuropathy. Although not worldwide approved yet, the recently published the ATTR-ACT trial showed that tafamidis was associated with reductions in all-cause mortality, cardiovascular-related hospitalizations and the decline in functional capacity and quality of life as compared with placebo, in patients with exclusively transthyretin amyloid cardiomyopathy.6363 Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(1):1007-16. This will soon extend the range of indications of the use of tafamidis in hereditary amyloidosis, previously restricted only to patients with polyneuropathy.
Although amyloid deposits are very stable, the body has limited capacity to eliminate them, particularly from myocardial deposits. The concept of passive immunotherapy to increase amyloid deposit clearance has been proven effective in experimental models and has been widely developed.1717 Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1(2):e000364. Experimental studies have shown that doxycycline, a widely used antimicrobial agent, affects the synthesis of amyloid fibrils, and when combined with biliary salts (taurodeoxycholic acid), shows a synergic effect in removing transthyretin deposits from tissues; these findings have led to studies in humans. In addition, the presence of a flavonoid abundant in green tea, has motivated studies on cardiac amyloidosis, since it has been demonstrated that this compound can inhibit amyloid fibril formation.1212 Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation. 2017;135(14):1357-77.
Cardiac transplantation has yielded disappointing results because of the multisystemic nature of amyloidosis; nevertheless, in highly selective cases undergoing suppression of light chain, and after treatment of extracardiac disease, prognosis may be good.1717 Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1(2):e000364.,5858 Tuzovic M, Yang EH, Baas AS, Depasquale EC, Deng MC, Cruz D, et al. Cardiac amyloidosis: diagnosis and treatment strategies. Curr Oncol Rep. 2017;19(7):46.
Conclusion
CMR can evaluate amyloid deposits in cardiac tissues by delayed enhancement technique, even when cardiac function is preserved. CMR is rewriting the knowledge about cardiac amyloidosis, leading to the development of new classification models and therapies as well as change in the prognosis of the patients.
-
Sources of fundingThere was no external funding for the study.
-
Study associationThis report is part of the "scientific initiation" research project of Vaneza Ferreira Ribeiro at the Department of Radiology of Fluminense Federal University Medical School, Niterói-RJ, Brazil.
-
Ethics approval and consent to participateThis article does not contain any studies with human participants or animals performed by any of the authors.
References
-
1Bulluck H, White SK, Rosmini S, Bhuva A, Treibel TA, Fontana M, et al. T1 mapping and T2 mapping at 3T for quantifying the area-at-risk in reperfused STEMI patients. J Cardiovasc Magn Reson. 2015 Aug 12;17:73.
-
2Meier-Ewert HK, Sanchorawala V, Berk JL, Ruberg FL. Cardiac amyloidosis: evolving approach to diagnosis and management. Curr Treat Options Cardiovasc Med. 2011;13(6):528-42.
-
3Ritts AJ, Cornell RF, Swiger K, Singh J, Goodman S, Lenihan DJ. Current Concepts of Cardiac Amyloidosis: Diagnosis, Clinical Management, and the Need for Collaboration. Heart Fail Clin. 2017;13(2):409-16.
-
4Sara L, Szarf G, Tachibana A, Shiozaki AA, Villa AV, de Oliveira AC, et al., Sociedade Brasileira de Cardiologia and Colegio Brasileiro de Radiologia. [II Guidelines on Cardiovascular Magnetic Resonance and Computed Tomography of the Brazilian Society of Cardiology and the Brazilian College of Radiology]. Arq Bras Cardiol. 2014;103(6 Suppl 3):1-86.
-
5Fontana M. Prognosis in cardiac amyloidosis by LGE: ready for prime time? JACC Cardiovasc Imaging. 2016;9(6):687-9.
-
6Fontana M, Martinez-Naharro A, Hawkins PN. Staging cardiac amyloidosis with CMR: understanding the different phenotypes. JACC Cardiovasc Imaging. 2016;9(11):1278-9.
-
7Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132(16):1570-9.
-
8Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Response to letters regarding article, "Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis". Circulation. 2016;133(12):e450-1.
-
9Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis. 2010;52(4):347-61. Erratum in: Prog Cardiovasc Dis. 2010;52(5):445-7.
-
10Sher T, Gertz MA. Recent advances in the diagnosis and management of cardiac amyloidosis. Future Cardiol. 2014;10(1):131-46.
-
11Maceira AM, Prasad SK, Hawkins PN, Roughton M, Pennell DJ. Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J Cardiovasc Magn Reson. 2008 Nov 25;10:54.
-
12Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing Common Questions Encountered in the Diagnosis and Management of Cardiac Amyloidosis. Circulation. 2017;135(14):1357-77.
