ABSTRACT
Introduction:
Minimally invasive surgery has revolutionized surgical management in the treatment of colorectal neoplasms, reducing morbidity and mortality, hospitalization, inactivity time and minimizing cost, as well as providing adequate oncological results when compared to the conventional approach. Robotic surgery, with Da Vinci Platform, emerges as a step ahead for its potentials. The objective of this article is to report the single institutional experience with the use of Da Vinci Platform in robotic colorectal surgeries performed at a reference center in oncological surgery in Brazil.
Materials and methods:
A retrospective cohort study was conducted based on the prospective database of patients from the institution submitted to robotic surgery for treatment of colorectal cancer from July 2012 to September 2017. Clinical and surgical variables were analyzed as predictors of morbidity and mortality.
Results:
A total of 117 patients underwent robotic surgery. The complications related to surgery occurred in 33 patients (28%), the most frequent being anastomotic fistula and surgical wound infection, which corresponded to 11% and 3%, respectively. Conversion rate was 1.7%. Median length of stay was 5 days. The only variable associated with increase of complications and death risk was BMI >30, with p-value of 0.038 and 0.027, respectively.
Conclusion:
Robotic surgery is safe and feasible for approaching colorectal cancer surgeries, presenting satisfactory results regarding length of hospital stay and rate of operative complications, as well as presenting a low rate of conversion. Obesity has been shown to be a risk factor for surgical complication in robotic colorectal surgery.
Keywords:
Colorectal robotic surgery; Morbidity and mortality in robotic surgery; Colon neoplasms; Rectal neoplasms
RESUMO
Introdução:
A cirurgia minimamente invasiva revolucionou o tratamento cirúrgico no manejo das neoplasias colorretais, reduzindo a morbidade e mortalidade, a hospitalização, o tempo de inatividade e minimizando os custos, além de fornecer resultados oncológicos adequados quando comparada à abordagem convencional. A cirurgia robótica, com a Plataforma Da Vinci, surge como um passo à frente por seus potenciais. O objetivo deste artigo é relatar a experiência institucional única com o uso da Plataforma Da Vinci em cirurgias robóticas colorretais realizadas em um centro de referência em cirurgia oncológica no Brasil.
Materiais e métodos:
Foi realizado um estudo de coorte retrospectivo, baseado na base de dados prospectiva de pacientes da instituição que foram submetidos à cirurgia robótica para tratamento de câncer colorretal, de julho de 2012 a setembro de 2017. As variáveis clínicas e cirúrgicas foram analisadas como preditores de morbidade e mortalidade.
Resultados:
Um total de 117 pacientes foram submetidos à cirurgia robótica. As complicações relacionadas à cirurgia ocorreram em 33 pacientes (28%), sendo as mais frequentes fístula anastomótica e infecção da ferida cirúrgica, correspondendo a 11% e 3%, respectivamente. A taxa de conversão foi de 1,7%. O tempo mediano de permanência foi de 5 dias. A única variável associada ao aumento de complicações e risco de óbito foi o IMC >30, com p-valor de 0,038 e 0,027, respectivamente.
Conclusão:
A cirurgia robótica é segura e viável para a abordagem de cirurgias de câncer colorretal, apresentando resultados satisfatórios quanto ao tempo de internação hospitalar e taxa de complicações operatórias, além de apresentar baixo índice de conversão. A obesidade tem se mostrado um fator de risco para complicações cirúrgicas na cirurgia colorretal robótica.
