Abstracts
According to the relevant literature, the Diels-Alder reaction of furan without a catalyst can last several weeks and shows a low yield due to the diene's low reactivity. The use of Lewis acid catalysts or high pressures is described as an effective method for improving the reaction yields. This paper describes our recent study on the use of niobium pentachloride as the catalyst in Diels-Alder reactions between furan and several reactive dienophiles, among which methyl acrylate showed good yields, especially at lower temperatures. Other dienophiles have shown lower yields because of problems such as byproduct formation and the high reversibility of the reaction.
niobium pentachloride; Diels-Alder; furan
De acordo com a literatura, a reação de Diels-Alder de furano sem uso de catalisador pode durar várias semanas e apresenta baixo rendimento devido à baixa reatividade do dieno. O uso de catalisadores ácidos de Lewis ou de altas pressões são descritos como métodos eficazes para melhorar o rendimento da reação. Este trabalho descreve nosso estudo recente sobre a utilização de pentacloreto de nióbio, como catalisador em reações de Diels-Alder entre furano e alguns dienófilos reativos, entre os quais o acrilato de metila apresentou bons rendimentos, especialmente sob temperaturas mais baixas. Outros dienófilos apresentaram rendimentos mais baixos devido a problemas, tais como a formação de produtos secundários e de elevado caráter de reversibilidade da reação.
ARTICLE
Niobium(V) chloride as catalyst in Diels-Alder reaction of furan ring
Deborah A. dos SantosI; Ludmila R. RodriguesI; Bruno H. ArpiniI; Valdemar Lacerda Jr.I, * * e-mail: vljuniorqui@gmail.com ; Sandro J. GrecoI; Reginaldo B. dos SantosI; Álvaro C. NetoI; Wanderson RomãoI, II; Eustaquio V. R. de CastroI
IChemistry Department, Federal University of Espírito Santo, Av. Fernando Ferrari, 514, 29075-910 Vitória-ES, Brazil
IIFederal Institute of Education, Science and Technology of Espírito Santo, Av. Ministro Salgado Filho, s/n, 29106-010 Vila Velha-ES, Brazil
ABSTRACT
According to the relevant literature, the Diels-Alder reaction of furan without a catalyst can last several weeks and shows a low yield due to the diene's low reactivity. The use of Lewis acid catalysts or high pressures is described as an effective method for improving the reaction yields. This paper describes our recent study on the use of niobium pentachloride as the catalyst in Diels-Alder reactions between furan and several reactive dienophiles, among which methyl acrylate showed good yields, especially at lower temperatures. Other dienophiles have shown lower yields because of problems such as byproduct formation and the high reversibility of the reaction.
Keywords: niobium pentachloride, Diels-Alder, furan
RESUMO
De acordo com a literatura, a reação de Diels-Alder de furano sem uso de catalisador pode durar várias semanas e apresenta baixo rendimento devido à baixa reatividade do dieno. O uso de catalisadores ácidos de Lewis ou de altas pressões são descritos como métodos eficazes para melhorar o rendimento da reação. Este trabalho descreve nosso estudo recente sobre a utilização de pentacloreto de nióbio, como catalisador em reações de Diels-Alder entre furano e alguns dienófilos reativos, entre os quais o acrilato de metila apresentou bons rendimentos, especialmente sob temperaturas mais baixas. Outros dienófilos apresentaram rendimentos mais baixos devido a problemas, tais como a formação de produtos secundários e de elevado caráter de reversibilidade da reação.
Introduction
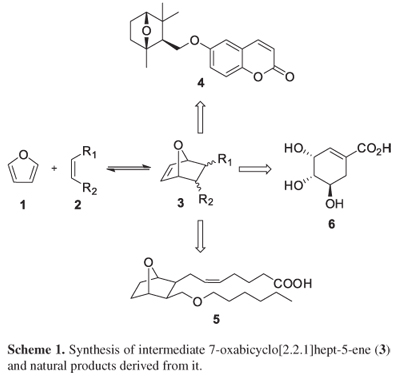
The Diels-Alder reaction of furan ring (1) with a reactive dienophile (2) provides 7-oxabicyclo[2.2.1] hept-5-ene (3), an important intermediate in the synthesis of several natural products such as terpenes (4), prostaglandins (5), shikimic acid (6), and its analogues (Scheme 1).1,2
Several researchers have described methods for synthesizing these cyclic intermediates. The Diels-Alder reaction using a furan ring is the simplest because it requires a single reaction stage. However, furan has low reactivity as a diene and requires the use of Lewis acid catalysts, high pressures, or highly reactive dienophiles. Another problem concerning this cycloaddition is how easily it undergoes a reverse reaction retro-Diels-Alder due to the stability of the diene. Therefore, high reaction temperatures are often avoided.2
Recent studies have shown the potential gains of using niobium compounds as Lewis acids in organic synthesis compared to other inorganic compounds of known acidity.3 The activity of niobium pentachloride as a catalyst has been described in several reactions such as epoxide opening,4-6 the Diels-Alder reaction,7,8 synthesis of pyranoquinoline derivatives,9 Mannich reactions,10 syntheses of α-aminonitriles,11 arylation of aromatic compounds,12 and cyanosilylation of carbonyl compounds,13 among others.14,15 Most of the time, NbCl5 provided higher yields and selectivity compared to the Lewis acids usually used in these reactions.
