Abstracts
The reaction of N-(p-tolyl)-maleimide with enaminones afforded succinimide-containing enaminones in moderate to good yields, and these compounds were evaluated against E. coli and S. aureus, but no significant antibacterial activity was observed. The regiochemistry of the compounds was examined mechanistically within frontier molecular orbital considerations. In the solid state three-dimensional structure there is the formation of cooperative inter- and intramolecular NH...O and CH...O hydrogen bonding.
enaminones; succinimide; maleimide
Succinimido enaminonas foram sintetizadas através da reação de enaminonas e a N-(p-toluil)-maleimida em rendimento moderado a bom. A ação antibacteriana destes compostos contra E. coli e S. aureus foi avaliada, mas a atividade antibacteriana não foi significativa. A regioquímica das succinimido enaminonas foi racionalizada empregando-se a teoria dos orbitais moleculares de fronteira. A estrutura cristalina de um dos compostos foi determinada e foram observadas várias pontes de hidrogênio inter- e intramolecular do tipo NH...O e CH...O.
Article
The Michael Reaction of Enaminones with N-(p-tolyl)-maleimide: Synthesis and Structural Analysis of Succinimide-enaminones
Silvio Cunha* * e-mail: silviodc@ufba.br ,# * e-mail: silviodc@ufba.br ,a, Waléria Rodovalhoa, Neucírio R. Azevedoa, Marcos de O. Mendonçab, Carlito Lariuccib and Ivo Vencatob
a Instituto de Química, b Instituto de Física, Universidade Federal de Goiás, CP 131, 74001-970 Goiânia - GO, Brazil
Succinimido enaminonas foram sintetizadas através da reação de enaminonas e a N-(p-toluil)-maleimida em rendimento moderado a bom. A ação antibacteriana destes compostos contra E. coli e S. aureus foi avaliada, mas a atividade antibacteriana não foi significativa. A regioquímica das succinimido enaminonas foi racionalizada empregando-se a teoria dos orbitais moleculares de fronteira. A estrutura cristalina de um dos compostos foi determinada e foram observadas várias pontes de hidrogênio inter- e intramolecular do tipo NH...O e CH...O.
The reaction of N-(p-tolyl)-maleimide with enaminones afforded succinimide-containing enaminones in moderate to good yields, and these compounds were evaluated against E. coli and S. aureus, but no significant antibacterial activity was observed. The regiochemistry of the compounds was examined mechanistically within frontier molecular orbital considerations. In the solid state three-dimensional structure there is the formation of cooperative inter- and intramolecular NH...O and CH...O hydrogen bonding.
Keywords: enaminones, succinimide, maleimide
Introduction
Over the years, the Michael addition of enaminones has been intensively studied and many synthetic achievements were described.1 For instance, the stereocontrolled elaboration of quaternary carbon centers followed by the aza-annulation of enaminones has been used in the synthesis of many nitrogen-containing compounds, where the Michael addition was the key step.2 Among the Michael acceptors studied (e.g. nitroolefins,3 maleic anhydrides4) the maleimides have the most complex reaction pattern, highly dependent of the nitrogen substituents at the maleimides and at the enaminones, Scheme 1.5
Additionally to the complex behavior, this kind of reaction caught our attention due its potential in the synthesis of succinimide-containing enaminones derivatives such as 4.5 The antibacterial activity of five-membered imides is well documented and some structural modifications to improve this one, such as the presence of N-aryl groups have been suggested.6 As part of our recent research program aimed at the synthesis of biological active substances7 such as anticonvulsant and antibacterial compounds and to provide insight into the reactivity of enaminones toward N-aryl-maleimides, we decided to study the Michael reaction of enaminones with N-(p-tolyl)-maleimide, in search for a general method to the synthesis of N-aryl-succinimide-containing enaminones. Although Michael addition of enaminones with N-H and N-alkyl-maleimides had been described three decades ago, studies with N-aryl maleimides are scarce.5 Afterward, to the best of our knowledge, there are no structural and biological studies with succinimide-enaminones derivatives.