Palavras-chave:
Cirurgia robótica colorretal; Morbidade e mortalidade em cirurgia robótica; Neoplasias do cólon; Neoplasias Retais
Introduction
Minimally invasive surgery has revolutionized surgical management in the treatment of colorectal neoplasms, reducing morbidity and mortality, hospitalization, inactivity time and minimizing costs, as well as providing adequate oncological results when compared to the conventional approach.11 Roy S, Evans C. Overview of robotic colorectal surgery: current and future practical developments. World J Gastrointest Surg. 2016;8:143-50. Robotic surgery, with Da Vinci Platform, emerges as a step ahead for its potentials. When compared to conventional laparoscopy, robotic surgery contributes with ergonomics, camera stability and articulated instruments, facilitating surgical dissection under spatially restricted anatomical conditions.22 Pędziwiatr M, Pisarska M, Kisielewski M, Major P, Mydlowska A, Rubinkiewicz M, et al. Eras protocol in laparoscopic surgery for colonic versus rectal carcinoma: are there differences in short-term outcomes? Med Oncol. 2016;33:56.–44 de Jesus JP, Valadão M, de Castro Araujo RO, Cesar D, Linhares E, Iglesias AC. The circumferential resection margins status: a comparison of robotic, laparoscopic and open total mesorectal excision for mid and low rectal cancer. Eur J Surg Oncol. 2016;42:808-12. The robotic approach in colorectal cancer offers the same oncologic results of conventional surgery associated with faster recovery due to the minimally invasive procedure without all the technical limitations of the laparoscopic surgery.55 Hazebroek EJ. Color Study Group Color: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Surg Endosc. 2002;16:949-53.
The main concern about robotic surgery is how much this technology costs. Recently a randomized, multi-centric study (ROLARR) has compared the robotic to laparoscopic approach on rectal cancer, reporting similar rates of conversion and also the larger costs of robotic surgery. The subgroup of male obese patients was benefited when compared to laparoscopy. Furthermore, robotic surgery for rectal cancer is still in full development worldwide.66 Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA. 2017;318:1569-80. In our hospital, it has been in use since 2013 under constant control, as to allow future evaluations of cost/effectiveness.
This article's objective is to report the single-center experience with the use of Da Vinci platform in colorectal robotic surgeries performed in a national reference surgical oncology center in Brazil.
Methods
This is a Retrospective Cohort study based on the prospective database of the Brazilian National Cancer Institute José de Alencar (INCA/MS) of patients who underwent robotic surgery as treatment for colorectal cancer between July 2012 and September 2017. All surgeries were performed at Hospital of Cancer 1/INCA by three staff surgeons from INCA's abdominal-pelvic team (JP, MV, EL), trained in laparoscopic and robotic surgery.
The clinical and surgical variables selected for distribution and frequency analysis were: age, gender, Body Mass Index (BMI), comorbidities, Length of stay, histopathologic staging, tumor location, distance from anal verge, neoadjuvant and adjuvant therapy, type of surgery, conversion to open surgery, distal and proximal surgical margins, and tumor recurrence.
Surgical complications were defined as all adverse events related to the surgical act according to the Clavien–Dindo Classification77 Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-13. taken place up to 60 days postoperative, and all deaths from surgical act complications in the same period. The Da Vinci Si platform is available on INCA and was used for all patients.
For the quantitative variables, mean and/or median were calculated and to verify the association between variables, Chi-square and Fisher's exact test were used. Significant p-value was considered <0.05. Software SPSS V. 17 was used for statistical analysis. Associations of surgical complications were verified with the following variables: age, comorbidities, gender, BMI, surgeon, neoadjuvant therapy and tumor localization.
The study was submitted to and approved by INCA's Ethics and Research Committee. Informed consent was obtained from the patients before the surgical procedure.