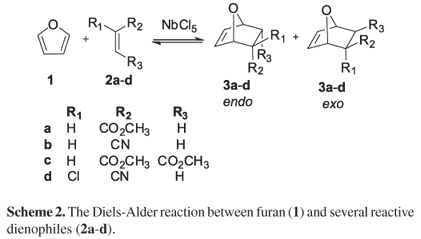
This study used niobium pentachloride as the Lewis acid in synthesizing 7-oxabicyclo[2.2.1]hept-5-ene (3) derivatives through the Diels-Alder reaction between furan (1) and reactive dienophiles (2a-d) (Scheme 2) to evaluate the catalyst activity in the reaction yields and the selectivity of the products.
Experimental
The starting materials are commercially available and were used without purification, except for furan, which was treated and distilled according to the literature.16 Infrared (IR) spectra were recorded on a FTLA2000-102-ABB BOMEM FT-IR spectrophotometer using Pike Technologies MIRacle Single Reflection ATR accessory, 45º, ZnSe, using atmosphere as blank. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded using a Varian 400 (400 MHz) spectrometer with tetramethylsilane (TMS) as the internal standard.
General experimental methods
The reactions were carried out in anhydrous medium, because NbCl5 is easily hydrolyzed. Ten millimoles of dienophile (2a-d) were added to a sealed round bottom flask with NbCl5 (0.05, 0.25, and 1.0 mmol). The mixture was stirred until a yellowish solution was achieved. Then, 11 mmol of diene (1) were slowly added, with the system kept under constant agitation all along the reaction length. The mixture obtained was diluted at the end of the reaction with 75 mL of ethyl acetate. This solution was washed with 100 mL of diluted sodium bicarbonate. The solvent was evaporated, and the products obtained were analyzed through IR spectroscopy and nuclear magnetic resonance of ¹H (400 MHz) and ¹³C (100 MHz). The reactions yields were calculated in terms of wt.%. The spectroscopic data agreed with the reports in the literature.17-19 Ratios of the endo and exo adducts were determined through the relation of the integral of the ¹H NMR signals from the corresponding double bond hydrogens of the endo and exo adducts.
Results and Discussion
The niobium pentachloride activity as a catalyst was investigated in Diels-Alder reactions between furan (1) and several reactive dienophiles methyl acrylate (2a), acrylonitrile (2b), dimethyl maleate (2c), and 2-chloro-acrylonitrile (2d) (Scheme 2) changing reaction parameters such as temperature, catalyst ratio, and reaction length.
The reactions were carried out without solvents, initially because the diene and dienophiles were liquid and to contribute to the application of environmentally friendly methodologies in organic synthesis, implying minimum residue generation, whereas the Diels-Alder reaction does not generate side products, and during the extraction of the adduct, the NbCl5 is hydrolyzed, providing Nb2O5.nH2O, which has low toxicity.
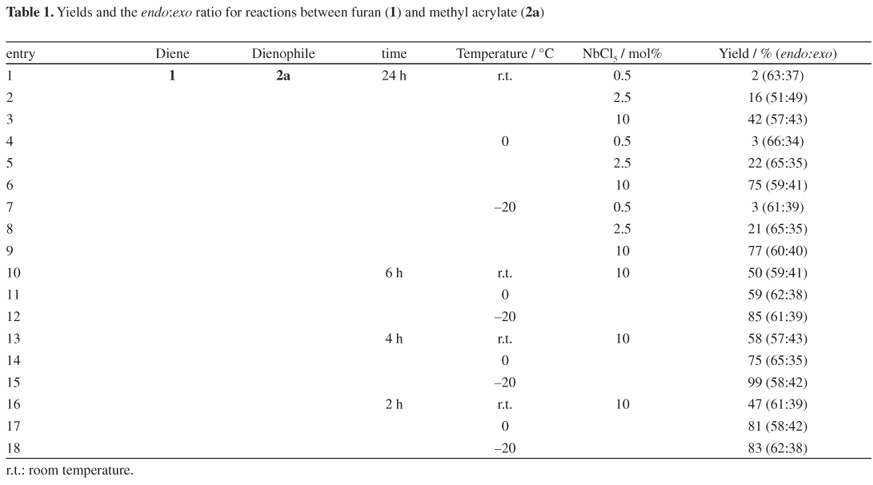
First, the optimum conditions for the reaction between furan (1) and methyl acrylate (2a) were studied (Table 1). Then, the ideal catalyst ratio was determined: 0.5 and 2.5 mol% of NbCl5 showed some activity in 24 reaction hours at different temperatures, whereas with 10 mol% of the catalyst, for the same reaction length, the yields were good (42-77%), especially at lower temperatures.
