Abstracts
OBJECTIVE: To present a wide-ranging review of the literature on bronchopulmonary dysplasia, covering new definitions, pathophysiology, prevention, treatment, prognosis and progression. SOURCES OF DATA: The most relevant articles published on the subject since it was first described in 1967 were selected from MEDLINE search results. SUMMARY OF THE FINDINGS: Bronchopulmonary dysplasia is considered one of the primary causes of chronic lung disease among infants. It is associated with frequent and prolonged hospital admissions, in particular for pulmonary diseases, with high rates of mortality and alterations to neuropsychomotor development and pondero-statural growth. Pathogenesis is complex, being primarily influenced by prematurity, infection, supplementary oxygen and mechanical ventilation. Prevention involves appropriate prenatal care, the prevention of premature delivery, prenatal corticosteroids, surfactant replacement therapy and "protective" ventilatory strategies. Treatment of bronchopulmonary dysplasia patients demands a multidisciplinary team. When indicated, oxygen supplementation is extremely important. Despite increased risk of morbidity and mortality during the first years of life, long term progress is favorable in the majority of cases. CONCLUSIONS: Bronchopulmonary dysplasia has been and continues to be studied in great depth with the objective of identifying its causes and possible prevention and treatment strategies. Controversies remain with respect of these issues and also about the prognosis of these patients, in particular when the subject is long-term progress of "new" bronchopulmonary dysplasia patients.
Bronchopulmonary dysplasia; oxygen therapy; mechanical ventilation; corticosteroid; prevention; treatment; progression
OBJETIVO: Apresentar uma ampla revisão da literatura sobre displasia broncopulmonar, abordando novas definições, fisiopatologia, prevenção, tratamento, prognóstico e evolução. FONTE DOS DADOS: Foram selecionados os artigos mais relevantes sobre o tema, desde a sua descrição inicial, em 1967, pesquisados na MEDLINE. SÍNTESE DOS DADOS: A displasia broncopulmonar é considerada uma das principais causas de doença pulmonar crônica em lactentes. Está associada a hospitalizações freqüentes e prolongadas, especialmente por doenças pulmonares, altos índices de mortalidade e alterações no desenvolvimento neuropsicomotor e no crescimento pôndero-estatural. A patogênese é complexa e influenciada principalmente por prematuridade, infecção, oxigênio suplementar e ventilação mecânica. A prevenção envolve o acompanhamento pré-natal adequado, a prevenção do parto prematuro, o uso pré-natal do corticosteróide, a terapia de reposição de surfactante e o uso de estratégias ventilatórias "protetoras". O tratamento do paciente com displasia broncopulmonar demanda uma equipe multidisciplinar. Quando indicada, a suplementação de oxigênio é de extrema importância. Apesar de maior risco de morbimortalidade nos primeiros anos de vida, a evolução em longo prazo é favorável na maioria das vezes. CONCLUSÕES: A displasia broncopulmonar vem sendo profundamente estudada na tentativa de identificação das suas causas e possibilidades de prevenção e de tratamento. Ainda existem controvérsias quanto a esses assuntos e também em relação ao prognóstico desses pacientes, especialmente quando se trata da evolução tardia da "nova" displasia broncopulmonar.