Results
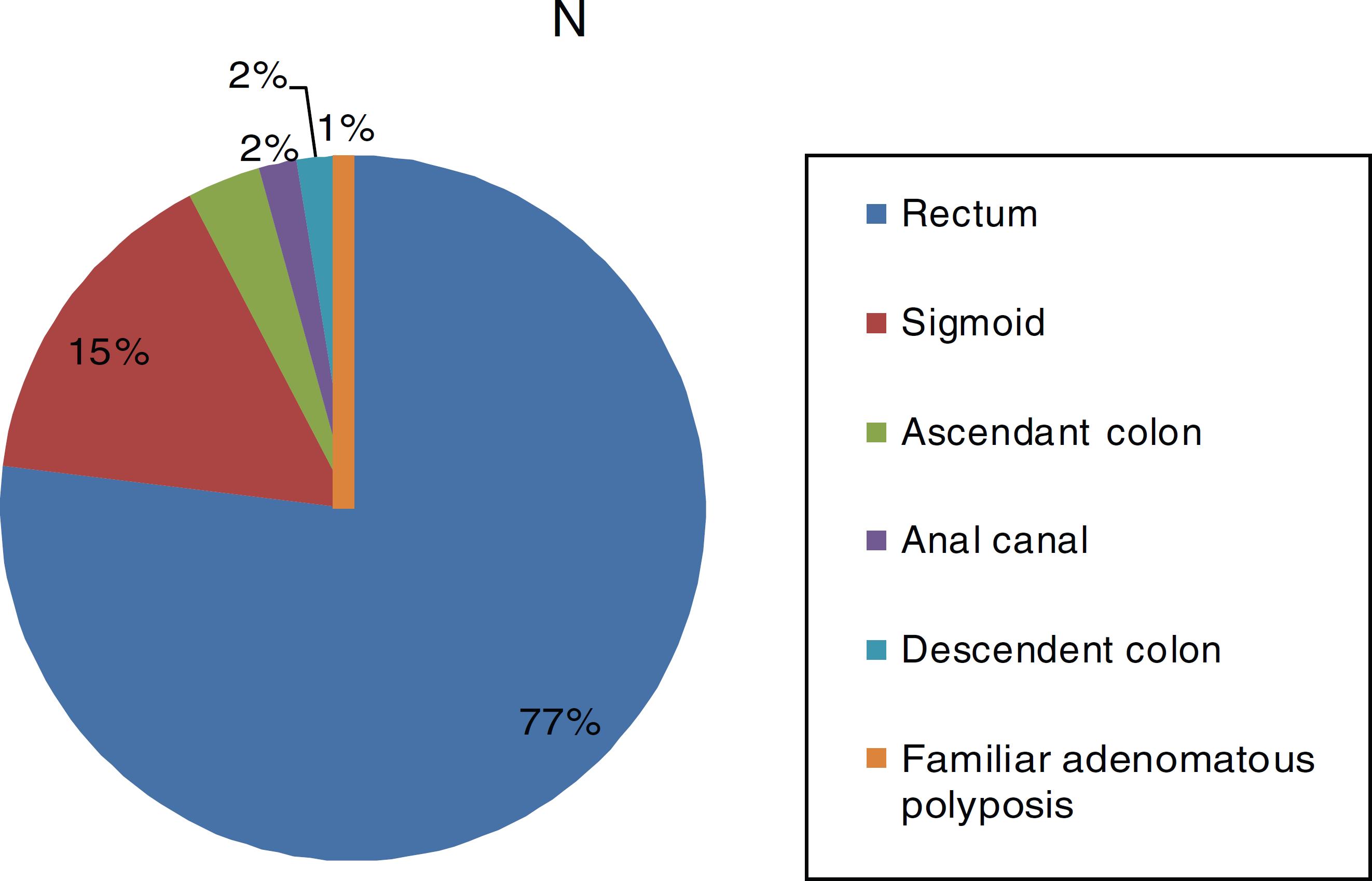
117 patients underwent robotic surgery, clinical and surgical variables frequency distributions are shown in Table 1. The most common histological type was adenocarcinoma, found in 113 patients, followed by epidermoid carcinoma in three patients, and 1 patient with Gastrointestinal Stromal Tumor (GIST). The most frequent location was the rectum, with 90 patients, and the most performed surgery was Low Anterior Resection (LAR) with primary anastomosis (Figs. 1 and 2). Regarding pathological staging (7th edition of the AJCC), the most frequent staging was IIA, in 31% of patients (Table 1).
