Abstracts
During the two-month rearing period, the effect of four water temperatures (15°C, 20°C, 25°C and 30°C) on survival rate, number of molts, and growth rate (molt increment and intermolt period) of juvenile Macrobrachium borellii Nobili, 1896 and Palaemonetes argentinus Nobili, 1901 prawns was evaluated in laboratory conditions. The two species showed some similarities in their both survival and growth pattern at different temperatures. The survival rate was highest at 20°C and 25°C, decreasing at the lowest temperature. The number of molts increased at higher temperatures, ranging the intermolt period from 22.2 days to 9.9 days, for M. borellii, and from 20.8 to 9.5 days for P. argentinus, corresponding those values to 15°C and 30°C, respectively. No difference between species was noted in the intermolt period. The size increment by molting increased significantly from 15°C to 25°C, whereas a reduction in the growth of prawns was observed at 30°C. Significant differences among temperatures were found in the slope of regressions between the size increment by molting and the cephalothorax length. M. borellii showed a significantly higher tolerance to elevated temperature and a faster growth (about twice at 25°C) than P. argentinus. These differences could provide M. borellii a competitive advantage for a better adaptation to the dynamic of freshwater environment, especially in areas with anthropogenic impact.
Decapods; molt increment; intermolt period; temperature
Durante un período de crianza de dos meses, se evaluó en condiciones de laboratorio el efecto de cuatro temperaturas (15°C, 20°C, 25°C y 30°C) sobre la supervivencia, número de mudas y crecimiento (incremento en muda y período de intermuda) de camarones juveniles de Macrobrachium borellii Nobili, 1896 y Palaemonetes argentinus Nobili, 1901. Ambas especies mostraron similitudes en la supervivencia y en el patrón de crecimiento, a las diferentes temperaturas. La supervivencia fue mayor a 20°C y 25°C, y disminuyendo a la menor temperatura. El número de mudas se incrementó con la temperatura, variando el período de intermuda entre 22,2 y 9,9 días en M. borellii y entre 20,8 a 9,5 días en P. argentinus, correspondiendo tales valores a 15°C y 30°C respectivamente. No se detectaron diferencias entre ambas especies en el período de intermuda. El incremento de tamaño por muda aumentó significativamente de 15°C a 25°C, reduciéndose en los camarones criados a 30°C. La pendiente de regresión entre el incremento de tamaño por muda y el largo del cefalotórax mostró variaciones significativas entre las temperaturas. El camarón M. borellii mostró una elevada tolerancia a la alta temperatura y un rápido crecimiento (casi el doble a 25°C) en comparación con P. argentinus. Estas diferencias podrían brindarle a M. borellii una ventaja competitiva para adaptarse a la dinámica de los ambientes dulceacuícolas, especialmente en áreas con impacto antropogénico.
Decápodos; incremento en muda; período de intermuda; temperatura
Effect of temperature on the survival and growth of freshwater prawns Macrobrachium borellii and Palaemonetes argentinus (Crustacea, Palaemonidae)
Efecto de la temperatura sobre la supervivencia y el crecimiento de los camarones dulciacuícolas Macrobrachium borellii y Palaemonetes argentinus (Crustacea, Palaemonidae)
Marcela C. Montagna
Instituto Nacional de Limnología (CONICET-UNL), Ciudad Universitaria, Paraje El Pozo s/n, 3000 Santa Fe, Argentina. marcela.montagna1@gmail.com
ABSTRACT
During the two-month rearing period, the effect of four water temperatures (15°C, 20°C, 25°C and 30°C) on survival rate, number of molts, and growth rate (molt increment and intermolt period) of juvenile Macrobrachium borellii Nobili, 1896 and Palaemonetes argentinus Nobili, 1901 prawns was evaluated in laboratory conditions. The two species showed some similarities in their both survival and growth pattern at different temperatures. The survival rate was highest at 20°C and 25°C, decreasing at the lowest temperature. The number of molts increased at higher temperatures, ranging the intermolt period from 22.2 days to 9.9 days, for M. borellii, and from 20.8 to 9.5 days for P. argentinus, corresponding those values to 15°C and 30°C, respectively. No difference between species was noted in the intermolt period. The size increment by molting increased significantly from 15°C to 25°C, whereas a reduction in the growth of prawns was observed at 30°C. Significant differences among temperatures were found in the slope of regressions between the size increment by molting and the cephalothorax length. M. borellii showed a significantly higher tolerance to elevated temperature and a faster growth (about twice at 25°C) than P. argentinus. These differences could provide M. borellii a competitive advantage for a better adaptation to the dynamic of freshwater environment, especially in areas with anthropogenic impact.