Displasia broncopulmonar; oxigenoterapia; ventilação mecânica; corticosteróide; prevenção; tratamento; evolução
REVIEW ARTICLE
Bronchopulmonary dysplasia
Luciana F. Velloso MonteI;Luiz Vicente F. da Silva FilhoII; Milton Harumi MiyoshiIII;Tatiana RozovIV
IMD. Fellow, Department of Pediatrics, Universidade de São Paulo (USP). Assistant physician, Pediatric Pulmonology Unit and Emergency Room of Instituto da Criança Professor Pedro de Alcântara, Hospital das Clínicas, School of Medicine, USP, São Paulo, SP, Brazil
IIMD, PhD. Assistant physician, Pediatric Pulmonology Unit, Instituto da Criança Professor Pedro de Alcântara, Hospital das Clínicas, School of Medicine, USP, São Paulo, SP, Brazil
IIIMD: Assistant professor, Departament of Pediatrics, Universidade Federal de São Paulo (UNIFESP/EPM). Medical consultant , Neonatal Intensive Care Unit, Hospital and Maternity Santa Joana, São Paulo, SP, Brazil
IVMD. Professor, Departament of Pediatrics and Pulmonary Rehabilitation, UNIFESP/EPM. Professor, Departament of Pediatrics, School of Medicine, USP, São Paulo, SP, Brazil
Correspondence Correspondence to Luciana de Freitas Velloso Monte SQSW 306, Bloco D - apto. 404, Sudoeste CEP 70673-434 - Brasília, DF, Brazil E-mail: lucifv@icr.hcnet.usp.br l-monte@ig.com.br
ABSTRACT
OBJECTIVE: To present a wide-ranging review of the literature on bronchopulmonary dysplasia, covering new definitions, pathophysiology, prevention, treatment, prognosis and progression.
SOURCES OF DATA: The most relevant articles published on the subject since it was first described in 1967 were selected from MEDLINE search results.
SUMMARY OF THE FINDINGS: Bronchopulmonary dysplasia is considered one of the primary causes of chronic lung disease among infants. It is associated with frequent and prolonged hospital admissions, in particular for pulmonary diseases, with high rates of mortality and alterations to neuropsychomotor development and pondero-statural growth. Pathogenesis is complex, being primarily influenced by prematurity, infection, supplementary oxygen and mechanical ventilation. Prevention involves appropriate prenatal care, the prevention of premature delivery, prenatal corticosteroids, surfactant replacement therapy and "protective" ventilatory strategies. Treatment of bronchopulmonary dysplasia patients demands a multidisciplinary team. When indicated, oxygen supplementation is extremely important. Despite increased risk of morbidity and mortality during the first years of life, long term progress is favorable in the majority of cases.
CONCLUSIONS: Bronchopulmonary dysplasia has been and continues to be studied in great depth with the objective of identifying its causes and possible prevention and treatment strategies. Controversies remain with respect of these issues and also about the prognosis of these patients, in particular when the subject is long-term progress of "new" bronchopulmonary dysplasia patients.
Key words: Bronchopulmonary dysplasia, oxygen therapy, mechanical ventilation, corticosteroid, prevention, treatment, progression.
Introduction
Bronchopulmonary dysplasia (BPD) is a chronic lung disease with peculiar clinical, radiological and histological characteristics. In general, it affects preterm infants submitted to oxygen therapy and to mechanical ventilation in the first days of life. Since it was first described in the 1960s, there has been considerable improvement in perinatal care, such as the widespread use of antenatal corticosteroids, exogenous surfactant therapy and the development of new techniques for noninvasive monitoring and for mechanical ventilation. Such facts have contributed to the increase in the survival of immature newborns and, as a consequence, to the higher incidence of BPD. Approximately 3,000 to 7,000 newborns have been estimated to suffer from this disease in the USA every year. Although the frequency of this disease has not decreased in the last few decades, the improvements made to the treatment of newborns with respiratory failure have minimized its severity. However, BPD is still extremely important clinically and is also a public health concern, as it is currently known as one of the major causes of chronic respiratory diseases among infants, resulting in frequent hospitalizations with high rates of mortality, impaired weight and height growth, and neuropsychomotor development disorders.1-3 For these reasons, there have been many ongoing studies to determine its causes and find alternatives to its prevention and treatment. The present study reviews the most relevant aspects related to the pathophysiology of BPD, in addition to important issues regarding its prevention, treatment and clinical follow-up.
