Abstracts
Total (HgT), reactive (HgR) and organic (HgORG) mercury species have been quantified in non-filtered waters collected from the Negro River Basin, Amazon (from January/2002 through January/2004), in both black and white water bodies. Black waters presented the highest HgT, HgORG and HgR concentration (29.1 ng L-1, 1.63 ng L-1 and 3.9 ng L-1, respectively), while, white waters presented the lowest HgT, HgORG and HgR concentration (8.8 ng L-1, 0.49 ng L-1 and 1.2 ng L-1, respectively). An inverse correlation between HgT and the water level over the basin was obtained, although the HgORG concentration has increased in the first rainy months and then decreased as the water level rose. Total mercury in surface sediments (0-10 cm) ranged from 70 to 271 mg kg-1 being the methylmercury 0.47-1.79 % of this stock.The results indicate that HgR and the labile dissolved organic matter were introduced into the aquatic environment during the flooding season, especially in the earlier stages, thus contributing to mercury methylation.
organic mercury; mercury speciation; Negro River Basin; black and white waters
Espécies de mercúrio total (HgT), reativo (HgR) e orgânico (HgORG) foram quantificadas em amostras de água não filtradas da Bacia do Rio Negro, Amazonia (de Janeiro/2002 a Janeiro/2004) em ambos corpos aquáticos de águas brancas e pretas. As águas pretas apresentaram as maiores concentrações de mercúrio total, orgânico e reativo (29,1; 1,63 e 3,9 ng L-1, respectivamente), sendo que as águas brancas apresentaram as menores concentrações de HgT, HgORG e HgR (8,8; 0,49 e 1,2 ng L-1, respectivamente). Uma correlação inversa entre HgT e o nível da água sobre a bacia foi obtido, embora a concentração de HgORG tenha aumentado nos primeiros meses chuvosos decrescendo com o aumento do nível da água. A concentração de HgT em sedimentos superficiais (0-10 cm) variou de 70 a 271 mg kg-1, com o metilmercúrio sendo 0,47-1,79% deste estoque. Os resultados indicaram que HgR e a matéria orgânica dissolvida e lábil estão sendo introduzidos no ambiente aquático durante a estação chuvosa, especialmente nos estágios iniciais contribuindo para a metilação de mercúrio.
ARTICLE
Seasonal behavior of mercury species in waters and sediments from the Negro River Basin, Amazon, Brazil
Márcia Cristina BisinotiI, * * e-mail: bisinoti@ibilce.unesp.br ; Ézio Sargentini JúniorII; Wilson F. JardimIII
IInstituto de Biociências, Letras e Ciências Exatas, Departamento de Química e Ciências Ambientais, Universidade Estadual Paulista, Rua Cristóvão Colombo, 2265, 15054-000 São José do Rio Preto-SP, Brazil
IICoordenação de Pesquisas em Produtos Naturais, Química Analítica Ambiental, Instituto Nacional de Pesquisas da Amazônia, C.P. 478, 69083-000 Manaus-AM, Brazil
IIIInstituto de Química, Departamento de Química Analítica, Universidade Estadual de Campinas, C.P. 6154, 13084-971 Campinas-SP, Brazil
ABSTRACT
Total (HgT), reactive (HgR) and organic (HgORG) mercury species have been quantified in non-filtered waters collected from the Negro River Basin, Amazon (from January/2002 through January/2004), in both black and white water bodies. Black waters presented the highest HgT, HgORG and HgR concentration (29.1 ng L-1, 1.63 ng L-1 and 3.9 ng L-1, respectively), while, white waters presented the lowest HgT, HgORG and HgR concentration (8.8 ng L-1, 0.49 ng L-1 and 1.2 ng L-1, respectively). An inverse correlation between HgT and the water level over the basin was obtained, although the HgORG concentration has increased in the first rainy months and then decreased as the water level rose. Total mercury in surface sediments (0-10 cm) ranged from 70 to 271 mg kg-1 being the methylmercury 0.47-1.79 % of this stock.The results indicate that HgR and the labile dissolved organic matter were introduced into the aquatic environment during the flooding season, especially in the earlier stages, thus contributing to mercury methylation.