The Diels-Alder reaction is reversible, and due to the easy way in which cycloadditions with 1 undergo a reverse reaction, we started a study on the ideal reaction length using 10 mol% of the catalyst. Shorter times increased the yields, and temperature was again an important factor in the conversion rates; the lower the temperature, the higher the yields. In general, product selectivity was higher for the endo adduct in all reaction conditions.
Comparing the best result achieved for the reaction between furan (1) and methyl acrylate (2a) catalyzed by NbCl5 to what has been reported in the literature (Table 2), higher product yields were achieved under shorter reaction time and without using solvent. Most methods reported show higher selectivity for the endo adduct compared to our results.
When acrylonitrile (2b) was used as the dienophile (Table 3), low yields were achieved even with 10 mol% of NbCl5 and at low temperatures. A problem observed during this reaction was the formation of byproducts mainly at 20 ºC, possibly resulting from polymerization of acrylonitrile. Reaction times shorter than those used in the reaction between 1 and 2a were tested to try to decrease the byproduct formation; however, our attempts were unsuccessful. Adduct selectivity was in general lower than that observed for product 3a.
Reaction times longer than those we used are required to achieve higher yields for product 3b as shown in Table 4.
Although the dienophilicity of dimethyl maleate (2c) is increased due to the β-carboalkoxy group, reactions using 2c as the dienophile did not show any conversion even in the presence of up to 10 mol% of NbCl5 in 24 h, at room temperature or 0 ºC. Tests using the solvent (CH2Cl2) were unsuccessful. At these temperatures, retro-Diels-Alder must be favored, Hayashi et al.24 observed higher yields and selectivity in reactions carried out at 20 and 50 ºC using HfCl4 as the Lewis acid catalyst. Thus, experiments at lower temperatures were performed to try to improve the results.
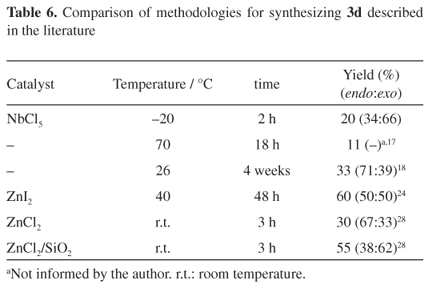
For reactions between furan (1) and 2-chloro-acrylonitrile (2d) (Table 5), we adopted conditions that had provided good conversion rates for other adducts. Since 2d is a more reactive dienophile, two catalyst ratios were tested (2.5 and 10 mol%) for 2 hours of reaction. In general, the yields were low. At 0 ºC and 20 ºC the yields were higher with 10 mol% of catalyst. At room temperature, however, the yields were lower with higher amounts of catalyst (entry 2, Table 5). To explain this behavior, the reaction was carried out for 1 and 4 h, and the yields achieved were 12 and 6%, respectively. Thus, a reverse reaction is favored by time under this temperature, which can also justify the higher ratio of the exo adduct.
The yields obtained for 3d were lower than those achieved using other Lewis acids (Table 6). However, higher selectivity for the exo adduct was observed when NbCl5 was used.
Conclusions
NbCl5 has been shown to be an active catalyst for the Diels-Alder reaction between furan (1) and methyl acrylate (2a), with good yields and reasonable selectivity for the endo adduct. When the dienophile was dimethyl maleate (2c), NbCl5 had no activity. In the reactions that used acrylonitrile (2b) as the dienophile, lower yields and selectivity were achieved, and byproducts formed. With 2-chloro-acrylonitrile (2d), the yields were also low; however, higher selectivity for the exo adduct was observed.
Supplementary Information (SI)
Supplementary data (NMR and FTIR spectra) are available free of charge at http://jbcs.sbq.org.br as a PDF file.
Acknowledgements
The authors thank CBMM for providing the niobium pentachloride used in this work, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenadoria de Aperfeiçoamento de Pessoal do Nível Superior (CAPES), and Fundação de Amparo à Pesquisa do Espírito Santo (FAPES) for financial support.