Definition
BPD was first described by Northway et al.4 in 1967 as a chronic lung disease that affected preterm newborns with respiratory distress syndrome (RDS), or hyaline membrane disease, submitted to prolonged mechanical ventilation at high blood pressure levels and inspired oxygen fractions (FiO2). At that time, the disease was classified into four stages according to its clinical, radiological and pathoanatomical characteristics. The patients also presented with tachydyspnea and room-air hypoxemia, hyperlucent cystic lesions with radiopaque images on the chest X-ray, cardiomegaly and cor pulmonale, in addition to metaplasia and pulmonary fibrosis. Most of them depended on oxygen therapy for months or even years. In 1969, Pursey et al.5 described diffuse lung disorders related to prolonged mechanical ventilation in newborns with pulmonary disease of several etiologies, including those who did not have RDS, suggesting that this condition did not necessarily predisposed to BPD.
In 1979, Bancalari et al.6 defined BPD as respiratory failure in newborns who required at least three days under mechanical ventilation and who depended on oxygen therapy for over 28 days, showing signs of increased breathing effort and pulmonary radiological findings. This was the most widely used criterion in the 1980s and early 1990s.
Nevertheless, improvements in perinatal care in the last few decades have increased the survival of extremely preterm infants, especially of those weighing less than 1,000 grams at birth. These patients often depend on oxygen therapy and/or mechanical ventilation up to 28 days of life, but they do not present with remarkable pulmonary involvement. On top of that, many of these patients become asymptomatic and are weaned from oxygen supplementation before their hospital discharge. Bearing these findings in mind, Shennan et al.,7 in 1988, suggested a new definition with the aim of identifying newborns at higher risk for the development of chronic lung disorders. The authors introduced the term chronic lung disease in preterm infants, regarding it as the persistence of respiratory signs and symptoms accompanied by pulmonary radiological findings,with necessity of oxygen supplementation for longer than 36 weeks' postconceptional age, which might be a better predictor of long-term respiratory outcomes.
However, none of these definitions accurately determines the severity of lung injury. Due to changes in the epidemiological, clinical and histopathological characteristics of the disease, a recent consensus conference has been held in the USA, organized by the National Institute of Child Health and Human Development (NICHD) and the National Heart, Lung, and Blood Institute (NHLBI) in partnership with the Office of Rare Diseases (ORD), with the aim of standardizing the terminology, defining severity criteria and implementing strategies for the prevention and treatment of BPD.8 In order to avoid terminological confusion, the consensus conference decided on the use of "bronchopulmonary dysplasia" instead of "chronic lung disease," due to the clear distinction of this disease, in epidemiological, etiopathogenic and prognostic terms, from other forms of chronic lung diseases that occur in infants and children. Moreover, new diagnostic and severity criteria were proposed for BPD. Thus, BPD should be considered in any newborn that depends on oxygen supplementation at levels greater than 21% for 28 days or longer. These patients, according to their gestational age, must be submitted to diagnostic reassessment and to disease severity analysis (see Table 1). Radiological findings, albeit extremely common, were considered to be inconsistently interpreted, and therefore should not be used for the definition or evaluation of BPD severity, according to the consensus.
Incidence
The incidence of BPD is inversely proportional to gestational age and birthweight. It rarely affects newborns with over 34 weeks' gestational age, although some cases have been described in full-term infants. Due to improvements in perinatal care, the incidence of the severe or "classic" form of BPD has decreased considerably. Furthermore, case descriptions vary substantially from one health center to another, due to the different populations analyzed, to the lack of standardized ventilatory strategies for the treatment of acute lung disease, to the great variability regarding water intake and the care of critically ill newborns and to the different diagnostic criteria used to define the disease (Table 2).9-12 No data about the incidence of BPD were found in Latin America. In our setting, Cunha et al.13 found a 26.6% incidence of BPD when assessing 124 infants weighing less than 1,500 g, who survived at 28 days of life between 2000 and 2002.
Pathophysiology
BPD is a multifactorial disease (Figure 1). These various factors are believed to act in a cumulative and synergic fashion, causing inflammation and lung injury.8 Injury to the lung tissue results in fibrosis and in an abnormal maturation process.
