ABSTRACT
Using camera traps and capture/recapture analyses we recorded the presence and abundance of cat species at Turvo State Park, in southern Brazil. Ocelot [Leopardus pardalis (Linnaeus, 1758)] population density was estimated for two areas of the park, with differing management profiles. Density estimates varied from 0.14 to 0.26 indiv. km2. Another five cat species were recorded at very low frequencies, precluding more accurate analyses. We estimate 24 to 45 ocelots occur in the reserve, which is probably too small for long-term maintenance of the population, if isolated. However, if habitat integrity and connectivity between the Park and the Green Corridor of Misiones is maintained, an estimated ocelot population of 1,680 individuals should have long-term viability.
KEYWORDS
Panthera; Puma; sympatric cats; camera trap; capture/recapture
RESUMO
Utilizando armadilhas fotográficas e análises de captura/marcação/recaptura, registramos a presença e abundância de felinos no Parque Estadual do Turvo, sul do Brasil. A densidade de jaguatiricas [Leopardus pardalis (Linnaeus, 1758)] foi estimada para duas áreas dentro do Parque, que apresentam diferentes formas de uso. A densidade estimada variou de 0,14 a 0,26 indivíduos por km². Outras cinco espécies de felinos foram registradas em frequências muito baixas, impedindo analises mais acuradas. Estimamos que 24 a 45 jaguatiricas devam utilizar a Unidade de Conservação, o que provavelmente representa uma população muito pequena para se manter ao longo prazo, caso isolada. Contudo, se a integridade e conectividade de habitat entre o Parque e o Corredor Verde de Misiones forem mantidas, uma população de jaguatiricas estimada em 1.680 indivíduos deve ser viável a longo prazo.
PALAVRAS-CHAVE
Panthera; Puma; felinos simpátricos; armadilha fotográfica; captura/recaptura
The ocelot [Leopardus pardalis (Linnaeus, 1758)] is a medium sized cat (7 - 16 kg) with a broad geographic distribution in the Americas, ranging from the southwestern United States to northern Argentina and southern Brazil (Eisenberg & Redford, 1999Eisenberg, J. F. & Redford, K. H. 1999. Mammals of the Neotropics: The Central Neotropics. Vol. 3. Chicago and London, The University of Chicago Press. 609p.). Although it is common over large areas, such as within the Amazon basin, this felid is regionally threatened and included in some local red lists. This is notably the case in the highly threatened biodiversity hot spot of the Atlantic Forest, where the ocelot is classified as Vulnerable on the red list of threatened species of Rio Grande do Sul State (FZB, 2014FZB. 2014. Táxons da fauna silvestre do Rio Grande do Sul ameaçados de extinção. Avaliable at < Avaliable at http://www.fzb.rs.gov.br
>. Accessed on 15 April 2015.
http://www.fzb.rs.gov.br...
), among several other species. Ocelot use many habitat types, including seasonally flooded forests, cerrado and Atlantic Forest (Wilson & Mittermeier, 2009Wilson, D. E. & Mittermeier, R. A. 2009. Handbook of the mammals of the world, Vol. 1: Carnivores. Barcelona, Lynx, 727p.). It is the best known of the smaller Neotropical cats, with studies of its home range (Ludlow & Sunquist, 1987Ludlow, M. E. & Sunquist, M. E. 1987. Ecology in behavior of ocelots in Venezuela. National Geographic Research 3:447-461.; Emmons, 1988_____. 1988. A Field study of ocelot (Felis pardalis) in Peru. Revue d'Ecologie Terre et la Vie 43:133-157.; Crawshaw & Quigley, 1989Crawshaw, P. G. & Quigley, H. B. 1989. Notes on ocelot movement and activity in the pantanal region, Brazil. Biotropica 21:377-379.), diet (e.g., Emmons, 1987Emmons, L. H. 1987. Comparative feeding ecology of felids in a neotropical rainforest. Behavioral Ecology and Sociobiology 20:271-283.; Konecny, 1989Konecny, M. J. 1989. Movement patterns and food habits of four sympatric carnivore species in Belize, Central America. In Redford, K. H. & Eisemberg, J. F. eds. Advances in Neotropical Mammalogy. Gainsville, Sandhill Crane Press, p. 243-264.) activity patterns, (e.g., Ludlow & Sunquist, 1987Ludlow, M. E. & Sunquist, M. E. 1987. Ecology in behavior of ocelots in Venezuela. National Geographic Research 3:447-461.; Di Bitetti et al., 2006Di Bitetti, M. S.; Paviolo, A. & De Angelo, C. 2006. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. Journal of Zoology 270(1):153-163.) and habitat use (e.g., Ludlow & Sunquist, 1987Ludlow, M. E. & Sunquist, M. E. 1987. Ecology in behavior of ocelots in Venezuela. National Geographic Research 3:447-461.; Di Bitetti et al., 2006Di Bitetti, M. S.; Paviolo, A. & De Angelo, C. 2006. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. Journal of Zoology 270(1):153-163.).