Keywords: organic mercury, mercury speciation, Negro River Basin, black and white waters
RESUMO
Espécies de mercúrio total (HgT), reativo (HgR) e orgânico (HgORG) foram quantificadas em amostras de água não filtradas da Bacia do Rio Negro, Amazonia (de Janeiro/2002 a Janeiro/2004) em ambos corpos aquáticos de águas brancas e pretas. As águas pretas apresentaram as maiores concentrações de mercúrio total, orgânico e reativo (29,1; 1,63 e 3,9 ng L-1, respectivamente), sendo que as águas brancas apresentaram as menores concentrações de HgT, HgORG e HgR (8,8; 0,49 e 1,2 ng L-1, respectivamente). Uma correlação inversa entre HgT e o nível da água sobre a bacia foi obtido, embora a concentração de HgORG tenha aumentado nos primeiros meses chuvosos decrescendo com o aumento do nível da água. A concentração de HgT em sedimentos superficiais (0-10 cm) variou de 70 a 271 mg kg-1, com o metilmercúrio sendo 0,47-1,79% deste estoque. Os resultados indicaram que HgR e a matéria orgânica dissolvida e lábil estão sendo introduzidos no ambiente aquático durante a estação chuvosa, especialmente nos estágios iniciais contribuindo para a metilação de mercúrio.
Introduction
The Negro River, situated in the northwest part of Brazil, covers an extension of approximately 1700 km and its basin spreads over an area of 696,800 km2, which represents 14% of the total area of the Brazilian Amazon region.1 According to Sioli,2,3 Amazon tributaries are generally classified according to their collor: black water, white water and intermediate water rivers. Black water rivers, such as the Negro River, drain highly weathered sandy soils of the central Amazon basin, which show low sediment and nutrient concentrations, brown-colored acid waters rich in dissolved humic substances and acidic pH. White waters, such as, Branco, Solimões and Madeira Rivers originate in the Andes and are characterized by a high suspended sediment load, neutral to slightly acidic pH, low organic matter concentration (bellow 9 mg C L-1) and rich in dissolved nutrients due to rapid weathering in piedmont regions. Intermediate water bodies present characteristics between black and white waters.
Moreover, the Negro River basin is characterized by a diversified type of soil, vegetation, deforested areas and mainly by the hydrological cycle, showing two distinct hydrological seasons: the dry period (September to February) and the wet period (rainy season from March to August), when the water level can increase up to 15 m. In the rainy season, rivers tend to flood, covering the forest and surrounding areas forming the so-called "igapós", which act as an important source of labile organic carbon to the water bodies, thus markedly affecting both the major nutrient cycles and mercury chemistry.
The mercury biogeochemical cycle in the Amazon is a topic of interest covered by several recent papers,4-16 but it is still poorly understood. High mercury concentrations have been found in soil (average value of 170 µg kg-1)4,5 and in other matrices5-12 in the Negro River basin. Different from other regions in the Amazon, this basin shows little gold-mining activity and there have been many indications that, in this region, naturally occurring mercury leaching from soil (99.7%) is the major pathway to mercury enrichment.4 Podzolization can be pointed to as the major mechanism controlling the dynamics of Hg in soils and its release and transport to the aquatic system because constant rains and plains relief.5
In waters and sediments, mercury can be transformed into methylmercury under both biotic and abiotic conditions.17-22 Methylmercury concentrations in water rarely exceed 10% the mercury amount, while in sediments this value is lower,19,23-25 ranging from 0.1 to 2 %. Methylmercury is a species of particular interest because of its biomagnification in the food chain, its high toxicity, bioavailability and persistence in the environment.19,24
The biogeochemistry of mercury is complex, and its understanding demands a consistent study of other processes, such as chemical, atmospherical, geological, hydrological, microbial, physiological and ecological ones. This complexity has been recognized since the early studies on the occurrence of mercury in natural aquatic ecosystems, where high concentrations were found in fish despite the extremely low mercury concentration in water.5-16 Organic mercury concentrations in surface waters depend on the balance between methylation and demethylation and, therefore, show both spatial and temporal dependence.24 Generally the amount of methylmercury tends to increase in the presence of organic matter and to decrease as the concentration of the inorganic particulates increases.19,24
Sampling was undertaken as part of a broader project examining the mercury cycle in this basin, to evaluate possible sources and seasonal behavior of mercury species in the water column and sediments. Assessing methylmercury concentration and trends in aquatic bodies is important to understand the mercury cycle and to predict possible threats for both biota and humans. Besides, data related to the presence of organic mercury in the Negro River are still scarce. The major contribution of this study is to provide data on seasonal variations observed for both organic and total mercury obtained over three years of monitoring these species in this basin.