Factors associated with lung injury
Preterm birth: the prevalence of BPD is inversely proportional to gestational age at birth. Lung immaturity is therefore one of the most important factors in the etiopathogenesis of the disease. BPD represents the response of immature lungs to acute lung injury induced by mechanical ventilation, oxygen, and other factors. It is commonly knowledge that antioxidant systems develop in the last trimester of gestation and, therefore, antioxidant enzymes have a less intense activity in preterm infants. In addition, these patients also have lower antiprotease levels. These are some of the reasons why it is difficult to control inflammation in these patients. Moreover, the regulation of repair mechanisms is impaired, favoring the development of fibrosis in the affected segments. Nevertheless, it is still unclear how lung immaturity interferes with the "inflammation x repair" process, producing irreversible lesions.1,2,14
Oxygen: Oxygen-induced lung injury occurs due to the production of toxic radicals, such as superoxide, hydrogen peroxide and free radicals. As previously reported, newborns, especially preterm ones, are more susceptible to these lesions because antioxidant systems (e.g.: catalase and superoxide dismutase) are not fully developed yet. The active oxygen metabolites cause extensive tissue damage due to enzyme oxidation, protease inhibition, DNA synthesis inhibition, decrease in surfactant synthesis, and lipid peroxidation, in addition to acting as chemotactic factors for inflammatory cells.1,2,14
Mechanical ventilation: the two major factors related to mechanical ventilation-induced lung injury are alveolar instability, which causes atelectasis (atelectatic trauma) and regional alveolar and airway hyperdistension (barotrauma/volutrauma). Atelectatic trauma is an injury produced by repeated cycles of alveolar collapse and re-expansion during mechanical ventilation. The development of nonrecruited areas (atelectasis) is not only a consequence, but also a cause of lung injury. Some evidence exists that ventilatory strategies that use low positive end-expiratory pressure (PEEP) are associated with more pronounced lung injuries and with less efficient exogenous surfactant therapy. Therefore, ventilation should aim at alveolar recruitment, maximizing the functional residual capacity. Thus, the lung is protected from atelectatic trauma; there is no need for high oxygen concentrations; and consequently, pulmonary damage is reduced. Volutrauma is the injury associated with hyperdistension of pulmonary structures due to the use of large tidal volumes during mechanical ventilation. This causes pulmonary damage due to the "stretching" of alveoli, airways, basement membrane and of pulmonary vascular endothelium. Therefore, vascular permeability is increased and there is extravasation of fluids, proteins, and blood, resulting in edema and inflammation. Additionally, the rupture of the alveolar-capillary barrier caused by volutrauma may allow inflammatory mediators and bacteria to enter the bloodstream and produce systemic inflammatory reaction and infection in other organs. Atelectatic trauma and volutrauma synergically contribute to pulmonary damage. Nowadays, it is known that insufficient PEEP (predisposing to atelectatic trauma) and the use of large tidal volumes are the major causes of acute lung injury induced by mechanical ventilation. Barotrauma is the lung injury produced by high pressures during mechanical ventilation. Studies with experimental models using high ventilatory pressures without large tidal volumes caused relatively less lung injury. Currently, it is believed that pressure alone is not a major factor for the development of lung injury.2,8,14,15
Infection: There is evidence that prenatal and postnatal infection may contribute to the development of BPD due to the release of inflammatory mediators and to the influx of inflammatory cells into the lung. Some studies correlate the occurrence of chorioamnionitis with the development of BPD. Others suggest the association of BPD with Ureaplasma urealyticum infection, but it has not been confirmed whether this is a causal relationship. In addition to causing the release of proinflammatory mediators, lung infection, either congenital or nosocomial, may result in the necessity for ventilatory support, which deteriorates pulmonary damage even further.1,4-17
Patent Ductus Arteriosus (PDA): PDA causes an increase in pulmonary blood flow and interstitial edema, reducing lung compliance and increasing airway resistance. This may result in a more aggressive and prolonged ventilatory strategy, elevating the risk for the development of BPD.1
Genetic factors: There may a genetic predisposition to the development of BPD. The mechanism has not been elucidated yet, but it may be related to the lung repair process, with higher or lower stimulation to the reconstruction of normal lung tissue or to the proliferation of fibroblasts and fibrosis. It has been suggested that growth regulatory genes undergo the action of inflammatory mediators found in the affected lung (e.g.: transforming growth factor - TGF-ß), probably stimulating the gene expression of mediators that predispose to the development of fibrosis.1,8