Camera traps are becoming increasingly important for wildlife studies (Wemmer et al., 1996Wemmer, C.; Kunz, T. H.; Lundie-Jenkins, G. & McShea, W. 1996. Mammalian Sign. In: Wilson, D. E.; Cole, F. R.; Nichols, J. D.; Rudran, R. & Foster, M. S. eds. Measuring and Monitoring Biological Diversity: Standard Methods for Mammals. Washington, London, Smithsonian Institution Press, p. 157-176.). They are especially useful for rare or difficult to observe species (Tomas & Miranda, 2003Tomas, W. M. & Miranda, G. H. B. 2003. Uso de armadilhas fotográficas em levantamentos populacionais. In;Cullen Jr., L. Rudran, R. & Valladares-Padua, C. orgs. Métodos de Estudo em Biologia da Conservação e Manejo da Vida Silvestre., Curitiba, Editora UFPR p. 243-267.). Additionally, for many animals, photographs allow individual identification to be made via unique pelage markings. Accordingly, capture-recapture statistical methods can be applied to such animals without their actual physical capture (Karanth et al., 2003Karanth, U.; Nichols, J. D. & Cullen Jr., L. 2003. Armadilhamento fotográfico de grandes felinos: Algumas considerações importantes. In: Cullen Jr., L. Rudran, R. & Valladares-Padua, C. org.. Métodos de Estudo em Biología da Conservação e Manejo da Vida Silvestre. Curitiba, Editora UFPR, p. 269-284. ). Using such methods, camera traps have been used to estimate population densities of large cats, such as tiger Panthera tigris (Linnaeus, 1758) (Karanth, 1995Karanth, U. 1995. Estimating Tiger Panthera tigris populations from camera-trap data using capture-recapture models. Biological Conservation 71:333-338.; Karanth & Nichols, 1998Karanth, U. & Nichols, J. D. 1998. Estimation of Tiger in India using photographic captures and recaptures. Ecology 79:2852-2862.) and jaguar Panthera onca (Linnaeus, 1758) (Wallace et al., 2003Wallace, R. B.; Gómez, H.; Ayala, G. & Spinoza, F. 2003. Camera trapping for jaguar (Panthera onca) in the Tuichi Valley, Bolivia. Mastozoologia Neotropical/Journal of Neotropical Mammalogy 10:133-139. ; Silver et al., 2004Silver, S. C.; Ostro, L. E. T.; Marsh, L. K.; Maffei, L.; Noss, A. J.; Kelly, M. J.; Wallace, R. B.; Gómez, H. & Ayala, G. 2004. Te use of camera traps for estimating jaguar Panthera onca abundance and diversity using capture/recapture analysis. Oryx 38:148-154.; Maffei et al., 2004Maffei, L.; Cuéllar, E. & Noss, A.2004. One thousand jaguars (Panthera onca) in Bolivia's Chaco? Camera trapping in the Kaa-Iya National Park. Journal of Zoology262:295-304. ; Soisalo & Cavalcanti, 2006Soisalo, M. K. & Cavalcanti, S. M. C. 2006. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture-recapture sampling in combination with GPS radio-telemetry. Biological Conservation129:487-496.; Paviolo et al., 2008Paviolo, A.; De Ângelo, C. D.; Di Blanco, Y. E.& Di Bitetti, M. 2008. Jaguar Panthera onca population decline in the Upper Paraná Atlantic Forest of Argentina and Brazil. Orix 42:554-561.). However, in the Neotropics, camera trap studies have been applied to only a few species of the smaller cats such L. pardalis (Trolle & Kéry, 2003Trolle, M. & Kéry, M. 2003. Estimation of Ocelot density in the Pantanal using capture-recapture analysis of camera-trapping data. Journal of Mammalogy 84:607-614., 2005_____. 2005. Camera-trap study of oceolot and other secretive mammals in northern Pantanal. Mammalia 69:405-412.; Maffei et al., 2005Maffei, L.; Noss, A. J.; Cuéllar, E.& Rumiz, D. I. 2005. Ocelot (Felis pardalis) population densities, activity, and ranging behavior in the dry forests of eastern Bolivia: data from camera trapping. Journal of Tropical Ecology21:1-6.; Di Bitetti et al., 2006Di Bitetti, M. S.; Paviolo, A. & De Angelo, C. 2006. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. Journal of Zoology 270(1):153-163.; Dillon & Kelly, 2007Dillon, A. & Kelly, M.2007. Ocelot Leopardus pardalis in Belize: the impact of trap spacing and distance moved on density estimates. Oryx 41(4):469-477.; Di Bitetti et al., 2008Di Bitetti, M. S.; Paviolo, A. De Angelo, C.D. & Di Blanco, Y. E. 2008. Local and continental correlates of the abundance of a neotropical cat, the ocelot (Leopardus pardalis). Journal of Tropical Ecology 24:189-200.; Maffei & Noss, 2008Maffei, L. & Noss, A. J. 2008. How Small is too Small? Camera trap survey and density estimates for Ocelots in the Bolivian Chaco. Biotropica 40:71-75.; Goulart et al., 2009Goulart, F. V. B.; Graipel, M. E.; Tortato, M. A.; Ghizoni-Jr., I. R.; Oliveira-Santos, L. G. & Cáceres, N. C. 2009. Ecology of the ocelot (Leopardus pardalis) in the Atlantic Forest of Southern Brazil. Neotropical Biology and Conservation 4:137-143.; Fusco-Costa et al., 2010Fusco-Costa, R.; Ingberman, B.; Couto, H. T. Z.; Nakano-Oliveira, E. & Monteiro-Filho, E. L. A. 2010. Population density of a costal island population of the ocelot in Atlantic Forest, Southeastern Brazil. Mammalian Biology 75:358-362.), Leopardus geoffroyi (d'Orbigny & Gervais, 1844) (Cuellar et al., 2006Cuellar, E.; Maffei, L.; Arispe, R. & Noss, A. 2006. Geoffroy's cats at the northern limit of their range: activity patterns and density estimates from camera trapping in Bolivian dry forests. Studies on Neotropical Fauna and Environment 41:169-177.) and Leopardus guttulus (Schreber, 1775) (Tortato & Oliveira, 2005Tortato, M. & Oliveira, T. G.2005. Ecology of oncilla () at Serra do Tabuleiro State Park, Southern Brazil. Cat News42:28-30.).