Keywords: Decapods, molt increment, intermolt period, temperature.
RESUMEN
Durante un período de crianza de dos meses, se evaluó en condiciones de laboratorio el efecto de cuatro temperaturas (15°C, 20°C, 25°C y 30°C) sobre la supervivencia, número de mudas y crecimiento (incremento en muda y período de intermuda) de camarones juveniles de Macrobrachium borellii Nobili, 1896 y Palaemonetes argentinus Nobili, 1901. Ambas especies mostraron similitudes en la supervivencia y en el patrón de crecimiento, a las diferentes temperaturas. La supervivencia fue mayor a 20°C y 25°C, y disminuyendo a la menor temperatura. El número de mudas se incrementó con la temperatura, variando el período de intermuda entre 22,2 y 9,9 días en M. borellii y entre 20,8 a 9,5 días en P. argentinus, correspondiendo tales valores a 15°C y 30°C respectivamente. No se detectaron diferencias entre ambas especies en el período de intermuda. El incremento de tamaño por muda aumentó significativamente de 15°C a 25°C, reduciéndose en los camarones criados a 30°C. La pendiente de regresión entre el incremento de tamaño por muda y el largo del cefalotórax mostró variaciones significativas entre las temperaturas. El camarón M. borellii mostró una elevada tolerancia a la alta temperatura y un rápido crecimiento (casi el doble a 25°C) en comparación con P. argentinus. Estas diferencias podrían brindarle a M. borellii una ventaja competitiva para adaptarse a la dinámica de los ambientes dulceacuícolas, especialmente en áreas con impacto antropogénico.
Palabras-clave: Decápodos, incremento en muda, período de intermuda, temperatura.
Among littoral communities, Macrobrachium borellii Nobili, 1896 and Palaemonetes argentinus Nobili, 1901 are the most abundant palaemonids in the lagoons and streams of the Paraná River floodplain in South America (BOSCHI, 1981; LOPRETTO, 1995), having their entire life cycle in fresh water (MENU-MARQUE, 1973; JALIHAL et al., 1993). In these environments, several factors such as seasonal changes in temperature and hydrometric level influence the biology and ecology of decapods fauna (COLLINS, 1999). Several records show that water temperature fluctuates during the year between values higher than 30°C and lower than 10°C, following the climate pattern described for latitudes of 28°-32°S (DRAGO & PAIRA, 1987; BONETTO & WAIS, 1995; DRAGO, 2007). Also according to these authors, at those latitudes the water level of the Paraná oscillates about 2.5 m throughout the year. In period of high water (i.e., from September to March), the high density of floating hydrophytes in the lagoons and streams influences the diurnal water temperature fluctuation by a reduction in the radiant energy, resulting in changes in the temperature profile of the water column below them (DRAGO, 2007; SABATTINI & LALLANA, 2007). Moreover, the period of low water occurs during the winter, in coincidence with lower temperatures and regional precipitation (COLLINS, 2005). The permanent presence of decapods in these fluctuating environments suggests that they should have developed several adaptive mechanisms.