-
13Perfetto F, Cappelli F, Bergesio F, Ciuti G, Porciani MC, Padeletti L, et al. Cardiac amyloidosis: the heart of the matter. Intern Emerg Med. 2013;8(3):191-203.
-
14Desai HV, Aronow WS, Peterson SJ, Frishman WH. Cardiac amyloidosis: approaches to diagnosis and management. Cardiol Rev. 2010;18(1):1-11.
-
15Narotsky DL, Castano A, Weinsaft JW, Bokhari S, Maurer MS. Wild-type transthyretin cardiac amyloidosis: novel insights from advanced imaging. Can J Cardiol. 2016;32(9):1166.e1-1166.e10.
-
16Mesquita ET, Jorge AJ, Souza CV Junior, Andrade TR. Cardiac amyloidosis and its new clinical phenotype: heart failure with preserved ejection fraction. Arq Bras Cardiol. 2017;109(1):71-80.
-
17Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1(2):e000364.
-
18Esplin BL, Gertz MA. Current trends in diagnosis and management of cardiac amyloidosis. Curr Probl Cardiol. 2013;38(2):53-96.
-
19Rocha AM, Ferreira SG, Nacif MS, Ribeiro ML, Freitas MR, Mesquita CT. Speckle tracking and transthyretin amyloid cardiomyopathy. Arq Bras Cardiol. 2017;108(1):21-30.
-
20Patel AR, Dubrey SW, Mendes LA, Skinner M, Cupples A, Falk RH, et al. Right ventricular dilation in primary amyloidosis: an independent predictor of survival. Am J Cardiol. 1997;80(4):486-92.
-
21Porciani MC, Lilli A, Perfetto F, Cappelli F, Massimiliano Rao C, Del Pace S, et al. Tissue Doppler and strain imaging: a new tool for early detection of cardiac amyloidosis. Amyloid. 2009;16(2):63-70.
-
22Wechalekar AD, Gillmore JD, Wassef N, Lachmann HJ, Whelan C, Hawkins PN. Abnormal N-terminal fragment of brain natriuretic peptide in patients with light chain amyloidosis without cardiac involvement at presentation is a risk factor for development of cardiac amyloidosis. Haematologica. 2011;96(7):1079-80.
-
23Dispenzieri A, Kyle RA, Gertz MA, Therneau TM, Miller WL, Chandrasekaran K, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet. 2003;361(9371):1787-9.
-
24Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990;323(8):508-13.
-
25de Miguel C, Llorente L, de Haro-Del Moral FJ, Garcia-Pavia P, Gonzalez-Lopez E, Segovia J, et al. Myocardial uptake of (99m)Tc-DPD in patients with AL amyloidosis. Amyloid. 2017;24(supl1):48-9.
-
26Hutt DF, Fontana M, Burniston M, Quigley AM, Petrie A, Ross JC, et al. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur Heart J Cardiovasc Imaging. 2017;18(12):1344-50.
-
27Moore PT, Burrage MK, Mackenzie E, Law WP, Korczyk D, Mollee P. The utility of (99m)Tc-DPD scintigraphy in the diagnosis of cardiac amyloidosis: an Australian experience. Heart Lung Circ. 2017;26(11):1183-90.
-
28Sachchithanantham S, Hutt DF, Quigley AM, Hawkins P, Wechalekar AD. Role of (99m) Tc-DPD scintigraphy in imaging extra-cardiac light chain (AL) amyloidosis. Br J Haematol. 2017 Oct 30. [Epub ahead of print].
-
29Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, et al. Noncontrast T1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013;6(4):488-97.
-
30Bluemke DA, Boxerman JL, Mosher T, Lima JA. Segmented K-space cine breath-hold cardiovascular MR imaging: Part 2. Evaluation of aortic vasculopathy. AJR Am J Roentgenol. 1997;169(2):401-7.
-
31Bluemke DA, Boxerman JL, Atalar E, McVeigh ER. Segmented K-space cine breath-hold cardiovascular MR imaging: Part 1. Principles and technique. AJR Am J Roentgenol. 1997;169(2):395-400.
-
32Barkhausen J, Ruehm SG, Goyen M, Buck T, Laub G, Debatin JF. MR evaluation of ventricular function: true fast imaging with steady-state precession versus fast low-angle shot cine MR imaging: feasibility study. Radiology. 2001;219(1):264-9.
-
33Tyler DJ, Hudsmith LE, Petersen SE, Francis JM, Weale P, Neubauer S, et al. Cardiac cine MR-imaging at 3T: FLASH vs SSFP. J Cardiovasc Magn Reson. 2006;8(5):709-15.
-
34Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171(3):841-5.
-
35Biglands JD, Radjenovic A, Ridgway JP. Cardiovascular magnetic resonance physics for clinicians: Part II. J Cardiovasc Magn Reson. 2012 Sep 20;14:66.