Complications related to surgery occurred in 33 patients (28%), the most frequent being anastomotic fistula and surgical wound infection, corresponding to 11% and 3%, respectively (Table 2). Regarding the severity of complications, according to the Clavien–Dindo classification, Classes II and IIIb were the most frequent, with 36% and 34% occurrence respectively (Table 3). Regarding the association between clinical and surgical variables and the occurrence of complications and death, the only variable that showed statistical significance was the BMI ≥30, with higher risk of complications and death, with a p-value of 0.038 and 0.027, respectively. The other mentioned variables did not show a statistically significant p-value (Table 4).
Surgical conversions were necessary in two patients (1.7%), re-interventions were needed in 16 patients (13.6%) and 3 patients came to death (2.5%) related to post-operative complications. The subgroup analysis of rectal cancer showed conversion rate of 2.1%, anastomotic fistula of 11% and death rate of 2.1% (two patients) (Table 5).
Discussion
Minimally invasive surgery is a fundamental tool in colorectal cancer treatment due to its associated advantages, such as less surgical trauma, extended visualization and technological association for better identification of vascular and nervous structures.44 de Jesus JP, Valadão M, de Castro Araujo RO, Cesar D, Linhares E, Iglesias AC. The circumferential resection margins status: a comparison of robotic, laparoscopic and open total mesorectal excision for mid and low rectal cancer. Eur J Surg Oncol. 2016;42:808-12.,88 Mak TW, Lee JF, Futaba K, Hon SS, Ngo DK, Ng SS. Robotic surgery for rectal cancer: a systematic review of current practice. World J Gastrointest Oncol. 2014;6:184-93.,99 Rodríguez-Sanjuán JC, Gómez-Ruiz M, Trugeda-Carrera S, Manuel-Palazuelos C, López-Useros A, Gómez-Fleitas M. Laparoscopic and robot-assisted laparoscopic digestive surgery: present and future directions. World J Gastroenterol. 2016;22:1975-2004. With the advent of robotic surgery, some new technologies were added to the minimally invasive surgery arsenal, such as tridimensional imaging, articulated and precise tweezers, better ergonomics and comfort for the surgeon.1010 Bandar MHA. The current scope of robotic surgery in colorectal cancer. Adv Robot Autom. 2015.,1111 Park S, Kim NK. The role of robotic surgery for rectal cancer: overcoming technical challenges in laparoscopic surgery by advanced techniques. J Korean Med Sci. 2015;30:837-46. Being so, robotic surgery has come with the expectation to present satisfactory results compared to the already common surgical modalities such as laparoscopic and conventional surgery, with its high cost being justified through the generated benefits.
Comparing the results between the possible surgical modalities for colorectal cancer treatment, Uhrich et al. reported uncomfortable positions during laparoscopic surgery increased surgeon fatigue and brought a higher chance of iatrogenic injury, which was minimized in the robotic approach.1212 Uhrich ML, Underwood RA, Standeven JW, Soper NJ, Engsberg JR. Assessment of fatigue, monitor placement, and surgical experience during simulated laparoscopic surgery. Surg Endosc. 2002;16:635-9. Liao et al. conducted a meta analysis with 1074 patients, comparing the robotic approach versus conventional surgery for rectal cancer treatment and showed that robotic surgery is safe, presents with lower blood loss and transfusion, shorter time of hospital stay, return for normal intestinal activity, and eating. However, there was no statistical difference in evaluating the oncological resection and there was a longer surgery time for patients under robotic surgery. Xiong et al. have published a meta-analysis with 1229 cases (554 robotic surgeries and 675 laparoscopies), concluding that robotic surgery lowers the rates of hospitalization and surgical conversion. On the other hand, there was no difference on circumferential margin and sexual dysfunction when compared to laparoscopy.1313 Xiong B, Ma L, Huang W, Zhao Q, Cheng Y, Liu J. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Gastrointest Surg. 2015;19:516-26. Fernandez et al. have shown that there was no difference between robotic and laparoscopic surgery on post-operative morbidity or on quality of mesorectal excision, however, it was described that robotic surgery showed lower rates of conversion compared to laparoscopy (8% vs. 17%).1414 Fernandez R, Anaya DA, Li LT, Orcutt ST, Balentine CJ, Awad SA, et al. Laparoscopic versus robotic rectal resection for rectal cancer in a veteran population. Am J Surg. 2013;206:509-17. The studies made by Patriti et al., Lin et al., Trastulli et al. and Ortiz-Oshiro et al. corroborate on these data.1515 Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, et al. Robotic versus laparoscopic minimally invasive surgery for rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2018;267:1034-46.–1818 Ortiz-Oshiro E, Sánchez-Egido I, Moreno-Sierra J, Pérez CF, Díaz JS, Fernández-Represa JÁ. Robotic assistance may reduce conversion to open in rectal carcinoma laparoscopic surgery: systematic review and meta-analysis. Int J Med Robot. 2012;8:360-70.