Crustaceans grow by periodically shedding their hard exoskeleton, in a discontinuous process involving a succession of molts (or ecdysis) separated by intermolt periods (HARTNOLL, 1982). The size increase at molt (molt increment), as well as the duration of the intermolt period may change depending on many factors, being temperature one of the most relevant that may affect neurohormonal functions and the ecdysis cycle (PASSANO, 1960; KURATA, 1962). In addition, temperature has a great influence on metabolic rate, food conversion efficiency and energy flow, and hence the growth of crustaceans (DÍAZ-HERRERA et al., 1994; THOMAS et al., 2000; ANGILLETTA, 2001; WU & DONG, 2002; PAGLIANTI & GHERARDI, 2004). The influence of temperature on ectotherm organisms depends on the species distribution, range of thermal tolerance, and acclimatization occurring in response to the environmental fluctuations (SCHMIDT-NIELSEN, 1997). Despite of the extensive studies made in both laboratory and field, there is still a lack of information on the effect of temperature on the growth and survival of prawns of genus Macrobrachium and Palaemonetes (JEFFERIES, 1964; BOND & BUCKUP, 1988; DÍAZ et al., 2002; FELIX & PETRIELLA, 2003; MANUSH et al., 2006).
In the present study, the survival and growth of M. borellii and P. argentinus in laboratory were analyzed at different controlled water temperatures, under optimal food conditions. In addition, the most efficient temperatures for their growth were identified for each species.
MATERIAL AND METHODS
Juveniles of M. borellii and P.argentinus were collected during 2009 at the Salado River, a tributary of the Paraná River, in the Province of Santa Fe, Argentina (31°39'S and 60°41'W). They were preliminarily acclimated to indoor laboratory conditions for three days in two aerated glass containers (100 x 50 x 30 cm) of 150 L each, at 25°C. Subsequently, prawns of each species were randomly subdivided into four groups (25 animals each), and maintained separately in four aerated glass aquaria (60 x 25 x 30 cm) of 45 L each, for an eight-day temperature acclimation period. During this period, the water temperature of the aquaria was gradually adjusted (2°C per day) and maintained at the desired temperature for 3-5 days. Throughout the experiment, dissolved oxygen levels were maintained between 5.5 to 7.0 mg l-1, pH ranged from 7.6 to 8.1, conductivity ranged from 900 to 500 µS cm-1, while hardness averaged 110 mg l-1 (as CaCO3 equivalents). Temperature, dissolved oxygen, pH and conductivity were checked daily by means of HANNA recorders. Total hardness was measured by the EDTA titrimetric method (APHA et al., 2005). Photoperiod was set to 14 h light and 10 h darkness (light intensity: 20 µE m-2 s-1).
Four temperature regimes: 15°C, 20°C, 25°C, and 30°C (± 1°C) were chosen for these experiments, for both M. borellii and P. argentinus. In order to prevent cannibalism during the trials, prawns assigned to each glass aquarium (25 specimens) were kept individually within a plastic cage (8 cm diameter x 7 cm height), whose walls had small holes allowing water exchange, in order to ensure a similar temperature and water quality in the same treatment. In each aquarium, water temperature was fixed at a different value, checking it daily. A cooler was used to maintain a constant 15°C or 20°C water temperature, while no device was necessary for the 25°C treatment. Water temperature was maintained at 30°C by means of a thermostat-controlled immersion heater (100 W, Atman). In each temperature regime, cages were also numbered to recognise each prawn and therefore follow its growth individually.
During the two-month rearing period, all prawns were daily provided with food ad libitum, six to seven days a week, and also checked for molting and mortality. The food consisted of fresh fish and artificial diet previously prepared in laboratory. This artificial diet was prepared according to COLLINS & PETRIELLA (1996), using soy flour, starch, wheat gluten, corn meal, fish meal, prawn meal and vitamins (36% proteins and 10% lipids). Before feeding, excess of food from the previous day, as well as feces, were siphoned out from the aquariums; water lost (about 10%) was immediately refilled with water previously adjusted at the proper temperature. The cephalothorax length (CL, in mm) was used as the size reference measure. In order to reduce stress from handling, CL was determined by measuring the exuvia (CHUL-WOONG & HARTNOLL, 2000), from the tip of the rostrum to the posterior margin at the dorsal midline (standard length, FITZPATRICK, 1977), with an electronic calliper (± 0.01 mm, SCHWYS IP54) and by under a stereoscopic microscope (MOTIC). Mean CL at the beginning of the experiment was 7.07 (± 0.91) mm and 7.00 (± 0.81) for M. borellii and P. argentinus respectively. The initial length did not differ among species (ANOVA, p = 0.5711) nor between temperature treatments (ANOVA, p = 0.9946 for M. borellii and p = 0.9985 for P. argentinus).