Results and Discussion
N-(p-tolyl)-maleimide reacted smoothly with enaminones 6a-c in benzene under reflux. Crystalline solids were isolated and these materials were 1:1 adduct as indicated by mass spectrum and proton NMR integration. The formation of a succinimide-enaminone was proposed by comparison of spectral data with model compounds 3 and 4.5 Although these reported compounds present similar IR and 1H NMR spectra, their 13C NMR show significant differences. Thus, the chemical shifts of the α and β carbons relative to the carbonyl group of the enaminone moiety are 106.4 ppm and 154.8 ppm for 3, while for 4 they appear at 90.0 ppm and 161.3 ppm, respectively.5 In the adducts here obtained, the 13C chemical shifts of Cα and Cβ are in agreement with structure 8 analogous to 4 (see Experimental), Scheme 2. The 1H NMR spectra contained a low field N-H (8.64-9.94 ppm) which suggests its participation in intramolecular hydrogen bonding. Despite the reportedly obtained E configuration to the model compound 4,5 we assigned the Z configuration to 8 because E- and Z-configurational isomers of enaminones are well distinguished by typical N-H chemical shifts (E-isomer: 4.1-6.5 ppm; Z-isomer: 9.5-12.0 ppm).8 Moreover, the structure of the addition products 8a was unambiguously confirmed by X-ray analysis and the Z configuration corroborated, as shown in Figure 1. This reaction proved to be sensitive to N-acyl substitution in 6. While moderated to good yields were obtained with enaminones 6a-c, N-(p-tolyl)-maleimide failed to react with enaminone 6d under the condition employed, even at prologated heating, Scheme 2.
The formation of 8a-c may be visualized as occurring by reaction of the enaminone-Cα at the electrophilic C-3 of the N-(p-tolyl)-maleimide, followed by proton transfer. However, at this point it is not clear the controlling factors that modulate maleimide and enaminone reactivity to form succinimide-enaminones or other nucleus present in Schemes 1 and 2. With the objective of shedding new light on this question we undertook a frontier orbital treatment in combination with the hardness-softness concept. Electronic parameters of model maleimides and enaminones were calculated using the AM1 method,9 as implemented in the MOPAC 6.0 package,10 and are shown in Table 1. According to Parr-Pearson principle of absolute hardness,11N-(p-tolyl)-maleimide is softer than N-H and N-alkyl maleimides because of the smaller HOMO-LUMO energy gap. Thus, it would appear that reaction of soft enaminones and N-aryl maleimides is kinetically favored, and frontier orbital control, and not charge control, should govern the process. In this way, an attack of enaminone-Ca at the N-(p-tolyl)-maleimide-C3, wherein the largest frontier orbital coefficients are located (see Table 1), results in the regiochemistry observed in Scheme 2.
To unambiguously assign the structure of the obtained succinimide-enaminones and to gain insight into intramolecular and intermolecular interactions the crystal structure of 8a was determined, and several structural features emerged. The asymmetric unit has two independent molecules. As noted in Figure 1, which shows the unprimed molecule with labeled atoms, strong hydrogen bonding occurs between the carbonyl oxygen and the NH group (intramolecular hydrogen bond: N16-H16A...O15 [2.699(3) Å] and N16'-H16C...O15' [2.661(3) Å]) providing a pseudo six-membered ring. In addition, a weak CH...O intramolecular H-bond was also observed: C4-H4A...O12[2.953(3)Å] and C4'-H4'2...O12'[2.965(3)Å].