Conversely, the Da Vinci system has some technical disadvantages compared to the other surgical modalities. First, there is lack of tactile sensation and tension for the surgeon, which can lead to tissue damage during the robotic arm traction and instrument movement. Besides, the technology costs are higher than conventional laparoscopy, suture material can be cut because there is no tension return during surgery and the docking process is time consuming and requires trained assistance.1919 Liao G, Li Y-B, Zhao Z, Li X, Deng H, Li G. Robotic-assisted surgery versus open surgery in the treatment of rectal cancer: the current evidence. Sci Rep. 2016;6:26981.,2020 Park EJ, Baik SH. Robotic surgery for colon and rectal cancer. Curr Oncol Rep. 2016;18:5. Recently, the ROLARR, a multi-centric and randomized study, questioned and opposed other studies about the potential benefit of robotic surgery on rectal cancer treatment, showing that this technology increases costs significantly while showing no potential benefits on conversion rates, circumferential margins or complication rates.66 Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA. 2017;318:1569-80. One criticism of the ROLARR study is that the robotic surgeon group had a 25 procedures experience in average, versus almost 100 for the laparoscopic surgeon group, which probably contributed to the findings in the study. We believe that these findings of clear non-superiority of robotic surgery may be overcome by surgeon and team experience, and that there are many benefits to the robotic approach on treating colorectal cancers, as mentioned in previous studies.22 Pędziwiatr M, Pisarska M, Kisielewski M, Major P, Mydlowska A, Rubinkiewicz M, et al. Eras protocol in laparoscopic surgery for colonic versus rectal carcinoma: are there differences in short-term outcomes? Med Oncol. 2016;33:56.–55 Hazebroek EJ. Color Study Group Color: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Surg Endosc. 2002;16:949-53.
Concerning our study, when compared with literature, the median of hospital stay was 5 days, which corroborates with Park et al. and Casillas et al., who found a median of 4 days for robotic surgery and 7 days for laparoscopy.1111 Park S, Kim NK. The role of robotic surgery for rectal cancer: overcoming technical challenges in laparoscopic surgery by advanced techniques. J Korean Med Sci. 2015;30:837-46.,2121 Casillas MA, Leichtle SW, Wahl WL, Lampman RM, Welch KB, Wellock T, et al. Improved perioperative and short-term outcomes of robotic versus conventional laparoscopic colorectal operations. Am J Surg. 2014;208:33-40. The conversion rate specific to rectal cancer found on our study was 2.1%, consistent with the literature findings that vary between 0% and 9% (Table 5).2222 Pai A, Melich G, Marecik SJ, Park JJ, Prasad LM. Current status of robotic surgery for rectal cancer: a bird's eye view. J Minim Access Surg. 2015;11:29-34.