In each temperature treatment, survival, number of molts, CL, and intermolt period of prawns were recorded. Survival was analysed by comparing the percentage of animals that survived until the end of the rearing period. The number of molts at each temperature was calculated as (Nm / Ni), where Nm is the number of molted prawns, and Ni is the number of alive individuals per treatment, at the end of the experiment. Molt increment (MI) was defined as MI (%) = (CL - CLt) / CLt) x 100 (HARTNOLL, 1982), where CLt and CLt+1 are the premolt and postmolt cephalothorax length, respectively. The intermolt period (IP, in days) was determined by the interval time between successive molting events.
The statistical comparison among temperature treatments was carried out by means of a one-way analysis of variance (ANOVA). Prior to ANOVA, data were examined for normality and homogeneity, using the chi square and Bartlett's tests. Differences were tested using Tukey's test, according to ZAR (1996). The IP-CL (premolt) and MI (%)-CL (premolt) relationships were fitted by regression to obtain the temperature response curves. Analyses of covariance (ANCOVA) were performed to compare among temperatures the slopes in IP-CL (premolt), and MI (%)-CL (premolt) regressions. Statistical analyses were run by using PAST (Paleontological Statistics), version 2.02 (HAMMER et al., 2001).
RESULTS
In both species, survival was high in prawns reared at 20°C and 25°C, significantly decreasing at lower or higher temperatures (p < 0.0001, Fig. 1). Only at 30°C, there was a significant (p = 0.0110) difference in survival between species, with a survival reduction more evident in P. argentinus (52%) with respect to M. borellii (24%).,
In both species, there was a significant (p < 0.05) increase in the number of molts with the increment of temperature (Fig. 2). For P. argentinus, the highest number of molts was detected at 30°C. Moreover, the number of molts was significantly different between species, at either 20°C or 25°C (p < 0.0001 for 20°C, and p = 0.0047 for 25°C). At these temperatures, P. argentinus molted, on average, more than four times during the two-month period, while M. borellii molted, on average, near three times.
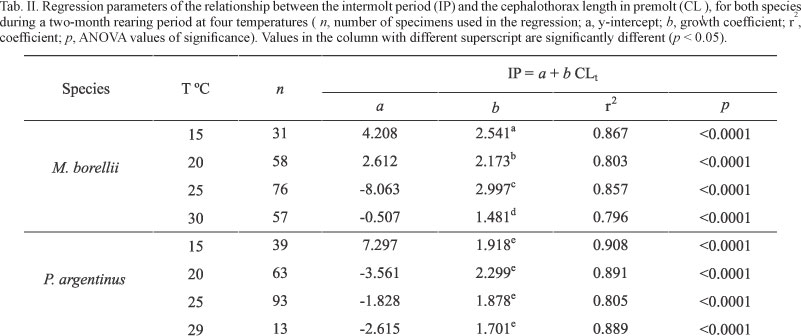
Temperature determined significant differences in the duration of intermolt period for each species (p < 0.0001). On the other hand, Tukey's test showed significant differences between species at each temperature, except at 30°C (p < 0.05) (Tab. I).
Duration of the intermolt period increased significantly in the successive molt cycles only in M. borellii at 20°C, 25°C, and 30°C (p = 0.0006 for 20ºC, p = 0.0222 for 25°C, and p = 0.0176 for 30°C), whereas no differences were found for P. argentinus (p < 0.05). On the other hand, the duration of the intermolt period was directly proportional to the increase in size, with a significant linear relationship among both variables, at any temperature (Tab. II). Significant differences (p < 0.05) between temperatures were observed in growth coefficients of M. borellii, while no differences (p > 0.05) were detected in P. argentinus (Tab. II).