Population parameter estimates are fundamentally important for wildlife conservation and management, especially for those carnivores that are sensitive to disturbance. Here we use camera traps to evaluate the relative abundance and when possible, estimate density of felids at Turvo State Park, southern Brazil.
MATERIAL AND METHODS
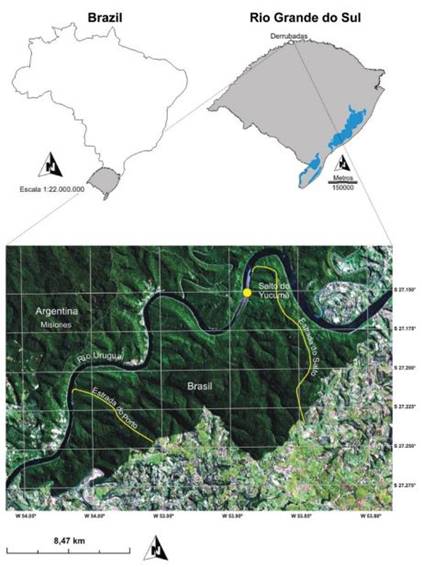
Turvo State Park is located in southernmost Brazil on the border of Argentina (27°00'S - 53°40'W to 27°20'S - 54°10'W). The park comprises 17,500 ha of rainforest of the Upper Uruguay River basin, and is part of the Atlantic Forest domain. The park lies within the Misiones Green Corridor, a well conserved area that extends from Iguaçu National Park (in the State of Paraná, in Brazil) to the Misiones region of Argentina. This area actually represents the southern range limit for several species, such as Panthera onca and Tapirus terrestris (Oliveira, 1994Oliveira, T. G. 1994. Neotropical Cats: ecology and conservation. São Luís, EDUFMA. 220p.; Eisenberg & Redford, 1999Eisenberg, J. F. & Redford, K. H. 1999. Mammals of the Neotropics: The Central Neotropics. Vol. 3. Chicago and London, The University of Chicago Press. 609p.), and a variety of other vertebrates (Fontana et al., 2003Fontana, C. S.; Bencke, G. A. & Reis, R. E. 2003. Livro Vermelho da Fauna Ameaçada de Extinção no Rio Grande do Sul. Porto Alegre, Edipucrs. 632p.). Turvo State Park is surrounded by an agricultural landscape dominated by soybean, wheat and dairy cattle. The original forest now consists of small fragments of secondary or altered primary forest, often associated with Eucalyptus spp. cultivation. The only connection of the park with forested areas is through the adjacent region in the Misiones province of Argentina, crossing the Uruguay River (Fig. 1). There are two internal roads in the park (1) the "Estrada do Salto" (Salto Road) is 15 km long and is open to the public from 8 AM to 5 PM and (2) "Estrada do Porto" (Porto Road) is 8 km long and its use is restricted to researchers. Camera trapping was carried out along these two roads.
Location of Turvo State Park at southern Brazil, and its connection with the region of Misiones, Argentina.
In 2005, eight camera stations were set on Salto road operating for four consecutive nights each month, from February to September. This sampling effort resulted in 312 trap nights that were considered eight (monthly) capture events. Four camera stations were set on Porto road. On Porto road, cameras were used until the end of photographic film, which varied from four to 14 days, monthly, from January to August. This sampling effort resulted in 412 trap nights, and was considered eight (monthly) capture events. The camera stations were separated from each other by 2 km (mean distance) in each road, and were composed by pairs of cameras placed facing each other to obtain the record of both sides all animals photographed.
In 2006 we set eight pairs of camera traps on each road, with four along the main road and four along trails perpendicular to the roads. Cameras were placed at intervals of 300 meters and were in operation for 60 - 70 consecutive days. Salto road was monitored from June to August resulting in a sample effort of 456 trap nights, consisting in 11 capture events of six days. Porto road was monitored from March to June resulting in a sample effort of 442 trap nights of 12 capture events of six days.
Animals were identified by their unique pelage patterns of spots and rosettes. We analyzed capture events with the program Capture (Rexstad & Burnham, 1991Rexstad, E. & Burnham, K. P. 1991. User's guide for interactive program CAPTURE: Abundance estimation of closed animal populations. Colorado, Colorado State University. 29p.). This program compares different models to determine the most parsimonious, and examines differences in capturability among individuals (sex, age, etc.) over time, and combinations thereof (Rexstad & Burnham, 1991Rexstad, E. & Burnham, K. P. 1991. User's guide for interactive program CAPTURE: Abundance estimation of closed animal populations. Colorado, Colorado State University. 29p.).