Malnutrition and vitamin A deficiency: the preserved nutritional status and vitamin A contribute to the differentiation, regeneration and reepithelization of lung tissue, and some authors suggest the association of these components with BPD.1,18,19
Inflammation is the end product of the factors that produce lung injury. The inflammatory process results from injury to the respiratory epithelium and to the pulmonary vascular epithelium, causing the release of proinflammatory mediators and chemotactic cytokines. There is an influx of inflammatory cells, especially granulocytes, which potentiate the inflammatory reaction by producing more cytokines (interleukin-8, macrophage inflammatory protein-1, TGF-ß, among others) and release of enzymes (e.g.: elastase). These substances increase vascular permeability, which contributes to interstitial, alveolar and airway edema. One should recall that pulmonary circulation receives all the cardiac output and also has a neutrophil store. Therefore, the lung contributes to the circulation of cells and inflammatory mediators through the whole body.1,14,15,20,21
Healing
There are two outcomes for the inflammatory process: repair of pulmonary structures with restoration of normal lung function, or fibrosis, causing impairment of respiratory function. The outcome to be achieved depends on several factors, such as nutritional status, inflammatory mediators, genetic factors, among others, as previously described.
Lung repair consists of reepithelization and reconstruction of the lung parenchyma. Type II pneumocytes play a key role in the differentiation of type I pneumocytes by replacing the damaged cells. In fibrosis, alveolar macrophages are regarded as the main cells in charge of releasing cytokines that lead to the proliferation of fibroblasts and collagen deposition. There seems to be a variable spectrum between these two processes in the same patient in different pulmonary regions, which may explain the great clinical variability of the disease.1,8,15
Lung development and BPD
A series of perinatal events have been associated with the occurrence of BPD, mainly the development of the respiratory system. Lung development is completed in the first days of life, so even full-term newborns experience the alveolarization of lung tissues in an intense manner. Preterm births may occur before the cannalicular period of lung development, which comprises from the 17th to the 24th weeks of gestation, or during terminal and alveolar sac formation (after the 25th week). In the cannalicular period, the maturation of lung airways occurs, followed by the development of terminal respiratory units, which will be mature in the saccular and alveolar periods, preparing the lung for gas exchanges. Injury during the development of the lung parenchyma may alter the alveolarization process, causing alveolar simplification. Thus, BPD represents a rupture in the normal development and repair of lungs in response to acute lung injury.1,8,14,15
Clinical picture
The clinical picture encompasses respiratory symptoms associated with oxygen dependence and radiological findings in newborns, usually preterm ones, submitted to mechanical ventilation. Pulmonary auscultation may be poor, and just tachydyspnea may be observed. In more severe cases, hypoxemia may be accompanied by hypercapnia. Many patients show chest deformity, different levels of tachydyspnea and less tolerance to physical exercises. Cough and wheezing are frequent. Symptoms vary considerably and depend on the severity of BPD.1,15
Radiological findings
Radiological findings range from pulmonary hyperinflation with thickening of bronchi and atelectasis to the presence of radiopaque images of fibrosis, large cysts, and interstitial emphysema. The pulmonary artery trunk may be evident due to associated pulmonary hypertension and, in extremely severe cases, enlargement of the cardiac region may be observed. Over 90% of BPD patients have radiological findings at high-resolution computed tomography.1,15,22
Pathological aspects
In the "pre-surfactant era" pathoanatomical findings of BPD were characterized by chronic inflammation and fibrosis of the lung parenchyma, besides squamous epithelial metaplasia and airway smooth muscle hypertrophy. Since lung injury and the clinical picture were more severe, the involvement of the cardiovascular system was also more pronounced, with proliferation of the tunica intima, hypertrophy of the muscle layer and of the right ventricle (cor pulmonale). This "classic BPD" is quite uncommon nowadays, due to the use of antenatal corticosteroids, to neonatal surfactant replacement, to less aggressive ventilatory strategies and to the improved support care of preterm infants. However, it may still be observed in patients who require aggressive and prolonged ventilatory support, as in severe meconium aspiration syndrome, pulmonary hypoplasia associated with diaphragmatic hernia and congenital pneumonia accompanied by pulmonary hypertension, among other disorders.