The most common post operative complications reported in our study were anastomotic fistula in 11% and surgical wound infection in 3% of patients, within Clavien–Dindo II and IIIb.77 Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-13. Table 5 shows studies of Hellan et al. and Pigazzi et al. with rates of anastomotic fistula, specific to rectal cancer, from 12.1% to 10.5% respectively, which are compatible to the rates we have found.2323 Hellan M, Anderson C, Ellenhorn JD, Paz B, Pigazzi A. Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol. 2007;14:3168-73.,2424 Pigazzi A, Garcia-Aguilar J. Robotic colorectal surgery: for whom and for what?. Dis Colon Rectum. 2010;53:969-70. However, the meta-analysis conducted by Liao et al. showed 6.5% of anastomotic fistula on robotic surgery versus 5% on conventional surgery, and rates of around 2% of surgical wound infection both in conventional and robotic approaches.1919 Liao G, Li Y-B, Zhao Z, Li X, Deng H, Li G. Robotic-assisted surgery versus open surgery in the treatment of rectal cancer: the current evidence. Sci Rep. 2016;6:26981. We found higher rates of anastomotic fistula in robotic surgery than that described in literature, but wound infection rates, the second most frequent complication, are consistent with the ones described. This fact may be associated to the higher percentage of patients with advanced tumors (T3 and T4) than the ones reported in Asian, American and European studies, besides the fact that most rectal cancer patients underwent neoadjuvant therapy and, consequently, had a higher chance of anastomotic fistula occurrence.44 de Jesus JP, Valadão M, de Castro Araujo RO, Cesar D, Linhares E, Iglesias AC. The circumferential resection margins status: a comparison of robotic, laparoscopic and open total mesorectal excision for mid and low rectal cancer. Eur J Surg Oncol. 2016;42:808-12. In addition, BMI ≥30, presented in 11% of subjects, was shown as a risk factor for surgical complications in robotic surgery with a p-value of 0.038.66 Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA. 2017;318:1569-80.,2525 Harr JN, Luka S, Kankaria A, Juo YY, Agarwal S, Obias V. Robotic-assisted colorectal surgery in obese patients: a case-matched series. Surg Endosc. 2017;31:2813-9. Harr et al. described higher rates of complications on robotic surgeries on obese patients, especially when BMI ≥30, such as we have found in our study.
Conclusion
Robotic surgery is safe and feasible for surgical approach of colorectal cancer, showing satisfactory results in hospital stay and complication rates, added to a low surgical conversion rate. Obesity has been shown as a risk factor for surgical complications in robotic colorectal surgery. In relation to advanced tumors, more in depth studies are necessary to denote their relationship with higher complication rates.
Compliance with ethical standards
The content has not been published or submitted for publication elsewhere.
The protocol for the research project has been approved by a suitably constituted Ethics Committee of the Brazilian National Cancer Institute within which the work was undertaken and that it conforms to the provisions of the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000).
The study had a statement that the subject gave informed consent and patient anonymity should was preserved.
References
-
1Roy S, Evans C. Overview of robotic colorectal surgery: current and future practical developments. World J Gastrointest Surg. 2016;8:143-50.
-
2Pędziwiatr M, Pisarska M, Kisielewski M, Major P, Mydlowska A, Rubinkiewicz M, et al. Eras protocol in laparoscopic surgery for colonic versus rectal carcinoma: are there differences in short-term outcomes? Med Oncol. 2016;33:56.
-
3Bhama AR, Obias V, Welch KB, Vandewarker JF, Cleary RK. A comparison of laparoscopic and robotic colorectal surgery outcomes using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Surg Endosc. 2016;30:1576-84.
-
4de Jesus JP, Valadão M, de Castro Araujo RO, Cesar D, Linhares E, Iglesias AC. The circumferential resection margins status: a comparison of robotic, laparoscopic and open total mesorectal excision for mid and low rectal cancer. Eur J Surg Oncol. 2016;42:808-12.
-
5Hazebroek EJ. Color Study Group Color: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Surg Endosc. 2002;16:949-53.
-
6Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA. 2017;318:1569-80.
-
7Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-13.
-
8Mak TW, Lee JF, Futaba K, Hon SS, Ngo DK, Ng SS. Robotic surgery for rectal cancer: a systematic review of current practice. World J Gastrointest Oncol. 2014;6:184-93.