The effective sampling area was estimated by the sum of buffers added around each trap station resulting in a polygon formed by the union of the buffer zones. The size of the buffer was defined as the mean of the maximum distance moved (MMDM) by the animals of each species. This value was calculated considering the mean of the maximum distance moved by animals that were recorded by different cameras. For comparative purposes, we also show estimates using 1/2 MMDM buffer, previously used by several authors (Trolle & Kery, 2005_____. 2005. Camera-trap study of oceolot and other secretive mammals in northern Pantanal. Mammalia 69:405-412.; Dillon & Kelly, 2007Dillon, A. & Kelly, M.2007. Ocelot Leopardus pardalis in Belize: the impact of trap spacing and distance moved on density estimates. Oryx 41(4):469-477.; Goulart et al., 2009Goulart, F. V. B.; Graipel, M. E.; Tortato, M. A.; Ghizoni-Jr., I. R.; Oliveira-Santos, L. G. & Cáceres, N. C. 2009. Ecology of the ocelot (Leopardus pardalis) in the Atlantic Forest of Southern Brazil. Neotropical Biology and Conservation 4:137-143.).
RESULTS
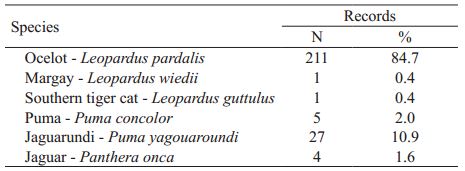
We recorded six species of felids in the Park: southern tiger cat Leopardus guttulus, margay Leopardus wiedii, ocelot L. pardalis, jaguarundi Puma yagouaroundi, puma Puma concolor and jaguar P. onca. Only the ocelot was common, comprising 87.7% (n= 211) of all cat records. Jaguarundi showed intermediate abundance, with 10.9% of the felid records from cameras. For puma, we obtained only five records of three different animals, while for jaguar only one individual was recorded four times. Margay and southern tiger cat were each recorded only once (Tab. I).
Photographic records of felids in the forests of Turvo State Park, Rio Grande do Sul state, Brazil.
There were no meaningful differences in density estimates between 2005 and 2006 for either Porto and Salto areas (Tab. II). In 2005, we obtained 98 ocelot records, 30 on the Porto road and 68 on the Salto road. We identified nine individuals, five males and four females, at Porto and 12 individuals, six males and six females, at Salto. In 2006, we obtained 113 records of ocelots, 31 at Porto and 82 at Salto We identified five individuals at each road site: three males and two females at Porto, and three males, one female and one unknown sex at Salto. During the two years of study a total of 26 individuals were recorded using the sampled area.
Via photographs from different camera stations we recorded distance moved for 15 animals. The mean maximum distance moved (MMDM) was 2.7 km. There were no statistical difference in either movement of males and females (t = 0.18, df = 7, p = 0.8605), or in the distance moved by individuals from the different road samples (t = 1.41, df = 9, p = 0.1852).
Based on MMDM, the effectively sampled area in 2005 was 58.12 km² on Porto road, and 92.09 km² on Salto road, and 29.99 km² on Port road and 35.25 km2 on Salto road in 2006. Density estimates varied between 0.14 - 0.26 individuals per km², with lower estimated densities for the Salto road site (Tab. II). Density estimates using 1/2 MMDM were three times greater than using the full MMDM.
Sampling areas, population size estimates (selected model) with 95% confidence interval (CI), effectively sampled area and density estimates (individuals / km²) at Turvo State Park, Rio Grande do Sul state, Brazil.
DISCUSSION
Although six species of cats were recorded at Turvo State Park, only ocelot was relatively abundant. This felid was recorded six times more than jaguarundi, the second most frequent cat in the park. The other smaller cats as well the larger species, jaguar and puma, seems occur in low abundance, recorded only sporadically.
The lack of differences between the years for the two areas is suggestive that camera arrangements did not affect results. The slightly higher density estimates for the Porto over the Salto site might be related to the latter area's public use of the roads, which could negatively impact ocelot. Although we do not know if prey availability is different between the two areas, all other environmental conditions appeared equal. In any event, this is highly indicative that abundance can change for varying reasons among sites within the same area/region.