-
36Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988;169(1):59-63.
-
37Kuetting DL, Homsi R, Sprinkart AM, Luetkens J, Thomas DK, Schild HH, et al. Quantitative assessment of systolic and diastolic function in patients with LGE negative systemic amyloidosis using CMR. Int J Cardiol. 2017 Apr 1;232:336-41.
-
38Oda S, Utsunomiya D, Nakaura T, Yuki H, Kidoh M, Morita K, et al. Identification and assessment of cardiac amyloidosis by myocardial strain analysis of cardiac magnetic resonance imaging. Circ J. 2017;81(7):1014-21.
-
39Diesbourg LD, Prato FS, Wisenberg G, Drost DJ, Marshall TP, Carroll SE, et al. Quantification of myocardial blood flow and extracellular volumes using a bolus injection of Gd-DTPA: kinetic modeling in canine ischemic disease. Magn Reson Med. 1992;23(2):239-53.
-
40Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94(12):3318-26.
-
41Saeed M, Wendland MF, Masui T, Higgins CB. Reperfused myocardial infarctions on T1- and susceptibility-enhanced MRI: evidence for loss of compartmentalization of contrast media. Magn Reson Med. 1994;31(1):31-9.
-
42Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218(1):215-23.
-
43Raina S, Lensing SY, Nairooz RS, Pothineni NV, Hakeem A, Bhatti S, et al. Prognostic value of late gadolinium enhancement CMR in systemic amyloidosis. JACC Cardiovasc Imaging. 2016;9(11):1267-77.
-
44Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, et al. T1 mapping of the myocardium: intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson. 2012 May 1;14:27.
-
45Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, et al. T1 mapping of the myocardium: intra-individual assessment of post-contrast T1 time evolution and extracellular volume fraction at 3T for Gd-DTPA and Gd-BOPTA. J Cardiovasc Magn Reson. 2012 Apr 28;14:26.
-
46Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, et al. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011 Nov 28;13:75.
-
47Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;62(14):1280-7.
-
48Liu S, Han J, Nacif MS, Jones J, Kawel N, Kellman P, et al. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: sample size considerations for clinical trials. J Cardiovasc Magn Reson. 2012 Dec 28;14:90.
-
49Nacif MS, Raman FS, Gai N, Jones J, van der Geest RJ, T Sibley C, et al. Myocardial T1 mapping and determination of partition coefficients at 3 tesla: comparison between gadobenate dimeglumine and gadofosveset trisodium. Radiol Bras. 2018;51(1):13-9.
-
50Nacif MS, Turkbey EB, Gai N, Nazarian S, van der Geest RJ, Noureldin RA, et al. Myocardial T1 mapping with MRI: comparison of look-locker and MOLLI sequences. J Magn Reson Imaging. 2011;34(6):1367-73.
-
51Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, et al. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265(3):724-32.
-
52Banypersad SM, Fontana M, Maestrini V, Sado DM, Captur G, Petrie A, et al. T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015;36(4):244-51.
-
53Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010 Nov 19;12:69.
-
54Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, et al. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011 Mar 4;13:16.
-
55Boynton SJ, Geske JB, Dispenzieri A, Syed IS, Hanson TJ, Grogan M, et al. LGE immunoglobulin light chain. JACC Cardiovasc Imaging. 2016;9(6):680-6.
-
56Mohty D, Boulogne C, Magne J, Varroud-Vial N, Martin S, Ettaif H, et al. Prognostic value of left atrial function in systemic light-chain amyloidosis: a cardiac magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2016;17(9):961-9.
-
57Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, et al. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol. 2017;70(4):466-77.
-
58Tuzovic M, Yang EH, Baas AS, Depasquale EC, Deng MC, Cruz D, et al. Cardiac amyloidosis: diagnosis and treatment strategies. Curr Oncol Rep. 2017;19(7):46.
-
59Bellavia D, Pellikka PA, Abraham TP, Al-Zahrani GB, Dispenzieri A, Oh JK, et al. 'Hypersynchronisation' by tissue velocity imaging in patients with cardiac amyloidosis. Heart. 2009;95(3):234-40.
-
60Zumbo G, Sadeghi-Alavijeh O, Hawkins PN, Fontana M. New and developing therapies for AL amyloidosis. Expert Opin Pharmacother. 2017;18(2):139-49.
-
61Adams D, Gonzalez-Duarte A, O,Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an a RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11-21.
-
62Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22-31.
-
63Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(1):1007-16.
Publication Dates
-
Publication in this collection
Mar-Apr 2019
History
-
Received
20 Dec 2017 -
Reviewed
27 Mar 2018 -
Accepted
07 May 2018