Experimental
Sampling periods and stations
The Negro River has an average flow of 29,000 m3 s-1 in Manaus where, after meeting the Solimões River, it forms the Amazon River. Rainfall in the Negro River basin varies from 3500 mm year-1 in the upper basin to 2137 mm year-1 in the lower basin. The average temperature of the region varies from 24 to 33ºC.1
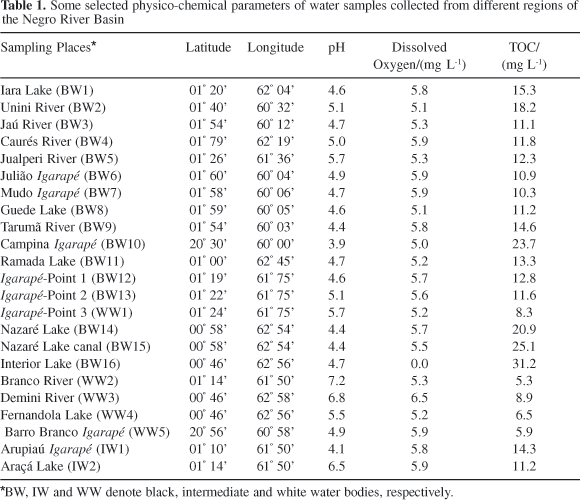
Several surface water samples (n=23) were collected in the Negro River basin (Figure 1) in January/2002 (dry season), January/2003 (dry season), June/2003 (rainy season) and January/2004 (dry season). Most samples (16 sampling points) were collected from black water bodies (BW1 to BW16), 5 samples were collected from white water bodies (WW1 to WW5) and two samples were collected in aquatic bodies with intermediate characteristics between black and white waters (IW1 and IW2). Table 1 presents a description of the sampling points with latitude and longitude references.
The behavior of mercury species in water has been evaluated through vertical profiles (0, 1, and 3 m depth) in black water bodies. Moreover, waters from the Tarumã River were collected monthly during the period June 2003 to June 2004 to investigate the seasonal behavior of HgORG, HgR and HgT.
Sediment samples were collected using an Ekman dredge or a gravity cylindrical collector in different sampling places indicated by SED1 to SED6 in the Figure 1 (Araçá and Iara Lakes; Unini, Jualperi, Jaú and Caurés Rivers).
Cleaning and sampling procedures
Precautions were taken in order to avoid contamination during sampling. The labware were placed in a HCl:water (1:4 v/v) bath for 48 h at 80 ºC on a hot plate.25 For water collection, bottles of polyethylene terephthalate (PET) commercialized for mineral water were used following the recommended procedure of Fadini and Jardim.26 These authors validated the use of PET bottles, compared with the traditional Teflon bottles for both sampling and storage of low concentrations of total and reactive mercury. The bottles were washed several times at the sampling site and filled 10 cm below the surface. For both total and reactive mercury analysis, water samples were collected and preserved according to the procedure above mentioned prior to be transported to the laboratory in Campinas, São Paulo State, Brazil.26 Water samples for organic mercury analysis were extracted using methylene choride and stored in a refrigerator for transportation.27
Triplicate samples for total, organic and reactive Hg in non-filtered water samples were collected at each sampling point and the coefficient of variation for the results of Total and Reactive Hg was bellow 5%. Because organic mercury concentrations are much lower than the ones obtained for total and reactive mercury, a lower precision among triplicate samples was observed (15%).
Sediment samples were homogenized, stored in plastic bags and placed in a freezer at 5º C until reaching the laboratory where carbon, methyl and total mercury concentrations were determined.
Reagents and standards
Analytical high-purity grade reagents were used throughout. Ultrapure water was obtained from a MilliQ System (Millipore). Mercury (II) chloride (Merck) and methylmercury chloride (Spectra Pure) were used as standards. A stock solution (1000 ng L-1) in ultrapure water of each individual mercury compound was prepared daily. Methylmercury solutions were protected against the light by storing in the dark at 4 ºC. SnCl2 (Nuclear) solution was made by dissolving the salt in HCl (Mallinckrodt) to obtain a 10% (v/v) solution. All solutions were prepared using ultrapure water.
Analytical procedures
Reactive, total, and organic mercury present in non-filtered waters were quantified using double-stage gold amalgamation followed by Cold Vapor Atomic Fluorescence Spectrometry (CVAFS from Brooks Randâ model 2). Total mercury in sediment samples was quantified using the CVAAS technique adapted to FIA as described elsewhere.28 The limit of detection was 0.1 µg L-1 when using 600 µL of sample at an analytical frequency of 60 samples h-1. Methylmercury present in sediment was quantified using a gas chromatograph equipped with an electron-capture detector (GC-ECD).29,30
Water samples
Reactive mercury (HgR) is an operationally defined parameter that embraces all mercury species amenable to be reduced by stannous chloride. Aliquots of 100 mL of water samples were placed in a wash bottle provided with a four-way valve and received the addition of 2 mL of 10% (m/v) stannous chloride solution. The elemental Hg formed was stripped out of the solution with argon and trapped onto a gold column. The first column was connected to the analytical column in the CVAFS, and the mercury was transferred to the second column by heating. From the analytical column, mercury was again released by heating and measured. The detection limit of this method is 40 pg.