Using 2005 data, we estimated ocelot densities of 0.14 to 0.26 individuals per km2. These population density estimates (using the full MMDM) are the largest reported for the ocelot in the southern Atlantic Forest, and similar to that observed by Fusco-Costa et al. (2010Fusco-Costa, R.; Ingberman, B.; Couto, H. T. Z.; Nakano-Oliveira, E. & Monteiro-Filho, E. L. A. 2010. Population density of a costal island population of the ocelot in Atlantic Forest, Southeastern Brazil. Mammalian Biology 75:358-362.) in southeastern Brazil. In Misiones, density estimates (also using full MMDM) were much smaller, with 0.03 individuals/km2 (in altered areas) to 0.12 indiv./km2 in preserved areas (Di Bitetti et al., 2008Di Bitetti, M. S.; Paviolo, A. De Angelo, C.D. & Di Blanco, Y. E. 2008. Local and continental correlates of the abundance of a neotropical cat, the ocelot (Leopardus pardalis). Journal of Tropical Ecology 24:189-200.). Another study in Atlantic Forest also found low densities, with 0.04 indiv./km² (Goulart et al., 2009Goulart, F. V. B.; Graipel, M. E.; Tortato, M. A.; Ghizoni-Jr., I. R.; Oliveira-Santos, L. G. & Cáceres, N. C. 2009. Ecology of the ocelot (Leopardus pardalis) in the Atlantic Forest of Southern Brazil. Neotropical Biology and Conservation 4:137-143.). In the Pantanal, estimates using the same method found 0.07 indiv./km2 (Trolle & Kéry, 2005_____. 2005. Camera-trap study of oceolot and other secretive mammals in northern Pantanal. Mammalia 69:405-412.). However, our estimate seem perfectly plausible considering that we found unless 21 different animals using the Park during 2005. Another strong indication that Turvo State Park has high ocelot densities is the notable capture rate of our study. We obtained 13.1 records/100-trap-nights-1 in this study, a value considerably higher than the 2.5 - 8.1 records/100-trap-nights obtained by Di Bitetti et al. (2006)Di Bitetti, M. S.; Paviolo, A. & De Angelo, C. 2006. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. Journal of Zoology 270(1):153-163., or 0.99 records/100-trap-nights (Goulart et al., 2009) and 3.2 records/100-trap-nights (Trolle & Kéry, 2005). There is some discussion if capture rates reflect population density (Carbone et al., 2001Carbone, C.; Christie, S.; Conforti, K.; Coulson, T.; Franklin, N.; Ginsberg, J. R.; Griffiths, M.; Holden, J.; Kawanishi, K.; Kinnaird, M.; Laidlaw, A.; Lyanam, A.; Macdonald, D. W.; Martyr, D.; Macdougal, C.; Nath, L.; O'Brien, T.; Seidensticher, J.; Smith, D. J. L.; Sunquist, M.; Tilson, R. & Wan Shahruddin, W. N. 2001. The use of photographic rates to estimate densities of tigers and other cryptic mammals. Animal Conservation 4(1):75-79.; Jennelle et al., 2002Jennelle, C. S.; Runge, M. C. & MacKenzie, D. I. 2002. The use of photographic rates to estimate densities of tigers and other cryptic mammals: a comment on misleading conclusions. Animal Conservation5:119-120.), but we consider that comparisons of relative abundance using similar methods with the same species are more than justified for making inferences between areas. Another aspect that can be associated to density is movement pattern of individuals. Average maximum movement distances were smaller in this study (2.7 km) than the 3.96 km from Misiones (Di Bitetti et al., 2006Di Bitetti, M. S.; Paviolo, A. & De Angelo, C. 2006. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. Journal of Zoology 270(1):153-163.), which may suggest that ocelot have smaller home ranges at Turvo State Park and, hence, higher density.
Altough has been used by several authors, effective sampling area estimates based on 1/2 MMDM model is probably very inaccurate. This model may substantially underestimate the area involved (Trolle & Kéry, 2005_____. 2005. Camera-trap study of oceolot and other secretive mammals in northern Pantanal. Mammalia 69:405-412.; Maffei & Noss, 2008Maffei, L. & Noss, A. J. 2008. How Small is too Small? Camera trap survey and density estimates for Ocelots in the Bolivian Chaco. Biotropica 40:71-75.), and consequently, overestimate population density (Soisalo & Cavalcanti, 2006Soisalo, M. K. & Cavalcanti, S. M. C. 2006. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture-recapture sampling in combination with GPS radio-telemetry. Biological Conservation129:487-496.). To avoid, or reduce, the problem of underestimating the sampled area, we adopted the full MMDM model, which gives more conservative density estimates. Also, full MMDM generated similar estimations of density observed for the species in the same habitat (Di Bitetti et al., 2008Di Bitetti, M. S.; Paviolo, A. De Angelo, C.D. & Di Blanco, Y. E. 2008. Local and continental correlates of the abundance of a neotropical cat, the ocelot (Leopardus pardalis). Journal of Tropical Ecology 24:189-200.). In our study, the use of 1/2 MMDM generates estimations three times bigger than estimated by MMDM, which seems unrealistic. (Tab. III).
Habitat, method used to estimate effective sampled areas and density estimates (individuals / km²), using camera traps, in chronological order.
The suggestion that ocelot density is positively correlated with rainfall and negatively correlated with latitude (Di Bitetti et al., 2008Di Bitetti, M. S.; Paviolo, A. De Angelo, C.D. & Di Blanco, Y. E. 2008. Local and continental correlates of the abundance of a neotropical cat, the ocelot (Leopardus pardalis). Journal of Tropical Ecology 24:189-200.), is not supported. The current study was carried out in the same region as that of Di Bitetti et al. (2008)Di Bitetti, M. S.; Paviolo, A. De Angelo, C.D. & Di Blanco, Y. E. 2008. Local and continental correlates of the abundance of a neotropical cat, the ocelot (Leopardus pardalis). Journal of Tropical Ecology 24:189-200., but showed considerably higher density estimates. There are other variables, such as prey density or type of vegetation cover that could have a stronger influence (Oliveira et al., 2010Oliveira, T. G.; Tortato, M. A.; Silveira, L.; Kasper, C. B.; Mazim, F. D.; Lucherini, M.; Jacomo, A. T.; Soares, J. B. G.; Marques, R. V. & Sunquist, M. 2010. Ocelot ecology and its effect on the small-felid guild in the lowland neotropics. In MacDonald, D. & Loveridge, A. org. Biology and Conservation of Wild Felids. Oxford, Oxford University Press. 784p.). Consequently, we believe that more studies on ocelot density are needed across the species range before further generalization can be made regarding the patterns and their causes and effects.