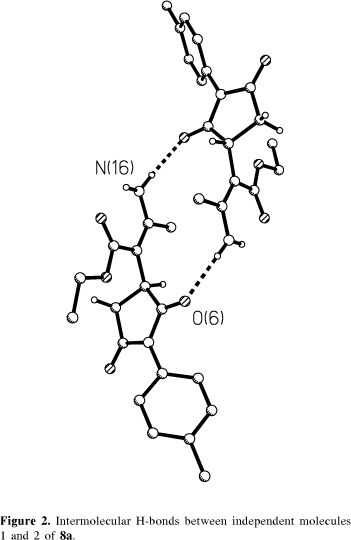
Figure 2 shows the observed intermolecular NH...O hydrogen bonds between molecules 1 and 2 of 8a: N16-H16B...O6' [x, 1+y, z, 2.968(3) Å], N16'-H16D...O6 [x, y-1, z, 2.988(3) Å]. Molecule 1 of 8a is involved in a CH...O H-bond with another one due to an inversion symmetry operation: C3-H3...O7[1-x, 1-y, -z, 3.199(3)Å], as indicated in Figure 3, leading to a structural feature like a dimer through this intermolecular interaction. This kind of interaction and its influence in the conformation was recently described.12
The molecules present small differences in the torsion angles O15-C11-C8-C3 of -173.1(2) and 170.6(2), O15-C11-C8-C9 of 4.0(4) and -9.3(4), O15-C11-O12-C13 of 4.0(3) and -6.1(3)0 for the unprimed and primed molecules, respectively. The five-membered imide rings are twisted on C3-C4 bond, with some distortions as can be seen from the Cremer and Pople13 parameters: N1→C2→...C5 [Q = 0.162(3) Å, ø = 94.1(8)o; Q = 0.151(2) Å, ø = 266.4(8)o ] for the unprimed and primed molecules, respectively. The O7 atom is 0.13(4) Å from the least-square plane through the atoms C2 N1 C5 C4 and the O7' atom is only 0.05(4) Å from the corresponding primed plane. Therefore, the intermolecular hydrogen bond where O7 is involved is also related to the twisting on C3-C4 bond. In the unprimed and primed molecules of 8a, no pyramidalization of the imide nitrogen N(1) was observed, with the sum of the angles at N(1) of 359.7 and 360.00, respectively.
The antibacterial activity of succinimide-enaminones 8a-c was evaluated against Escherichia coli ATCC 8739 (gram-negative) and Staphilococus aureus ATCC 6538 (gram-positive), and the minimal inhibitory concentration (MIC) was determined. However, no activity was observed to compounds 8a-c even at 1000 mg mL-1. Efforts are underway to elucidate the structural-activity relationship that can improve the antibacterial activity of succinimide-enaminones derivatives.
Experimental
Melting points were determined on a Karl Kolb apparatus and are uncorrected. Infrared spectra were recorded as KBr discs on a FT-IR BOMEM MB100 instrument. NMR spectra were obtained for 1H at 300 MHz and for 13C at 75 MHz using a Varian Gemini 300 or a Bruker AC300P spectrometers at Instituto de Química/UNICAMP. Chemical shifts are reported in ppm units downfield from reference (internal TMS). MS spectra were measured on a SHIMADSU CG-MS QP-5050 spectrometer at 70 eV. Elemental analyses were performed on a 2400 CHN Perkin Elmer instrument at Instituto de Química/UNICAMP. The single crystal X-ray data collection was done with a Nonius CAD-4 diffractometer at Instituto de Física-SC/USP. Enaminones 6a, 6b, 6c, 6d and N-(p-tolyl)-maleimide were prepared according to known procedures.14
Ethyl (Z)-3-amino-2-[1-(4-methylphenyl)-2,5-dioxo-2,5-dihydro-1H-3-azolyl]-2-butenoate (8a). A solution of 393.0 mg (3.0 mmol) of enaminone 6a and 597.0 mg (3.0 mmol) of N-(p-tolyl)-maleimide 7 in 10 cm3 of benzene was heated at reflux for 1 day (the solution turned yellow and a precipitate began to form after 30 min.), after which time the reaction mixture was cooled at room temperature, and the solvent was evaporated. The residue was recrystallized from CH2Cl2 /petroleum ether to give 433.8 mg (43%) of 8a as colorless solid, mp 178-179 ºC. IR (KBr) νmax/cm-1 3407, 1772, 1702, 1666, 1620, 1378, 1172. 1H NMR (CDCl3): 1.18 (3H, t, J 7.1 Hz); 2.09 (3H, s); 2.38 (3H, s); 2.77 (1H, dd, J 18.3 and J 5.5 Hz); 3.04 (1H, dd, J 18.3 and J 9.5 Hz); 3.76 (1H, dd, J 9.5 and J 5.5 Hz); 4.14 (2H, m); 4.88 (1H, sl); 7.19 (2H, d, J 8.1 Hz); 7.28 (2H, d, J 8.1 Hz); 8.64 (1H, sl). 13C NMR (CDCl3): 14.3 (CH3); 21.1 (CH3); 21.4 (CH3); 36.1 (CH2); 39.7 (CH); 59.5 (CH2); 90.6 (C); 126.1 (CH); 129.8 (CH); 138.4 (C); 159.6 (C); 168.4 (C); 176.3 (C); 179.1 (C). MS, m/z (%): 318 (1.5) [M+ +2], 317 (11) [M+ +1], 316 (52) [M+], 209 (23), 181 (100), 126 (26), 107 (85), 81 (41). Anal. Calcd. for C17H20N2O4 : C, 64.56%; H, 6.33%; N, 8.86%. Found: C, 64.32%; H, 6.21%; N, 8.73%.