Total mercury (HgT) represents the sum of all mercury species present in water samples. The procedure involves the oxidation of all mercury species to Hg2+ by BrCl oxidation.31 The excess of this compound was eliminated by adding 30% (m/v) hydroxylamine chloride. All mercuric species are then reduced to elemental mercury by adding a 10% (m/v) stannous chloride solution, and HgT is quantified according to the procedure described for reactive mercury. The detection limit of this method is 60 pg.
HgORG represents the stock of all organic mercury species present in the water samples, with methylmercury being the predominant one. The procedure for extraction and quantification of HgORG from water samples is based on acidic extraction using 10% (m/v) HCl followed by step wise CH2Cl2 extraction and back-extraction into water as described by Bisinoti and co-authors.27 The method consists in taking a 300 mL aliquot of the sample in a 500 mL Teflon bottle, followed by the addition of 15 mL of a saturated KCl solution in 10.0 % (m/v) HCl and 30 mL of methylene chloride (Mallinckrodt) previously saturated with ultrapure water. The sample is shaken for 12 h in an orbital shaker at 150 rpm. The organic phase (methylene chloride) is transferred to another Teflon bottle containing 100 mL of MilliQ water. In a hood, the sample was then heated to 45 ºC to speed up the evaporation of methylene chloride and then purged with N2 to remove the remaining methylene chloride at room temperature. The organic mercury remaining was transferred to a Teflon bottle containing ultrapure water and oxidized by adding bromine chloride, letting the reaction occur for 30 min. The excess of the oxidizing agent was destroyed with 500 µL of a 30 % (m/v) hydroxylamine chloride solution. After 5 minutes, the organic mercury was quantified as already described for reactive mercury. The detection limit of this method is 160 pg.
The determinations of HgORG, HgR, and, HgT were carried out in triplicate. The accuracy of the analytical method has been checked through mercury recovery ranges (90 to 100%).
Sediment samples
Total mercury concentrations were determined using the conventional CVAAS procedure after the reduction of inorganic mercury with SnCl2 in acidic medium.28 The method consists in taking 1-2 g of wet sediment sample in 10 mL of MilliQ water, followed by the addition of 5 mL of concentrated sulfuric acid, 2.5 mL of concentrated nitric acid and 15 mL of 7% (m/v) potassium permanganate solution. After 15 min, 10 mL of 8% (m/v) potassium persulfate solution was added and the sample was heated at 80 ºC for 2 h. After cooling, the excess of permanganate was eliminated by adding 6 mL of 15% (m/v) hydroxylamine hydrochloride solution, and the final volume was adjusted to 50 mL with ultrapure water.
Mercury standard solutions and blanks used in the calibration procedure were prepared following this same procedure.4,27 Sediment samples were analyzed on a wet basis to avoid artifacts and losses associated with drying. A separate aliquot was analysed for percent humidity, in order to establish the appropriate correction for dry-basis measurements.
Methylmercury in sediment samples was determined by digesting samples in an alcoholic potassium hydroxide solution followed by a dithizone-toluene extraction. After a series of clean-up steps, methylmercury dithizonate was identified and quantified using the Akagi and Nishimura method,29 which consists in the quantification of mercury present in the toluene layer using a gas chromatograph, Shimadzu model GC-14 B equipped with an electron-capture detector (ECD). Analytical quality control was based on the analysis of certified reference material (BCR-580) for total and methylmercury in sediment.30
Other physical and chemical parameters
Analysis of the water samples included the determination of other parameters such as pH, redox potential, temperature, conductivity and dissolved oxygen (DO) using a multiparameter equipment (ATI-ORION - PerpHect 370).32 TOC was measured using a Shimadzu TOC 5000 via the catalytic oxidation process (HTCO). Determination of organic carbon in sediments was carried out by means of an Elemental Analyzer (Perkin Elmer, 2004).
Results and Discussion
Water samples
According to Sioli,2 black water tributaries, as described before, are characterized by low concentrations of both nutrients and suspended sediments and by dark coloured waters due to a high content of humic compounds, leading to a water with low pH. White water tributaries present higher concentrations of major elements, a close to neutral pH, lower concentrations of DOC, and a high suspended sediment burden coming from the Andean mountains. In this work (Table 1) the black waters were characterized by high organic carbon contents (10.3-31.2 mg C L-1), low pH (3.9-5.7), temperatures typical of tropical waters (29-33 ºC), Dissolved Oxygen (DO) of 0.0-5.9 mg L-1 and EH of 100-300 mV. In comparison, white water bodies showed low organic carbon (5.3-8.9 mg C L-1), neutral to slightly acidic pH (4.9-7.2), similar values for temperature (29-33 ºC), higher DO (5.2-6.5 mg L-1) and similar EH values (100-300 mV). These results are in good agreement with previous data found in the literature,4-12 and are very important to explain the behavior of mercury species in these two different aquatic systems. Some selected physico-chemical parameters of water samples are presented in Table 1.