From the 1990s on, with the improvements in perinatal care, the survival of extremely preterm infants has increased. The lungs of these patients are in the cannalicular or saccular stage of development, as previously described. At birth, even in the presence of few injuries, there is discontinuation or inhibition of normal lung development and growth. An interference is believed to occur in the indicative processes of lung development, impairing terminal maturation and the course of alveolarization, probably due to secondary septal defect. Then, one may observe simplification of distal areas, with a smaller number of alveoli. There is less fibrosis, more elastic tissue and less injury to the airways. In addition, the development of pulmonary microvasculature is inhibited, which contributes to the increase in vascular resistance. This picture is currently known as "new BPD" (Table 3).1,8,14,15
Lung function
Abnormal findings regarding lung function have different intensity and include:
- increase in airway resistance and restriction of airflow, leading to bronchial hyperresponsiveness;
- increase in breathing effort;
- decrease in lung compliance due to fibrosis, hyperinflation, and atelectasis;
- hypoxemia, aggravated by factors, such as sleep, agitation, feeding and infections;
- hypercapnia in more severe cases.
The persistence of these functional problems varies, as described further ahead.1,21-24
Prevention of BPD
Prevention of preterm birth, with adequate prenatal care, is the major form of prevention of BPD.2,8,14 Among other preventive measures, we highlight the following:
Corticosteroid therapy
Antenatal use: the beneficial effects of the antenatal use of corticosteroids is well established. The administration of this drug to mothers before preterm delivery reduces the incidence of RDS, peri-intraventricular hemorrhage and neonatal mortality. Corticosteroids seem to increase the amount of antioxidant enzymes and regulate the differentiation of several fetal tissues, preparing the conceptus for preterm birth. The effects of this drug seem to be enhanced when, combined with maternal corticosteroid therapy, preterm newborns receive exogenous surfactant replacement immediately after birth, which considerably reduces the need for aggressive ventilatory support. Although not confirmed by controlled studies, the use of this drug reduces the incidence and severity of BPD. Thus, the use of corticosteroids should be considered in all expectant mothers who go into labor prematurely between 24 and 34 weeks of gestational age.
Postnatal use: in the literature, the recommendations for systemic corticosteroid therapy in the postnatal period are extremely controversial and vary across different health services.25-27 There is some evidence that the inflammatory process plays a crucial role in the pathogenesis of BPD. This has allowed for the use of therapies that are able to reduce or regulate the pulmonary inflammatory process as an attempt to reduce the incidence or severity of BPD. Corticosteroid therapy reduces the inflammatory process, edema and fibrosis, in addition to inducing bronchodilation. The therapy quickly improves lung function, allowing for the discontinuation of mechanical ventilation. For this reason, corticosteroids have been considered a good alternative to the prevention and treatment of BPD. Studies have shown that systemic corticosteroids actually reduce the incidence of BPD and allow for earlier extubation. However, late neurological disorders have been observed in these patients, especially with the early use of dexamethasone in high doses. A recent meta-analysis28 has concluded that the use of corticosteroids in preterm newborns, despite the above-mentioned benefits, did not improve mortality rates or the length of hospital stay. Short-term benefits may not outweigh the long-term severe risks of postnatal corticosteroid therapy, especially with regard to neurological disorders. Therefore, the systemic administration of corticosteroids in the neonatal period should be limited to special situations and should only be initiated after informed consent from the parents.26,28-32 Nevertheless, controversy still exists over the postnatal use of corticosteroids.33 Its use is considered for preterm infants who depend on mechanical ventilation for over seven days and whose radiological findings are compatible with BPD, meeting the following criteria:
- necessity of FiO2 greater than 0.40 and mean airway pressure greater than 10 cmH2O;
- clinical and radiological evidence of recurrent atelectasis, recurrent pulmonary edema refractory to diuretics or presence of bronchial hyperresponsiveness (wheezing, pulmonary hypersecretion).