Ethyl 2-(Z)-1-methyl-2-[1-(4-methylphenyl)-2,5-dioxo-2,5-dihydro-1H-3-azolyl]-3-oxo-1-butenylaminoacetate (8b). A solution of 206.3 mg (1.1 mmol) of enaminone 6b and 211.2 mg (1.1 mmol) of N-(p-tolyl)-maleimide 7 in 10 cm3 of benzene was heated at reflux for 12 h, after which time the reaction mixture was cooled at room temperature, and the solvent was evaporated. The residue was recrystallized from CH2Cl2/petroleum ether and the solid that formed was triturated with ethyl ether to give 214.3 mg (51%) of 8b as yellow solid, mp 127-130 ºC. IR (KBr) νmax/cm-1 3345, 1735, 1658, 1602, 1386, 1226. 1H NMR (Acetone-D6): 1.28 (3H, t, J 7.0 Hz); 2.26 (3H, s); 2.31 (3H, s); 2.40 (3H, s) 3.01 (2H, m); 3.71 (1H, m); 4.21 (2H, q, J 7.0 Hz); 4.32 (1H, d, J 17.9 Hz); 4.53 (1H, d, J 17.9 Hz); 7.05 (2H, d, J 8.1 Hz); 7.42 (2H, d, J 8.1 Hz); 9.01 (1H, sl). 13C NMR (Acetone-D6): 12.0 (CH3); 13.0 (CH3); 19.8 (CH3); 36.1 (CH2); 40.4 (CH); 42.6 (CH2); 60.4 (CH2); 95.3 (C); 116.2 (C); 118.8 (CH); 128.3 (CH); 131.7 (C); 135.7 (C); 151.8 (C); 167.2 (C); 177.0 (C); 191.6 (C). MS, m/z (%): 374 (1.4) [M+ +2], 373 (7.3) [M+ +1], 372 (30) [M+], 265 (19), 237 (79), 192 (41), 163 (26), 150 (19), 136 (23), 122 (22), 107 (100). Anal. Calcd. for C20H24N2O5 C, 64.52%; H, 6.45%; N, 7.53%. Found: C, 64.81%; H, 6.66%; N, 7.28%.
Ethyl (Z)-3-benzylamino-2-[1-(4-methylphenyl)-2,5-dioxo-2,5-dihydro-1H-3-azolyl]-2-butenoate (8c). A solution of 209.1 mg (1.0 mmol) of enaminone 6c and 182.8 mg (1.0 mmol) of N-(p-tolyl)-maleimide 7 in 10 cm3 of benzene was heated at reflux for 12 h, after which time the reaction mixture was cooled at room temperature, and the solvent was evaporated. The residue was triturated with ethyl ether to give 320.7 mg (79%) of 8c as colorless solid, mp 108-110 ºC. IR (KBr) νmax/cm-1 3463, 1776, 1699, 1654, 1383, 1183. 1H NMR (Acetone-D6): 1.15 (3H, t, J 7.0 Hz); 2.16 (3H, s); 2.37 (3H, s); 2.68 (1H, dd, J 17.7 and J 5.7 Hz); 3.09 (1H, dd, J 17.7 and J 9.7 Hz); 4.05 (2H, m); 4.15 (1H, m); 4.57 (2H, s); 7.19 (2H, d, J 7.9 Hz); 7.26 (2H, d, J 7.9 Hz); 7.35 (5H, m); 9.94 (1H, sl). 13C NMR (Acetone-D6): 13.2 (CH3); 14.0 (CH3); 19.6 (CH3); 35.5 (CH2); 39.0 (CH); 46.1 (CH2); 57.8 (CH2); 88.9 (C); 125.3 (CH); 126.0 (CH); 126.3 (C); 127.8 (CH); 128.3 (CH); 129.6 (C); 136.6 (C); 137.9 (C); 160.9 (C); 167.7 (C); 174.8 (C); 178.0 (C). MS, m/z (%): 286 (100), 257 (21), 239 (19), 119 (59). Anal. Calcd. for C24H26N2O4 : C, 59.11%; H, 6.40%; N, 6.90%. Found: C, 59.01%; H, 6.47%; N, 6.88%.