Total, reactive and organic mercury were measured in non-filtered surface water samples as previous attempts in working with filtered water samples showed consistent events of mercury cross-contamination. The results for total mercury ranged from <0.3 to 29.1 ng L-1, and the concentration found for the other species are presented in Table 2. In Brazil, there are no guidelines for methylmercury in natural waters. Rudd18 has suggested that a concentration < 0.1 ng L-1 of methylmercury should be representative of pristine waters although, in this case this background value cannot be used as the region is characterized by high concentrations of naturally occurring mercury4,5.
Major results obtained during all excursions (January/2002, January/2003, June/2003 and January/2004) showed detectable amounts of organic mercury in both white and black waters, with values oscillating from < 0.1% to 17.0% of the total mercury present for the black waters. For white waters, organic mercury content ranged from < 0.3% to 6.0% (Table 2). As pointed out by Fadini and Jardim,4 total mercury concentrations are higher in black waters when compared to those observed in white water bodies, which can be explained by the metal leaching from the soil caused by the acid pH and high organic content of black waters, as mentioned above.
According to Maurice-Bourgoin and co-authors,33 Amazonian black water bodies, including the Negro River show a very low turbidity (5 NTU) when compared to the one observed for the Solimões River (around 80 NTU). Despite the low concentration of suspended solids (5 mg L-1), the high concentration of organic matter in this material is responsible for the transport of 61% of the total mercury in the Negro River. Similar results concerrning the load of suspended solids in this basin was carried out by Moreira-Turcq et al.34
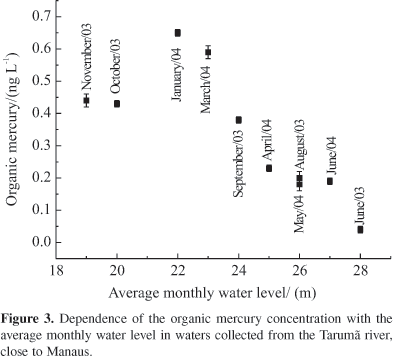
Total mercury concentrations can be also dependent on the hydrological conditions, here presented as average monthly water level (daily measure with the aid of a ruler). Unfortunatelly numerous efforts to perform water filtration in situ always resulted in mercury contamination, where the mass balance of dissolved plus particulate led to errors around 100%. The lack of speciation, however, does not impair mercury fluxes estimates, as this parameter refers to the total amount of the metal. As shown in Figure 2, throughout the dry season, total mercury values are higher than the ones obtained in the rainy season. The highest concentrations of HgT and HgORG have been observed at Interior Lake, which also presented high TOC (31.2 mg C L-1), acidic pH (4.7) and anaerobic conditions (EH = 0.0 mV), conditions essential to mercury methylation.
In most natural water bodies, HgORG concentrations do not show a correlation with the total mercury concentration. Methylmercury formation, from the toxicological point of view is considered the most important organic mercury species, is governed by a number of environmental factors, including temperature, pH, redox potential, bacterial activity, inorganic and organic complexing agents, organic matter and inorganic mercury content. In a review17 dealing with the interaction of mercury and organic matter, it has been demonstrated that organic matter plays an important role in the bioavailability of mercury to methylating bacteria and subsequent bioaccumulation of methylmercury in the aquatic food chain.
Organic mercury concentrations ranged from <0.01 to 1.63 ng L-1, corresponding to <0.1 and 17% of the total mercury found in these waters. The highest HgORG concentrations occurred in the Igarapé-Point 1, Igarapé-Point 2, Interior Lake, Nazaré Lake Canal, and Iara Lake, showing a different pattern from that observed for total mercury. High concentrations of organic mercury found in these points may be attributed to both high organic matter content and acidic conditions. Another aspect to possibly explain this behavior in flooded areas is due to the presence of sulfate reducing bacteria (SRB) and methanogenic bacteria, which can contribute to mercury methylation.24 Unfortunately, there have been few works focused on the Negro River basin exploring the real role of these bacteria in mercury methylation, thus demanding more detailed studies to better understand this mechanism. However, one important point to be highlited in this paper is the fact that the values obtained for HgORG are similar or even lower than the ones reported for freshwaters worldwide.35-47
Reactive mercury provides information on the availability of Hg for biogeochemical reactions.24 In general, the amount of HgR in all sampled sites varied between about 24% and 8% of the total Hg for black and white waters, respectively, indicating that very little of the transported Hg in this basin is present as the free ion or labile species able to be reduced by stannous chloride. Poor correlations between total and reactive mercury concentrations (R2 = 0.16) were observed in this study. The average results obtained in this work were compared with the results obtained in other areas of the Amazon Basin (Table 3) and were shown to be of the same order of magnitude.