Temperature variation had a significant effect on the percentage of molt increment only in M. borellii at 25°C and 30°C (Tukey's test, p < 0.0001). Furthermore, the molt increment showed significant differences between species at each temperature (Tab. I).
No significant differences (p > 0.05) were seen in the percentage of molt increment between the successive molt cycles of both species, for any temperature. In the regression between molt increment and cephalothorax length (premolt), increasing temperature produced significantly (p < 0.05) lower slopes for M. borellii, but higher slopes for P. argentinus. In all cases, though, slopes were negative (Tab. III).
DISCUSSION
The species investigated in this study, Macrobrachium borellii and Palaemonetes argentinus, showed a high survival at 20°C (88 and 92%, respectively) and 25°C (96% for both species) suggesting that these temperatures are within the optimum temperature range for these prawns. In addition, the reduced survival at 15°C, and 30°C especially in P. argentinus, could indicate that these temperatures are near the thermal tolerance limits. These results show the thermal environment needed for prawns to live, and reflect a possible adjustment to achieve habitat segregation. In this context, both M. borellii and P. argentinus living in the middle Paraná River have a similar geographical distribution (BOSCHI, 1981), but they modify their spatial arrangement within the lagoon and stream to segregate habitats. COLLINS (2005) reported that in winter, M. borellii is more frequently find near the lagoon coast, while P. argentinus moved further away from the coast, showing a greater tolerance to cold water. Additionally, in spring and summer, when the river rises and the limits of the water bodies are not defined, new habitats are formed due to the widening of the water-land transition zone, therefore causing increased movements of juveniles, post-larvae and sexually active specimens. Moreover, the greater tolerance to elevated temperatures observed in M. borellii can confer an adaptive advantage, allowing this species to survive to temperature rise in freshwater environments caused by anthropogenic influences like pollution, deforestation and eutrophication processes (BONETTO & WAIS, 1995).
Ecdysis would determine a high growth rate if occurred a decrease in the duration of the intermolt period (i.e. an increase in molt frequency), an increase in size after molting, or a combination of both (PASSANO, 1960). In the present study, these components of growth were strongly influenced by temperature, yielding the different growth rates observed at the temperatures evaluated. Time between molt events decreased from about 22.2 to 9.9 days in M. borellii and from 20.8 to 9.5 days in P. argentinus, in correspondence with temperature increasing from 15°C to 30°C. These results agree with previous studies performed in Brazil on M. borellii by BOND & BUCKUP (1988), which demonstrated that the relationship between temperature and intermolt shortening is high in small animals, while larger animals are less sensitive to temperature variations. A shortening of the intermolt period was also registered in Penaeus chinensis (Osbeck, 1765) (CHEN et al., 1996), P. semisulcatus (De Haan, 1844) (KUMLU et al., 2000), and Fenneropenaeus chinensis (Osbeck, 1765) (WU & DONG, 2002) at high temperatures. LOBÃO et al. (1996) observed a similar effect in M. amazonicum (Heller, 1862) and M. rosenbergii (De Man, 1879). In field studies, SPIVAK (1997) reported that small specimens of P. argentinus studied in Mar Chiquita coastal lagoon (Argentina) grew quickly in summer, but growth of juveniles diminished in winter and restarted in spring. However, FELIX & PETRIELLA (2003), although observed a marked annual variation in water temperature (4°C to 23°C) in the Los Padres Lagoon (Argentina), they did not notice any alteration in the molt frequency of P. argentinus prawns. This result could be attributed to the seasonal migration of the animals from the shore towards deeper zones in search of refuge from the adverse environmental conditions of winter, such as low temperatures and strong winds.