Crystal structure of 8a. C17H20N2O4 , Mw = 316.35, monoclinic, space group P21/c, Z = 8, a = 14.708(3), b = 9.561(2), c = 23.155(5) Å, β = 98.02(3)0, V = 3224.3(12) Å3, dc = 1.303 Mg m-3, l (Mo Kα) = 0.71073 Å, m = 0.094 mm-1, 70552 measured reflections, 5687 unique (Rint = 0.105) of which 4270 were considered as observed with I > 2σ(I). The single crystals were obtained by diffusion of petroleum ether into a solution of synthesized succinimide-enaminone 8a in CH2Cl2 at room temperature. The structure was solved with direct methods using SHELXS9715 and it was refined anisotropically with full-matrix least-squares on F2 using SHELXL97.16 The hydrogen atoms were placed at calculated position except those involved in H-bonds, found on difference maps and refined. Final indices: R1(Fo) = 0.054, wR2 (F2) = 0.146 for 434 refined parameters.
Crystallographic data (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 173681. Copies of the data can be obtained, free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK ; fax: +44 1223 336033 ; or e-mail: deposit@ccdc.cam.ac.uk).
Antibacterial assay. The bacterias used in this experiment were Escherichia coli ATCC 8739 (gram-negative) and Staphilococus aureus ATCC 6538 (gram-positive). Antibacterial activity was measured using a dilution in agar technique.17 The compounds (20 mg) were solubilized in 1 mL of dimethyl sulfoxide (DMSO) and serially two-fold diluted in nutrient agar (peptone, beef extract and agar), to obtain a concentration range of 62.51000 μg mL-1. The control was plates of nutrient agar containing only DMSO diluted in the same way, which did not influence the bacterial growth. The bacterias from broth medium were suspended in sterile physiological Tris buffer (pH 7.4, 0.05 mol mL-1), homogenised and adjusted to an OD (530 nm) of 0.05 (equivalent to 1 X 106 UFC mL-1). This suspension (3 μL) was inoculated using an automatic micropippete for the test in the agar plates (diameter: 10 cm). The plates were incubated at 37 oC for 24 h. The minimal inhibitory concentration (MIC) was defined as the minimal concentration of the compounds which completely inhibited the visible growth of the bacterias. The positive control was chloranphenicol used in the same technique. All assays were tested in duplicate.
Acknowledgments
The authors thank the Brazilian Agencies for fellowships to WR (PIBIC/CNPq ) and IV (CNPq), and financial support from FUNAPE-UFG. The authors also thank Instituto de Química-UNICAMP for NMR and elemental analyses measurements, and Instituto de Física-SC/USP for the X-ray single crystal data collection. Silvio Cunha would like to acknowledge his debt of gratitude to Professor Dr. Albert James Kascheres, now retired from Instituto de Química-UNICAMP, for help, advice and guidance in his career.
Received: January 6, 2002
Published on the web: August 28, 2002
# Present address: Instituto de Química, Universidade Federal da Bahia, Campus de Ondina, 40170-290 Salvador-BA, Brazil
- 1. Lue, P.; Greenhill, J. V. In Advances in Heterocyclic Chemistry; Katritzky, A. R., ed.; Academic Press: New York, 1997; Vol 67, p 207.
- 2. Benovsky, P.; Stepheson, G. A.; Stille, J. R.; J. Am. Chem. Soc 1998, 120, 2493.
- 3. Cimarelli, C.; Palmieri, G.; Tetrahedron 1998, 54, 915.
- 4. Desmaële, D.; Mekovar, K.; d'Angelo, J.; J. Org. Chem. 1997, 62, 3890.
- 5. Murphy, J. P.; Hadden, M.; Stevenson, P. J.; Tetrahedron 1997, 53, 11827.
- 6. Cook, G. R.; Beholz, L. G.; Stille, J. R.; J. Org. Chem. 1994, 59, 3575.
- 7. Paulvannan, K.; Stille, J. R.; J. Org. Chem. 1994, 59, 1613.
- 8. Barta, N. S.; Brode, A.; Stille, J. R.; J. Am. Chem. Soc. 1994, 116, 6201.