For organic mercury (Figure 3) one can observe that, when the rainy season starts, there is an increase in the concentration of HgORG (from June through September) despite the increase in the water level. From this point on, as the water level increases, a concomitant decrease of organic mercury concentration is observed, due to the dilution effect. It is believed that, in this basin, during the early flooding periods organic mercury (or its precursors) is flushed from the newly flooded soil (igapós) into the aquatic system, thus increasing the concentration in the receiving waters.
Figure 4 shows the correlation between reactive and organic mercury species (R=0.9568), revealing that organic mercury production is higher when the supply of reactive Hg is also high. This is another indication that, during rainy periods, inorganic mercury species and/or labile mercury-organic matter complexes leached from the soil are carried to rivers and lakes in this watershed. Input of reactive mercury and labile organic matter into the water column during the flooding period has already been observed by Bisinoti et al.53 In this work, the authors proposed the use of hydrogen peroxide consumption kinetics to infer organic matter lability in the water column and confirmed the input of this type of organic matter during flooding periods. 53
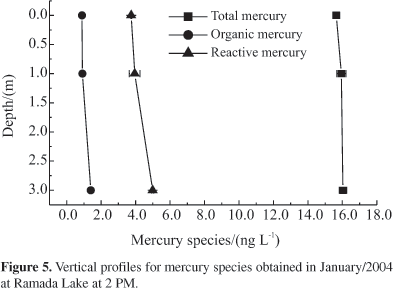
The distribution of the mercury species in Ramada Lake (black waters) is shown in Figure 5. The species HgT showed a constant vertical profile of concentration whereas, for HgORG and HgR, concentrations were slightly lower at surface compared to the deeper parts. This gradient is due to losses of the HgORG, possibly due to photodegradation of this species in the upper layer of the photic zone as already mentioned in the literature,54 or due to the solar driven reduction of Hg2+ to Hg0, thus lowering the concentration of the major substrate for the methylation process. A possible evasion of this species directly to the atmosphere cannot be excluded. According to Silva,12 the concentration of dissolved gaseous mercury in the Rio Negro is higher at the surface than at 3 m deeper, what causes an evasive water flux of elemental mercury under sunlight. Such behavior can be explained by assuming that solar radiation plays an important role in the destruction of methylmercury in the photic zone of these waters55. A similar trend has also been observed by Vandal et al.56
Sediment samples
Total and methylmercury concentrations obtained in sediment samples are shown in Table 4 and they are comparable to results found by other authors in other ecosystems throughout the world.57,58 Methylmercury concentrations in sediments ranged from 0.19 to 3.76 mg kg-1, representing 0.22% to 1.79% of the total mercury (Table 4). The pattern of methylmercury in sediments is similar to that of HgT, again showing higher concentrations in sediment from black water lakes and rivers.
Sediment cores show a production of methylmercury in the upper layers (0-10 cm) and a gradual decrease with depth (Table 4) for Iara and Araçá Lakes, which could be explained by the higher organic carbon concentrations. Much higher concentrations were found in the Iara Lake sediment where more favorable conditions (acidic pH conditions and higher organic matter content) for the methylation of Hg occur.
Conclusions
Despite the pristine conditions of the Negro River watershed, the concentrations of total mercury found in surface waters are similar to those observed in industrialized areas, which can be explained by assuming high background concentrations of this metal due to natural occurrence. The singular conditions observed for black waters such as low pH and oxygenated water, as well as, the high organic matter content favour mercury methylation.
All the mercury species observed are determined by the local and unique hydrological conditions. Organic and reactive mercury concentrations increased in the beginning of the rainy season, mostly due to leaching from the soil. After this early period, due to the dilution effect, the concentration of all mercury species decreased. Total mercury correlated inversely with the average monthly rainfall for samples collected close to Manaus (Tarumã River). The major factors responsible for the high organic mercury content found in black waters are the input of soil containing labile, freshly produced organic matter, rich in humic acid observed in the early stages of the flooding periods, mediated by acidic pH conditions, and the possible presence of methanogenic bacteria in these waters.
Acknowledgment
The authors would like to thank Prof. C. H. Collins for revising the manuscript and the comments of the two reviewers. This work was supported by FAPESP (02/03395-1; 00/13517-1).
Received: July 9, 2006
Web Release Date: April 27, 2007
FAPESP helped in meeting the publication costs of this article.