In several crustacean species, higher temperatures determine an increase in the molt increment during the juvenile phase, while during the mature growth phase the reverse is true (KURATA, 1962; HARTNOLL, 1982). In the present study, the juveniles of M. borellii significantly increased the percentage of molt increment from 15°C to 25°C, while P. argentinus did not. The greatest difference between species in the percentage of molt increment was seen at 25°C (2.31 ± 0.68 for M. borellii against 1.43 ± 0.49 for P. argentinus), which determined a faster growth rate with a few number of molts. This difference in growth could be an advantage for M. borellii in the use of both habitat and food resources during the juvenile phase. Previous studies indicate that theses prawns have similar trophic niche breadth, and when the most aggressive species (M. borellii) is found in high densities, especially in aquatic vegetation, the other one (P. argentinus) is found in a lower number (COLLINS & PAGGI, 1998; COLLINS, 2005). Aquatic vegetation develops in association with islands, shallow lakes and the riparian area of the floodplain, showing the highest growth in the months when temperature exceeds 15°C (LALLANA, 1980). This vegetation is related to an abundant trophic offer and refuges, due to its variety in fauna taxa and microhabits, including nematodes, oligochaetes, cladocerans, copepods, ostracods, amphipods, insects and molluscs (POI DE NEIFF & CARIGNAN, 1997). Possibly, the difference at the beginning of the reproductive period among species - ovigerous females occur from August in P. argentinus and from September in M. borellii (COLLINS et al., 2007) represents a type of adjustment to a competitive pressure.
At the highest temperature (30°C) the percentage of molt increment declined in both studied species, and a higher mortality occurred. This reduction in growth suggests that little energy was left for growth, despite of the prawns being fed in excess. In addition, this could be the result of an increased metabolic consumption requiring a higher nutrient content than that provided by given food. COMAN et al. (2002) reported a similar effect in Penaeus japonicus (Bate, 1888) reared at 31°C with respect to a greater growth rate observed at 27°C and 29°C. For HARTNOLL (1982), the depression of size increment in many crustaceans at elevated temperatures may be related to the abbreviation of the intermolt period, which perhaps permits the accumulation of only limited reserves for size increase. Besides, for this author, the higher respiratory rate at increased temperature may also contribute to this effect.
To conclude, this study shows that a temperature range between 20°C and 25°C is optimal for juveniles M. borellii and P. argentinus in terms of survival and growth rate. At these temperatures, the increment molt was high together with a relatively low intermolt period. However, in addition to the higher tolerance to elevated temperatures showed by M. borellii growth of this species was markedly higher than that of P. argentinus. The obtained results suggest interspecific variations in the growth patterns due to genetic differences, including the phenotypic plasticity or responsiveness to ecological factors. In M. borellii, rapid growth rate may be related to taking maximum advantage of abundant resources, and adaptation to dynamics of rivers and lakes.
Acknowledgements. I wish to thank Dr. Pablo Collins (Instituto Nacional de Limnología) for his constant help and valuable comments on the manuscript.
Recebido em 18 de janeiro de 2010.
Aceito em 16 de setembro de 2011.
- ANGILLETTA, M. J. 2001. Thermal and physiological constraints on energy assimilation in a geographically widespread lizard (Sceloporus undulatus). Ecology 82:3044-3056.
- APHA (American Public Health Association), AWWA (American Water Works Association) & WPCF (Water Pollution Control Federation). 2005. Standard Methods for the examination of water and wastewater. 21ed. American Public Health Association, Washington, D.C.
- BOND, G. & BUCKUP, L. 1988. O ciclo da intermuda em Macrobrachium borellii (Nobili, 1896) (Crustacea, Decapoda, Palaemonidae): a influência da temperatura e do comprimento do animal. Revista Brasileira de Zoologia 5(1):45-59.
- BONETTO, A. A. & WAIS, I. R. 1995. Southern South American streams and rivers. In: CUSHING, C. E.; CUMMINS, K. W. & MINSHALL, G. W. eds. Ecosystems of the World 22 River and stream ecosystems Amsterdam, Elsevier. p.257-293.
- BOSCHI, E. E. 1981. Decapoda Natantia Buenos Aires, Serie fauna de agua dulce de la República Argentina. 60p.