It is important that clinical intercurrent events be identified and treated before initiating corticosteroid therapy, since they may contribute to the deterioration of respiratory symptoms. These intercurrent events include: PDA with hemodynamic repercussion, air leak syndrome, infectious process, inefficient respiratory impulses (immaturity of the respiratory center or central nervous system injury), metabolic disorders, and malnutrition.
We recommend the intravenous or oral administration of dexamethasone in the following doses: 0.15 mg/kg/day every 12 hours for three days; then, 0.10 mg/kg/day every 12 hours for three days, and finally, 0.05 mg/kg/day every 12 hours for 3 days. If there is a positive response, with reduction of ventilatory parameters (pressure support ventilation and FiO2) after the initial doses (first 3 days), the treatment can be resumed. If the response is not satisfactory on the first days, corticosteroid therapy must be discontinued. On top of that, even if patients meet the criteria for corticosteroid therapy, repeated administration of the drug must be avoided, seeking to find alternatives to treat respiratory symptoms.
With regard to the use of inhaled corticosteroids in newborns, some authors have reported improvement in extubation rate and lower demand for systemic corticosteroid therapy. However, there are few studies on the use of inhaled corticosteroids for the prevention of BPD, thus hindering any conclusions.30,34-39
Surfactant therapy
Exogenous surfactant therapy causes a more homogeneous alveolar recruitment, stabilizes terminal airways, reducing the occurrence of atelectasis and helping reduce ventilatory parameters. In extremely preterm infants, there have been advantages of the prophylactic use (before two hours of life) over the therapeutic use for the reduction of mortality and of the incidence of BPD. Even though the use of exogenous surfactant did not reduce the incidence of BPD, its severity may have been reduced.2
Ventilatory strategies
The aim of mechanical ventilation should include the reduction of lung injury by the recruitment of collapsed areas and improvement of the functional residual capacity, without causing hyperdistension of already recruited areas. This way, one tries to minimize atelectatic trauma and barotrauma/volutrauma. Several ventilatory strategies aimed at the reduction of lung injury and consequently of BPD have been described, such as: conventional ventilation using lung protection strategies (low tidal volumes -4 to 6 ml/kg, improvement of PEEP and permissive hypercapnia), prone position, nasal continuous positive airway pressure (CPAP) and high-frequency ventilation. However, so far, controlled studies that used these strategies have not clearly shown reduction in the incidence of BPD. Moreover, permissive hypercapnia needs to be further investigated in preterm infants, due to the possible risk of central nervous system injury.2,8,14,40,41
Vitamin A
Vitamin A acts on the differentiation and maintenance of the integrity of the respiratory epithelium. Its deficiency leads to alveolarization and to the reduction in mucociliary transport with increase in squamous metaplasia. Based on these data, some studies have shown a reduction in the incidence of BPD in newborns at risk who receive high doses of vitamin A.1,18,19
Inhaled nitric oxide
Nitric oxide is a potent vasodilator and has been used in inhaled form during mechanical ventilation with the aim of reducing pulmonary shunt and inflammation. Several studies have been carried out using nitric oxide initially in low doses in preterm newborns with pulmonary hypertension for the prevention of BPD. Results have been inconclusive so far.8
Superoxide dismutase
Based on the evidence that preterm infants lack endogenous antioxidant systems and that oxygen free radicals play a key role in the etiopathogenesis of BPD, several studies have used antioxidant therapy, especially superoxide dismutase, for the prevention of BPD. Studies with experimental models have described beneficial effects of the tracheal administration of recombinant human superoxide dismutase, reducing the risk of hyperoxia-induced injury. Thus, the use of this drug may probably play a role in the prevention of BPD. Further studies are necessary because results are still controversial.2,8,42,43