- 1. Instituto Brasileiro de Geografia e Estatística, Amazônia Brasileira. Geography Edition National Council of X Congresso Brasileiro de Geografia, Rio de Janeiro, September 1944, http:\\www.inep.gov.br (in Portuguese).
- 2. Sioli, H.; Am. J. Trop. Med. Hyg. 1953, 2, 700.
- 3. Sioli, H.; Amazônia. Fundamentos da Ecologia da Maior Região de Florestas Tropicais, Editora Vozes: Petrópolis, 1985.
- 4. Fadini, P. S.; Jardim, W. F.; Sci. Total Environ. 2001, 275, 71.
- 5. Silva-Forsberg, M. C.; Forsberg, B. R.; Zeidemann, V. K.; Ambio 1999, 28, 519.
- 6. Malm, O.; Environ. Res 1998, 77, 73.
- 7. Miretzky, P.; Bisinoti, M. C.; Jardim, W. F.; Rocha, J. C.; Quim. Nova 2005, 28, 438.
- 8. Rocha, J. C.; Sargentini Júnior, E.; Zara, L. F.; Rosa, A. H.; Santos, A.; Burba, P.; Talanta 2000, 53, 551.
- 9. Barbosa, A. C.; Jardim, W. F.; Dorea, J.G.; Forsberg, B. R.; Souza, J.; Arch. Environ. Contam. Toxicol. 2001, 40, 439.
- 10. Barbosa, A. C.; Souza, J.; Dorea, J.G.; Jardim, W. F.; Fadini, P. S.; Arch. Environ. Contam. Toxicol. 2003, 45, 235.
- 11. Fadini, P. S.; PhD Thesis, Universidade Estadual de Campinas, Brazil, 1999.
- 12. Silva, G. S.; PhD Thesis, Universidade Estadual de Campinas, Brazil, 2004.
- 13. Cordeiro, R. C.; Turcq, B.; Ribeiro, M. G.; Lacerda, L. D.; Sifeddine, A.; Turcq, P. F.M.; Sci. Total Environ. 2002, 293, 247.
- 14. Lechler, J. M.; Lacerda, L. D.; Lyons, W. B.; Bonzongo, J.; Sci. Total Environ. 2000, 260, 173.
- 15. Bastos, W. R.; Gomes, J. P. O.; Almeida, R.; Oliveira, R. C.; Nascimento, E. L.; Bernardi, J. V. E.; Lacerda, L. D.; Silveira, E. G.; Pfeiffer, W. C.; Sci. Total Environ. 2006, 368, 344.
- 16. Lacerda, L. D.; Riveiro, M. G.; Souza, M.; Environ. Pollution 2004, 129, 247.
- 17. Ravichandran, M.; Chemosphere 2004, 55, 319.
- 18. Rudd, J. W. M.; Water, Air, Soil Pollut. 1995, 80, 697.
- 19. Bisinoti, M. C.; Jardim, W. F.; Quim. Nova 2004, 27, 593.
- 20. Martian-Doimeadios, R. C. R.; Wasserman, J. C.; Bermejo, L. F. G.; Amouroux, D.; Nevado, J. J. B.; Donard, O. F. X.; J. Environ. Monitor. 2000, 2, 360.
- 21. Subramanian, V.; Madhavan, N.; Saxena, R.; Lundin, L. C.; J. Environ. Monitor. 2003, 5, 427.
- 22. Steffan, R. J.; Korthals, E. T.; Winfrey, M. R.; Appl. Environ. Microbiol. 1988, 54, 2003.
- 23. Villas-Bôas, R. C.; Beinhoff, C.; Da Silva, A. R.; Mercury in the Tapajos Basin, CNPQ/CYTED, CETEM: Rio de Janeiro, 2001.
- 24. Ullrich, S. M.; Tanton, T. W.; Abdrashitova, S. A.; Crit. Rev. Environ. Sci. Technol. 2001, 31, 241.
- 25. Bisinoti, M. C.; Jardim, W. F.; J. Braz. Chem. Soc. 2003, 14, 244.
- 26. Fadini, P. S.; Jardim, W. F.; Analyst 2000, 125, 549.
- 27. Bisinoti, M. C.; Júnior, J. L. B.; Malm, O.; Guimarães, J. R.; Jardim, W. F.; Quim. Nova 2006, 29, 1169.
- 28. Pasquini, C.; Jardim, W. F.; Faria, L. C.; J. Automat. Chem. 1988, 10, 188.
- 29. Akagi, H.; Nishimura, H.; Advances in Mercury Toxicology Plenum Press: city ???, 1991, 53.
- 30. Kehrig, H. A.; Costa, M.; Moreira, I.; Olaf, O.; Mar. Pollut. Bull. 2002, 44, 1018.