- CHEN, J. C.; LIN, J. N.; CHEN, C. T. & LIN, M. N. 1996. Survival, growth and intermolt period of juvenile Penaeus chinensis (Osbeck) reared at different combinations of salinity and temperature. Journal of Experimental Marine Biology 204(1-2):169-178.
- CHUL-WOONG, O. & HARTNOLL, R. 2000. Effects of food supply on the growth and survival of the common shrimp, Crangon crangon (Linnaeus, 1758) (Decapoda, Caridea). Crustaceana 73:83-99.
- COLLINS, P. A. 1999. Feeding of Palaemonetes argentinus (Decapoda: Palaemonidae) from an oxbow lake of the Paraná River, Argentina. Journal of Crustacean Biology 19:485-492.
- _____. 2005. A coexistence mechanism for two freshwater prawns in the Paraná River floodplain. Journal of Crustacean Biology 25:219-225.
- COLLINS, P. A. & PAGGI, J. C. 1998. Feeding ecology of Macrobrachium borellii (Nobili) (Decapoda: Palaemonidae) in flood valley of river Parana Argentina. Hydrobiologia 362:21-30.
- COLLINS, P. & PETRIELLA, A. 1996. Crecimiento y supervivencia del camarón Macrobrachium borellii (Decapoda: Palaemonidae) alimentado con dietas artificiales. Neotropica 42:3-8.
- COLLINS, P.; WILLINER, V. & GIRI, F. 2007. Littoral communities. Macrocrustaceans. In: IRIONDO, M. H.; PAGGI, J. C. & PARMA, M. J. eds. The Middle Paraná River: Limnology of a Subtropical Wetland Berlin, Springer-Verlag. p.277-301.
- COMAN, G. J.; CROCOS, P. J.; PRESTON, N. P. & FIELDER, D. 2002. The effects of temperature on the growth, survival and biomass of different families of juvenile Penaeus japonicus Bate. Aquaculture 214:185-199.
- DÍAZ, F.; SIERRA, E.; RE, A. D. & RODRÍGUEZ, L. 2002. Behavioural thermoregulation and critical thermal limits of Macrobrachium acanthurus (Wiegman). Journal of Thermal Biology 27:423-428.
- DÍAZ-HERRERA, F.; SIERRA URIBE, E.; BUCKLE RAMÍREZ, L. F. & GARRIDO MORA, G. 1994. Critical thermal maxima and minima of Macrobrachium rosenbergii (Decapoda: Palaemonidae). Journal of Thermal Biology 23:381-385.
- DRAGO, E. C. 2007. The physical dynamics of the river-lake floodplain system. In: IRIONDO, M. H.; PAGGI, J. C. & PARMA, M. J. eds. The Middle Paraná River: Limnology of a Subtropical Wetland Berlin, Springer-Verlag. p.83-122.
- DRAGO, E. C. & PAIRA, A. R. 1987. Temperature and heat budget in a floodplain pond of the Middle Paraná River (Argentina). Revista de la Asociación de Ciencias Naturales del Litoral 18:193-201.
- FELIX, M. M. & PETRIELLA, A. M. 2003. Molt cycle of the natural population of Palaemonetes argentinus (Crustacea, Palaemonidae) from Los Padres lagoon (Buenos Aires, Argentina). Iheringia, Série Zoologia 93:399-411.
- FITZPATRICK, J. C. 1977. The statistical relationships of different tecniques of measurements in a crayfish species. Freshwater Crayfish 3:471-479.
- HAMMER, O.; HARPER, D. A. & RYAN, P. D. 2001. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia ElectronicaWeb. Available at: <http://palaeo-electronica.org/2001_1/past/issue1_01.htm>. Accessed on: 27.11.2010
- HARTNOLL, R. G. 1982. Growth. In: BLISS, D. E. ed. The biology of Crustacea New York, Academic. p.111-196.
- JALIHAL, D. R.; SANKOLLI, K. N. & SHENOY, S. 1993. Evolution of larval development patterns and the process of fresh waterization in the prawns genus Macrobrachium Bate, 1868 (Decapoda, Palaemonidae). Crustaceana 65:365-376.