-
9Rodríguez-Sanjuán JC, Gómez-Ruiz M, Trugeda-Carrera S, Manuel-Palazuelos C, López-Useros A, Gómez-Fleitas M. Laparoscopic and robot-assisted laparoscopic digestive surgery: present and future directions. World J Gastroenterol. 2016;22:1975-2004.
-
10Bandar MHA. The current scope of robotic surgery in colorectal cancer. Adv Robot Autom. 2015.
-
11Park S, Kim NK. The role of robotic surgery for rectal cancer: overcoming technical challenges in laparoscopic surgery by advanced techniques. J Korean Med Sci. 2015;30:837-46.
-
12Uhrich ML, Underwood RA, Standeven JW, Soper NJ, Engsberg JR. Assessment of fatigue, monitor placement, and surgical experience during simulated laparoscopic surgery. Surg Endosc. 2002;16:635-9.
-
13Xiong B, Ma L, Huang W, Zhao Q, Cheng Y, Liu J. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Gastrointest Surg. 2015;19:516-26.
-
14Fernandez R, Anaya DA, Li LT, Orcutt ST, Balentine CJ, Awad SA, et al. Laparoscopic versus robotic rectal resection for rectal cancer in a veteran population. Am J Surg. 2013;206:509-17.
-
15Prete FP, Pezzolla A, Prete F, Testini M, Marzaioli R, Patriti A, et al. Robotic versus laparoscopic minimally invasive surgery for rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2018;267:1034-46.
-
16Lin S, Jiang H-G, Chen Z-H, Zhou S-Y, Liu X-S, Yu J-R. Meta-analysis of robotic and laparoscopic surgery for treatment of rectal cancer. World J Gastroenterol. 2011;17:5214-20.
-
17Trastulli S, Cirocchi R, Desiderio J, Coratti A, Guarino S, Renzi C, et al. Robotic versus laparoscopic approach in colonic resections for cancer and benign diseases: systematic review and meta-analysis. PLOS ONE. 2015;10:e0134062.
-
18Ortiz-Oshiro E, Sánchez-Egido I, Moreno-Sierra J, Pérez CF, Díaz JS, Fernández-Represa JÁ. Robotic assistance may reduce conversion to open in rectal carcinoma laparoscopic surgery: systematic review and meta-analysis. Int J Med Robot. 2012;8:360-70.
-
19Liao G, Li Y-B, Zhao Z, Li X, Deng H, Li G. Robotic-assisted surgery versus open surgery in the treatment of rectal cancer: the current evidence. Sci Rep. 2016;6:26981.
-
20Park EJ, Baik SH. Robotic surgery for colon and rectal cancer. Curr Oncol Rep. 2016;18:5.
-
21Casillas MA, Leichtle SW, Wahl WL, Lampman RM, Welch KB, Wellock T, et al. Improved perioperative and short-term outcomes of robotic versus conventional laparoscopic colorectal operations. Am J Surg. 2014;208:33-40.
-
22Pai A, Melich G, Marecik SJ, Park JJ, Prasad LM. Current status of robotic surgery for rectal cancer: a bird's eye view. J Minim Access Surg. 2015;11:29-34.
-
23Hellan M, Anderson C, Ellenhorn JD, Paz B, Pigazzi A. Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol. 2007;14:3168-73.
-
24Pigazzi A, Garcia-Aguilar J. Robotic colorectal surgery: for whom and for what?. Dis Colon Rectum. 2010;53:969-70.
-
25Harr JN, Luka S, Kankaria A, Juo YY, Agarwal S, Obias V. Robotic-assisted colorectal surgery in obese patients: a case-matched series. Surg Endosc. 2017;31:2813-9.
Publication Dates
-
Publication in this collection
13 June 2019 -
Date of issue
Apr-Jun 2019
History
-
Received
16 Dec 2018 -
Accepted
16 Jan 2019