Based on the area of Turvo State Park and our density estimates, we suggest a population of 24 to 45 individuals occurs within the Park. At least 26 different animals were recorded during two years of study, which is in accordance with our minimum estimate. We lack data on population turnover from migration or mortality, but seems clear that densities at Turvo Park are higher than previously observed for the Misiones region by Di Bitetti et al. (2008Di Bitetti, M. S.; Paviolo, A. De Angelo, C.D. & Di Blanco, Y. E. 2008. Local and continental correlates of the abundance of a neotropical cat, the ocelot (Leopardus pardalis). Journal of Tropical Ecology 24:189-200.).
Turvo State Park, the Misiones Green Corridor and ocelot conservation: The population size (24-45) estimated for ocelots in Turvo is small below the minimum viable population of Shaffer (1981Shaffer, M. L. 1981. Minimum population sizes for species conservation. BioScience 31:131-134), and the minimum effective population size proposed by Franklin (1980Franklin, I. R. 1980. Evolutionary change in small populations. In: Soule, M. E. & Wilcox, B. A. eds. Conservation Biology: An EvolutionaryEcological Perspective. Sunderland, Sinauer Associates. 345p.) (both of 50 individuals) required to prevent inbreeding and its deleterious effects. However, to date the park is not isolated. In fact Turvo State Park is part of the Misiones Region of Argentina, a forested area known as the Green Corridor. This region is part of a relatively well-preserved area of about 12,000 km² that extends up to Iguaçu National Park to the north. This area also represents the southernmost ranges of jaguar, tapir T. terrestris and white-lipped peccary Tayassu pecari. Movements of animals between the Misiones Region and Turvo State Park has been recorded for a variety of species, including jaguar (Paviolo et al., 2006Paviolo, A.; De Ângelo, C. D.;; Di Blanco, Y. E. Ferrari, C.; Di Bitetti, M.; Kasper, C. B.; Mazim, F.; Soares, J. B. G. & Oliveira, T. G. 2006. The need of transboundary efforts to preserve the southernmost jaguar population in the World. Cat News 45:12-14.) and ungulates. We believe that the Uruguay River do not represent a barrier to this felid. Consequently, the Park's and the Argentinean population are one. Additionally, ocelot is also found in fragmented areas (Oliveira, 1994Oliveira, T. G. 1994. Neotropical Cats: ecology and conservation. São Luís, EDUFMA. 220p.), which also suggests that movement between the park and nearby areas is possible.
Although ocelots are listed as least concern in Brazil and worldwide (Caso et al., 2015Caso, A.; Lopez-Gonzalez, C.; Payan, E.; Eizirik, E.; Oliveira, T. G.; Leite-Pitman, R.; Kelly, M. & Valderrama, C. 2015. Leopardus pardalis. The IUCN Red List of Threatened Species. Version 2014.3. Avaliable at < Avaliable at http://ww.iucnredlist.org
>. Downloaded on 15 April 2015.
http://ww.iucnredlist.org...
), this felid is threatened regionally. It was considered Vulnerable in Argentina (Diaz & Ojeda, 2000Diaz, G. B. & Ojeda, R. A. eds. 2000. Libro rojo de los mamíferos amenazados de la Argentina. Mendoza, SAREM (Sociedad Argentina para el Estudio de los Mamíferos). 106p.), and regionally in the state of Rio Grande do Sul (FZB, 2014FZB. 2014. Táxons da fauna silvestre do Rio Grande do Sul ameaçados de extinção. Avaliable at < Avaliable at http://www.fzb.rs.gov.br
>. Accessed on 15 April 2015.
http://www.fzb.rs.gov.br...
), which comprise the Green Corridor of the southernmost Atlantic Forest. Given the known densities for ocelots in the Green Corridor, we would expect a total population between 600 and 3,120 individuals (based in the lower values from Di Bitetti et al., (2008Di Bitetti, M. S.; Paviolo, A. De Angelo, C.D. & Di Blanco, Y. E. 2008. Local and continental correlates of the abundance of a neotropical cat, the ocelot (Leopardus pardalis). Journal of Tropical Ecology 24:189-200.) and our higher estimates). Using a reasonable average density of 0.14 ocelots/km2, we can estimate a population size around 1,680 individuals. A population of this size is nevertheless within the 1,250 effective minimum viable population size proposed for the long-term conservation of felids (Oliveira, 1994Oliveira, T. G. 1994. Neotropical Cats: ecology and conservation. São Luís, EDUFMA. 220p.). This is suggestive that as long as the gene flow is maintained and the area is not deforested, this, probably the southernmost population of ocelots, should endure.
However, the best estimate of 45 animals for the entire Park in a scenario of isolation would suggest a critical situation for the local ocelots. Hydroelectric plants are being planned for the region, which would probably cause the increase of river width, which depending on its size, could diminish or even halt gene flow between Turvo and Misiones. Thus, conservation of the ocelot and of all the large cats at Turvo State Park will only be guaranteed over the long term if the Misiones Green Corridor is preserved and remains connected, without barriers.
Acknowledgements
We thank the "Fundação Grupo Boticário de Proteção à Natureza" Foundation for the financial support, the CNPq for the student fellowship and NGO THERIS. We thank Marcos Tortato for his statistical help, Camila for her help with GIS, Flávio Henrique Guimarães Rodrigues, Mario Di Bitetti, Sandra Maria Hartz for their helpful suggestions about the manuscript, and Adrian A. Barnett for English review and comments to manuscript. We also thank the Conservation Unit Division of the SEMA-RS to allow research and provide conditions for study in the Turvo State Park.