- JEFFERIES, D. J. 1964. The moulting behaviour of Palaemonetes varians (Leach) (Decapoda, Palaemonidae). Hydrobiologia 24(4):457-488.
- KUMLU, M.; EROLDOGAN, O. T. & AKTAS, M. 2000. Effects of temperature and salinity on larval growth, survival and development of Penaeus semisulcatus. Aquaculture 188:167-173.
- KURATA, H. 1962. Studies on the age and growth of Crustacea. Bulletin of Hokkaido Regional Fisheries Research Laboratory 24:1-115.
- LALLANA, V. H. 1980. Productividad de Eichhornia crassipes (Mart.) Solms. En una laguna isleña de la cuenca del río Paraná medio II. Biomasa y dinámica de población. Buenos Aires, Ecología. v.5, p.1-16.
- LOBÃO, V. L.; ROVERSO, E. A.; LACE, M. & HORTENCIO, E. 1996. Ciclo de muda e crescimento em Macrobrachium amazonicum Heller, 1862 e Macrobrachium rosenbergii De Man (Decapoda, Palaemonidae). Boletim do Instituto de Pesca 23:31-45.
- LOPRETTO, E. C. 1995. Crustacea Eumalacostraca. In: LOPRETTO, E. C. & TELL, G. eds. Ecosistemas de aguas continentales Metodologías para su estudio. Buenos Aires, Ediciones Sur. p.1001-1039.
- MANUSH, S. M.; PAL, A. K.; DAS, T. & MUKHERJEE, S. C. 2006. The influence of temperatures ranging from 25 to 36°C on developmental rates, morphometrics and survival of freshwater prawn (Macrobrachium rosenbergii) embryos. Aquaculture 256:529-536.
- MENU-MARQUE, S. A. 1973. Desarrollo larval de Palaemonetes argentinus (Nobili, 1901) en el laboratorio (Crustacea, Caridea, Palaemonidae). Physis 32:149-169.
- PAGLIANTI, A. & GHERARDI, F. 2004. Combined effects of temperature and diet on growth and survival of young-of-year crayfhish: a comparison between indigenous and invasive species. Journal of Crustacean Biology 24:140-148.
- PASSANO, M. L. 1960. Molting and its control. In: WATERMAN, T. H. ed. The physiology of Crustacea New York, Academic. p.473-536.
- POI DE NEIFF, A. & CARIGNAN, R. 1997. Macroinvertebrates on Eichhornia crassipes roots in two lakes of the Paraná river floodplain. Hydrobiologia 345:185-196.
- SABATTINI, R. A. & LALLANA, V. H. 2007. Aquatic Macrophytes. In: IRIONDO, M. H.; PAGGI, J. C. & PARMA, M. J. eds. The Middle Paraná River: Limnology of a Subtropical Wetland Berlin, Springer-Verlag. p.205-226.
- SCHMIDT-NIELSEN, K. 1997. Animal physiology Adaptation and environment. Cambridge, University Press. 607p.
- SPIVAK, E. D. 1997. Life history of a brackish-water population of Palaemonetes argentinus (Decapoda: Caridea) in Argentina. Annals of Limnology 33:179-190.
- THOMAS, C. W.; CREAR, B. J. & HART, P. R. 2000. The effect of temperature on survival, growth, feeding and metabolic activity of the southern rock lobster, Jasus edwardsii Aquaculture 185:73-84.
- WU, L. & DONG, S. 2002. Compensatory growth responses in juvenile chinese shrimp, Fenneropenaeus chinensis, at different temperatures. Journal of Crustacean Biology 22:511-520.
- ZAR, J. H. 1996. Biostatistical Analysis New Jersey, Prentice Hall. 662p.
Publication Dates
-
Publication in this collection
13 Dec 2011 -
Date of issue
Sept 2011
History
-
Received
18 Jan 2010 -
Accepted
16 Sept 2011