- 31. Szakácss, O.; Lasztity, A.; Horváth, Z. S.; Anal. Chim. Acta 1980, 121, 219.
- 32. Clesceri, L. S.; Greenberg, A. E.; Eaton, A D.; Standard Methods for Examination of Water and Wastewater, 20th edition, APHA: Washington, 1998.
- 33. Maurice-Bourgoin, L.; Quiroga, I.; Chincheros, J.; Courau, P.; Sci. Total Environ. 2000, 260, 73.
- 34. Moreira-Turcq, P.; Seyler, P.; Guyot, J. L.; Etcheber, H.; Hydrol. Processes 2003, 17, 1329.
- 35. Kannan, K.; Smith, R. G.; Lee, R. F; Windom, H. L.; Heitmuller, P. T.; Macauley, J. M.; Summers, J. K.; Arch. Environ. Contam. Toxicol. 1998, 34, 109.
- 36. Domagalski, J.; Appl. Geochem. 2001, 16, 1677.
- 37. Gill, G. A.; Bruland, K. W.; Environ. Sci. Technol. 1990, 24, 1392.
- 38. Gilmour, C. C.; Henry, E. A.; Environ. Pollut. 1991, 71, 131.
- 39. Bonotto, D. M.; Da Silveira, E. G.; J. South Am. Earth Sci 2003, 15, 911.
- 40. Bloom, N. S.; Watras, C. J.; Sci. Total Environ. 1989, 87-8, 199.
- 41. Watras, C. J.; Morrison, K. A.; Host, J. S.; Bloom, N. S.; Limnol. Oceanogr. 1995, 40, 556.
- 42. Bloom, N. S.; Effler, S. W.; Water, Air, Soil Pollut. 1990, 53, 251.
- 43. Meuleman, C.; Leermakers, M.; Bayens, W.; Water, Air, Soil Pollut. 1995, 80, 539.
- 44. Parkman, H.; Ostlund, P.; Samulsson, M.; Iverfeldt, A.; Methylmercury in a Permanently Stratified Fjord. In: Watras, C.; Huckabee, J.; editors. Mercury pollution, integration and synthesis. Palo Alto, Lewis Publishers, 1995.
- 45. Nguyen, H. L.; Leermakers, M.; Kurunczi, S.; Bozo, L.; Baeyens, W.; Sci. Total Environ. 2005, 340, 231.
- 46. Bloom, N. S.; Effler, S. W.; Water, Air, Soil Pollut. 1990, 53, 251.
- 47. Lechler, P. J.; Miller, J. R.; Lacerda, L. D.; Vinson, D.; Bonzongo, J.-C.; Lyons, W. B.; Warwick, J. J.; Sci. Total Environ. 2000, 260, 87.
- 48. Nriagu, J. O.; Pfeiffer, W. C.; Malm, O.; Souza, C. M. M.; Mierle, G.; Nature 1992, 356, 389.
- 49. M. Roulet, M. Lucotte, R. Canuel, I. Rheault, S. Tran, Y. G. De Freitos Gog, N. Farella, R. Souza do Vale, C. J. Souza Passos, E. De Jesus da Silva, D. Mergler, M. Amorim, Sci. Total Environ. 1998, 213, 203.
- 50. Peleja, J. R. P.; PhD Thesis, Instituto Nacional de Pesquisa Amazônia, Brazil, 2002.
- 51. Roulet, M.; Lucotte, M.; Guimarães, J. R. D.; Rheault, I.; Sci. Total Environ. 2000, 261, 43.
- 52. Bonotto, D. M.; Da Silveira, E. G.; J. Soc. Am. Earth Sci 2003, 15, 911.
- 53. Bisinoti, M. C.; Fadini, P. S.; Jardim, W. F.;RMZ Materials and Geoenvironment 2004, 51, 821.
- 54. Sellers. P.; Kelly, C. A.; Rudd, J. W. M.; Machutchin, A. R.; Nature 1996, 380, 694.
- 55. Bisinoti, M. C.; Jardim, W. F.; Rmz Materials and Geoenvironment 2004, 51, 825.
- 56. Vandal, G. M.; Mason, R. P.; McKnight, D.; Fitzgerald, W.; Sci. Total Environ. 1998, 213, 229.
- 57. Watras, C. J.; Morrison, K. A.; Host, J. S.; Bloom, N. S.; Limnol. Ocean. 1995, 40, 556.
- 58. Nguyen, H. L.; Leermakers, M.; Kurunczi, S.; Bozo, L.; Baeyens, W.; Sci. Total Environ. 2005, 340, 231.
Publication Dates
-
Publication in this collection
28 June 2007 -
Date of issue
2007
History
-
Accepted
27 Apr 2007 -
Received
09 July 2006