REFERENCES
- Carbone, C.; Christie, S.; Conforti, K.; Coulson, T.; Franklin, N.; Ginsberg, J. R.; Griffiths, M.; Holden, J.; Kawanishi, K.; Kinnaird, M.; Laidlaw, A.; Lyanam, A.; Macdonald, D. W.; Martyr, D.; Macdougal, C.; Nath, L.; O'Brien, T.; Seidensticher, J.; Smith, D. J. L.; Sunquist, M.; Tilson, R. & Wan Shahruddin, W. N. 2001. The use of photographic rates to estimate densities of tigers and other cryptic mammals. Animal Conservation 4(1):75-79.
- Caso, A.; Lopez-Gonzalez, C.; Payan, E.; Eizirik, E.; Oliveira, T. G.; Leite-Pitman, R.; Kelly, M. & Valderrama, C. 2015. Leopardus pardalis. The IUCN Red List of Threatened Species. Version 2014.3. Avaliable at < Avaliable at http://ww.iucnredlist.org >. Downloaded on 15 April 2015.
» http://ww.iucnredlist.org - Crawshaw, P. G. & Quigley, H. B. 1989. Notes on ocelot movement and activity in the pantanal region, Brazil. Biotropica 21:377-379.
- Cuellar, E.; Maffei, L.; Arispe, R. & Noss, A. 2006. Geoffroy's cats at the northern limit of their range: activity patterns and density estimates from camera trapping in Bolivian dry forests. Studies on Neotropical Fauna and Environment 41:169-177.
- Diaz, G. B. & Ojeda, R. A. eds. 2000. Libro rojo de los mamíferos amenazados de la Argentina. Mendoza, SAREM (Sociedad Argentina para el Estudio de los Mamíferos). 106p.
- Di Bitetti, M. S.; Paviolo, A. & De Angelo, C. 2006. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. Journal of Zoology 270(1):153-163.
- Di Bitetti, M. S.; Paviolo, A. De Angelo, C.D. & Di Blanco, Y. E. 2008. Local and continental correlates of the abundance of a neotropical cat, the ocelot (Leopardus pardalis). Journal of Tropical Ecology 24:189-200.
- Dillon, A. & Kelly, M.2007. Ocelot Leopardus pardalis in Belize: the impact of trap spacing and distance moved on density estimates. Oryx 41(4):469-477.
- Eisenberg, J. F. & Redford, K. H. 1999. Mammals of the Neotropics: The Central Neotropics. Vol. 3. Chicago and London, The University of Chicago Press. 609p.
- Emmons, L. H. 1987. Comparative feeding ecology of felids in a neotropical rainforest. Behavioral Ecology and Sociobiology 20:271-283.
- _____. 1988. A Field study of ocelot (Felis pardalis) in Peru. Revue d'Ecologie Terre et la Vie 43:133-157.
- Fontana, C. S.; Bencke, G. A. & Reis, R. E. 2003. Livro Vermelho da Fauna Ameaçada de Extinção no Rio Grande do Sul. Porto Alegre, Edipucrs. 632p.
- Franklin, I. R. 1980. Evolutionary change in small populations. In: Soule, M. E. & Wilcox, B. A. eds. Conservation Biology: An EvolutionaryEcological Perspective. Sunderland, Sinauer Associates. 345p.
- Fusco-Costa, R.; Ingberman, B.; Couto, H. T. Z.; Nakano-Oliveira, E. & Monteiro-Filho, E. L. A. 2010. Population density of a costal island population of the ocelot in Atlantic Forest, Southeastern Brazil. Mammalian Biology 75:358-362.
- FZB. 2014. Táxons da fauna silvestre do Rio Grande do Sul ameaçados de extinção. Avaliable at < Avaliable at http://www.fzb.rs.gov.br >. Accessed on 15 April 2015.
» http://www.fzb.rs.gov.br - Goulart, F. V. B.; Graipel, M. E.; Tortato, M. A.; Ghizoni-Jr., I. R.; Oliveira-Santos, L. G. & Cáceres, N. C. 2009. Ecology of the ocelot (Leopardus pardalis) in the Atlantic Forest of Southern Brazil. Neotropical Biology and Conservation 4:137-143.
- Haines, A. M.; Janecka, J. E.; Tewes, M. E.; Grasman Jr, L. I. & Morton, M. 2006. The importance of private lands for ocelot Leopardus pardalis conservation in the United States. Oryx 40:90-94.
- Jennelle, C. S.; Runge, M. C. & MacKenzie, D. I. 2002. The use of photographic rates to estimate densities of tigers and other cryptic mammals: a comment on misleading conclusions. Animal Conservation5:119-120.
- Karanth, U. 1995. Estimating Tiger Panthera tigris populations from camera-trap data using capture-recapture models. Biological Conservation 71:333-338.
- Karanth, U. & Nichols, J. D. 1998. Estimation of Tiger in India using photographic captures and recaptures. Ecology 79:2852-2862.
- Karanth, U.; Nichols, J. D. & Cullen Jr., L. 2003. Armadilhamento fotográfico de grandes felinos: Algumas considerações importantes. In: Cullen Jr., L. Rudran, R. & Valladares-Padua, C. org.. Métodos de Estudo em Biología da Conservação e Manejo da Vida Silvestre. Curitiba, Editora UFPR, p. 269-284.