Submitted: December 14, 2013
Published online: March 14, 2014
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]
- 1. Kappe, O. C.; Murphree, S. S.; Padwa, A.; Tetrahedron 1997, 53, 14179.
- 2. Voguel, P.; Cossy, J.; Plumet, J.; Arjona, O.; Tetrahedron 1999, 55, 13521.
- 3. Lacerda Jr., V.; dos Santos, D. A.; da Silva-Filho, L. C.; Greco, S. J.; dos Santos, R. B.; Aldrichimica Acta 2012, 45, 19.
- 4. Constantino, M. G.; Lacerda Jr., V.; Aragão, V.; Synth. Commun. 2007, 37, 3529.
- 5. Constantino, M. G.; Lacerda Jr., V; Invernize, P. R.; da Silva-Filho, L. C.; da Silva, G. V. J.; Molecules 2001, 6, 770.
- 6. Constantino, M.; Lacerda Jr., V. ; da Silva, G. V. J.; J. Heterocycl. Chem. 2003, 40, 369.
- 7. Constantino, M. G.; Lacerda Jr., V.; da Silva, G. V. J.; Molecules 2002, 7, 456.
- 8. Silva-Filho, L. C.; Lacerda Jr., V.; Invernize, P. R.; Constantino, M. G.; da Silva, G. V. J.; Beilstein J. Org. Chem. 2005, 1, 14.
- 9. da Silva-Filho, L. C.; Lacerda Jr., V.; Constantino, M. G.; da Silva, G. V. J.; Synthesis 2008, 16, 2527.
- 10. Wang, R.; Li, B.; Huang, T.; Shi, L.; Lu, X.; Tetrahedron Lett. 2007, 48, 2071.
- 11. Majhi, A.; Sung, S. K.; Kim, H. S.; Appl. Organomet. Chem. 2008, 22, 466.
- 12. Yadav, J. S.; Bunia, D. C.; Krishna, V. K.; Srihari, P.; Tetrahedron Lett. 2007, 48, 8306.
- 13. George, S. C.; Kim, S. S.; Bull. Korean Chem. Soc. 2007, 28, 1167.
- 14. Barbosa, S. L.; Hurtado, G. R.; Klein, S. I.; Lacerda Jr., V.; Dabdoub, M. J.; Guimarães, C. F.; Appl. Catal., A 2008, 338, 9.
- 15. Constantino, M. G.; Lacerda Jr., V.; Silva-Filho, L. C.; Silva, G. V. J.; Lett. Org. Chem. 2004, 1, 360.
- 16. Armarego, W. L. F.; Chai, C. L. L.; Purification of Laboratory Chemicals, 6th ed.; Butterworth-Heinemann: Oxford, 2009, pp. 393.
- 17. Schmidt, R. R.; Beitzke, C.; Forrest, A. K.; J. Chem. Soc., Chem. Commun. 1982, 909.
- 18. Schuda, P. F.; Bennett, J. M.; Tetrahedron Lett. 1982, 23, 5525.
- 19. Chau, C. W.; Fawcett, A. H.; Mulemwa, J. N.; Tan, C. E.; Polymer 1985, 26, 1268.
- 20. Nelson, W. L.; Allen, D. R.; Vincenzi, F. F.; J. Med. Chem. 1971, 14, 698.
- 21. Dauben, W. G.; Krabbenhoft, H. O.; J. Am. Chem. Soc. 1976, 98, 1992.
- 22. Kotsuki, H.; Nishizawa, H.; Ochi, M.; Matsuoka, K.; Bull. Chem. Soc. Jpn. 1982, 55, 496.
- 23. Brion, F.; Tetrahedron Lett. 1982, 23, 5299.
- 24. Hayashi, Y.; Nakamura, M.; Nakao, S.; Inoue, T.; Shoji, M.; Angew. Chem., Int. Ed. 2002, 41, 4079.
- 25. Kotsuki, H.; Asao, K.; Ohnishi, H.; Bull. Chem. Soc. Jpn. 1984, 57, 3339.
- 26. Kienzle, F.; Helv. Chim. Acta 1975, 58, 1180.
- 27. Morton, C. J. H.; Gilmour, R.; Smith, D. M.; Lightfoot, P.; Slawin, A. M. Z.; MacLean, E. J.; Tetrahedron 2002, 58, 5547.
- 28. Fraile, J. M.; García, J. I.; Massam, J.; Mayoral, J. A.; Pires, E.; J. Mol. Catal. A: Chem. 1997, 123, 43.
Publication Dates
-
Publication in this collection
30 May 2014 -
Date of issue
May 2014
History
-
Received
14 Dec 2013 -
Accepted
14 Mar 2014