- 9. Revial, G.; Lim, S .; Viossat, B.; Lemoine, P.; Tomas, A.; Duprat, A. F.; Pfau, M.; J.Org. Chem. 2000, 65, 4593.
- 10. d'Angelo, J.; Cavé, C.; Desmaële, D.; Gassama, A.; Thominiaux, C.; Riche, C.; Heterocycles 1998, 47, 725.
- 11. Hadden, M.; Nieuwenhuyzen, M.; Potts, D.; Stevenson, P. J.; J. Chem. Soc., Perkin Trans. 1 1998, 3437.
- 12. Furukawa, I.; Ade, T.; Fujisawa, H.; Ohta, T.; Tetrahedron 1997, 53, 17643.
- 5. Caballero, E.; Madrigal, B.; Medarde, M.; Puebla, P.; Honores, Z.; Martín, E.; Feliciano, A. S.; ACH-Models Chem. 1998, 135, 457.
- 6. Gupa, A. K.; Ila, H.; Junjappa, H.; Synthesis 1988, 284.
- 7. Shah, K. R.; Blanton Jr., C. D.; J. Org. Chem 1982, 47, 502.
- 8. von Dobeneck, H.; Bruner, E.; Bunker, H.; Metzner, G.; Schmidt, R.; Weil, E.; Sonnenbichler, J.; Liebigs Ann. Chem. 1981, 410.
- 9. Blanton Jr., C. D.; Whidby, J. F.; Briggs, F. H.; J. Org. Chem. 1971, 36, 3929.
- 10. Robson, J. H.; Marcus, E.; Chem. Ind. 1971, 1022.
- 11. Andricopulo, A. D.; Yunes, R. A.; Nunes, R. J.; Savi, A. O. S.; Corrêa, R.; Cruz, A. B.; Cechinel Filho, V.; Quim. Nova 1998, 21, 573.
- 12. Cunha, S.; Costa, M. B.; Napolitano, H. B.; Lariucci, C.; Vencato, I.; Tetrahedron 2001, 57, 1671.
- 13. Zhuo, J.-C.; Magn. Reson. Chem. 1998, 36, 565.
- 14. Dewar, M. J. S.; Zoebisch, E. G.; Healy, E. F.; Stewart, J. J. P.; J. Am. Chem. Soc. 1985, 107, 3902.
- 15. Quantum Chemistry Program Exchange (QCPE), Bloomington, IN, No. 455, 1990, 506.
- 16. Parr, R. G.; Zhou, Z.; Acc. Chem. Res. 1993, 26, 256.
- 17. Parr, R. G.; Pearson, R. G.; J. Am. Chem. Soc. 1983, 105, 7512.
- 18. Kumaraswamy, S.; Kumar, K. S.; Raja, S.; Swamy, K.; Tetrahedron 2001, 57, 8181.
- 19. Cremer, D.; Pople, J. A.; J. Am. Chem. Soc 1975, 97, 1354.
- 20. Braibante, M. E. F.; Braibante, H. S.; Missio, L.; Andricopulo, A.; Synthesis 1994, 898.
- 21. Braibante, M. E. F.; Braibante, H. S.; Salvatore, S. J. S. A.; Quim. Nova 1990, 13, 67.
- 22. Rechsteiner, B.; Texier-Boullet, F.; Hamelin, J.; Tetrahedron Lett. 1993, 34, 5071.
- 23. Ovenden, S. P. B.; Capon, R. J.; Lacey, E.; Gill, J. H.; Friedel, T.; Wadsworth, D.; J. Org. Chem. 1999, 64, 1140.
- 24. Ramos, J. G.; Barralesrienda, J. M.; Chaves, M. S.; An. Quim. 1977, 139.
- 25. Sheldrick, G. M.; SHELXS97; Program for the Solution of Crystal Structures; University of Göttingen, Germany, 1990.
- 26. Sheldrick, G. M; SHELXL97; Program for the Refinement of Crystal Structures; University of Göttingen, Germany, 1997.
- 27. Alves, S. H.; Cury, A.; Rev. Pat. Trop. 1992 34, 259.
Publication Dates
-
Publication in this collection
08 Oct 2008 -
Date of issue
Sept 2002
History
-
Received
06 Jan 2002