- Kolowski, J. M. & Alonso, A. 2010. Density and activity patterns of ocelots (Leopardus pardalis) in northern Peru and the impact of oil exploration activities. Biological Conservation143(4):917-925.
- Konecny, M. J. 1989. Movement patterns and food habits of four sympatric carnivore species in Belize, Central America. In Redford, K. H. & Eisemberg, J. F. eds. Advances in Neotropical Mammalogy. Gainsville, Sandhill Crane Press, p. 243-264.
- Ludlow, M. E. & Sunquist, M. E. 1987. Ecology in behavior of ocelots in Venezuela. National Geographic Research 3:447-461.
- Maffei, L.; Cuéllar, E. & Noss, A.2004. One thousand jaguars (Panthera onca) in Bolivia's Chaco? Camera trapping in the Kaa-Iya National Park. Journal of Zoology262:295-304.
- Maffei, L. & Noss, A. J. 2008. How Small is too Small? Camera trap survey and density estimates for Ocelots in the Bolivian Chaco. Biotropica 40:71-75.
- Maffei, L.; Noss, A. J.; Cuéllar, E.& Rumiz, D. I. 2005. Ocelot (Felis pardalis) population densities, activity, and ranging behavior in the dry forests of eastern Bolivia: data from camera trapping. Journal of Tropical Ecology21:1-6.
- Oliveira, T. G. 1994. Neotropical Cats: ecology and conservation. São Luís, EDUFMA. 220p.
- Oliveira, T. G.; Almeida, L. B. & Campos, C. B. 2013. Avaliação do risco de extinção da Jaguatirica Leopardus pardalis (Linnaeus, 1758) no Brasil. Biodiversidade Brasileira 3:66-75.
- Oliveira, T. G.; Tortato, M. A.; Silveira, L.; Kasper, C. B.; Mazim, F. D.; Lucherini, M.; Jacomo, A. T.; Soares, J. B. G.; Marques, R. V. & Sunquist, M. 2010. Ocelot ecology and its effect on the small-felid guild in the lowland neotropics. In MacDonald, D. & Loveridge, A. org. Biology and Conservation of Wild Felids. Oxford, Oxford University Press. 784p.
- Paviolo, A.; De Ângelo, C. D.; Di Blanco, Y. E.& Di Bitetti, M. 2008. Jaguar Panthera onca population decline in the Upper Paraná Atlantic Forest of Argentina and Brazil. Orix 42:554-561.
- Paviolo, A.; De Ângelo, C. D.;; Di Blanco, Y. E. Ferrari, C.; Di Bitetti, M.; Kasper, C. B.; Mazim, F.; Soares, J. B. G. & Oliveira, T. G. 2006. The need of transboundary efforts to preserve the southernmost jaguar population in the World. Cat News 45:12-14.
- Rexstad, E. & Burnham, K. P. 1991. User's guide for interactive program CAPTURE: Abundance estimation of closed animal populations. Colorado, Colorado State University. 29p.
- Shaffer, M. L. 1981. Minimum population sizes for species conservation. BioScience 31:131-134
- Silver, S. C.; Ostro, L. E. T.; Marsh, L. K.; Maffei, L.; Noss, A. J.; Kelly, M. J.; Wallace, R. B.; Gómez, H. & Ayala, G. 2004. Te use of camera traps for estimating jaguar Panthera onca abundance and diversity using capture/recapture analysis. Oryx 38:148-154.
- Soisalo, M. K. & Cavalcanti, S. M. C. 2006. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture-recapture sampling in combination with GPS radio-telemetry. Biological Conservation129:487-496.
- Tomas, W. M. & Miranda, G. H. B. 2003. Uso de armadilhas fotográficas em levantamentos populacionais. In;Cullen Jr., L. Rudran, R. & Valladares-Padua, C. orgs. Métodos de Estudo em Biologia da Conservação e Manejo da Vida Silvestre., Curitiba, Editora UFPR p. 243-267.
- Tortato, M. & Oliveira, T. G.2005. Ecology of oncilla () at Serra do Tabuleiro State Park, Southern Brazil. Cat News42:28-30.
- Trolle, M. & Kéry, M. 2003. Estimation of Ocelot density in the Pantanal using capture-recapture analysis of camera-trapping data. Journal of Mammalogy 84:607-614.
- _____. 2005. Camera-trap study of oceolot and other secretive mammals in northern Pantanal. Mammalia 69:405-412.
- Wallace, R. B.; Gómez, H.; Ayala, G. & Spinoza, F. 2003. Camera trapping for jaguar (Panthera onca) in the Tuichi Valley, Bolivia. Mastozoologia Neotropical/Journal of Neotropical Mammalogy 10:133-139.
- Wemmer, C.; Kunz, T. H.; Lundie-Jenkins, G. & McShea, W. 1996. Mammalian Sign. In: Wilson, D. E.; Cole, F. R.; Nichols, J. D.; Rudran, R. & Foster, M. S. eds. Measuring and Monitoring Biological Diversity: Standard Methods for Mammals. Washington, London, Smithsonian Institution Press, p. 157-176.
- Wilson, D. E. & Mittermeier, R. A. 2009. Handbook of the mammals of the world, Vol. 1: Carnivores. Barcelona, Lynx, 727p.
Publication Dates
-
Publication in this collection
30 Sept 2015
History
-
Received
20 Apr 2015 -
Accepted
30 Sept 2015