Fluid restriction
Some studies revealed that a mild water restriction in extremely preterm infants may reduce the incidence of BPD, since pulmonary edema is important in the pathophysiology of the disease.2
Treatment
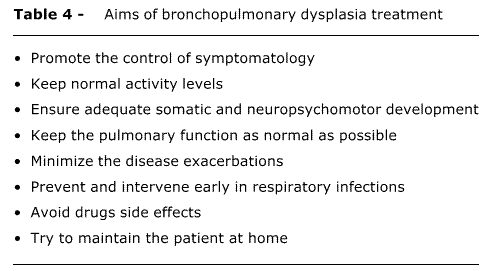
The clinical follow-up of BPD patients should preferably be made by a multidisciplinary team, including neonatologist and other pediatric subspecialties, such as pulmonology, cardiology, ophthalmology and neurology, besides physical therapist, nutritionist, speech therapist and occasionally other professionals. Treatment should be on a case-by-case basis, due to the different clinical manifestations and severity. The aims of the treatment are shown in Table 4.1,2,8,10,44
Oxygen therapy: one of the mainstays of the treatment of BPD patients is the maintenance of appropriate arterial oxygen levels. Hypoxemia is the main cause of cardiovascular disorders (pulmonary hypertension and cor pulmonale), in addition to interfering with weight gain and with brain development in BPD patients. If left untreated, hypoxemia is associated with a higher risk for sudden death and episodes of apnea in these infants.10,24 Oxygen should be preferably supplemented via a nasal tube in order to maintain the oxyhemoglobin saturation levels between 92 and 95%. In patients with cor pulmonale, higher values, between 95 and 96%, are recommended. Although there is some controversy about the best desirable oximetry level for BPD patients, these values should be kept stable during feeding, sleep or wake, thus avoiding intermittent supplementation.10,24
Nievas & Chernick10 in 2002, suggested an algorithm for oxygen supplementation in BPD (Figure 2). Initially, the requirement for oxygen is evaluated in alert patients. Pulse oximeter is used to measure oxygen saturation in an alert patient, every 2 or 3 weeks, after breathing room air for ten minutes. If saturation is equal to or greater than 92% (or 95%, if cor pulmonale), oxygen supplementation may be discontinued during wake periods, but kept during sleep periods. After that, oxygen saturation is measured during sleep, preferably using equipment that can record oximetry during the night.23 Oxygen therapy should be withheld when the above-mentioned levels have been achieved. In case of low weight gain at any of the stages described above, in case of good acceptance of a hypercaloric diet, full-time oxygen therapy should be restarted, since this is a strong sign of intermittent episodes of remarkable hypoxemia. If oxygen supplementation is still necessary for several months after discharge from the neonatal ICU, one should try to identify co-occurring events that might be worsening the symptoms of BPD or mimicking them, such as: untreated gastroesophageal reflux, episodes of microaspiration secondary to cricopharyngeal incoordination, undiagnosed congenital heart disease, among others.1,2,10,24
We recommend maintaining oxygen supplementation via nasal tube until medications, such as diuretics, can be discontinued, and until the patient is clinically stable, with oxygen saturation equal to or greater than 93% throughout the day. Discontinuation of nighttime oxygen therapy often occurs after the discontinuation of daytime supplementation.
Diuretics: some patients also present with recurrent pulmonary edema or impaired cardiac function. In these situations, the use of diuretics, mainly furosemide, is recommended, since some authors believe that, besides the diuretic effects, this drug has a direct effect on the lungs, improving compliance and airway resistance. However, special attention should be paid to the occurrence and correction of side effects, such as hydroelectrolytic disorders, hypercalciuria, nephrocalcinosis, among others. Short-term treatment is efficient in controlling pulmonary edema, but the efficacy of prolonged use of diuretics is controversial.1,2,10,24,45,46 Thus, furosemide should be considered in cases of recurrent and symptomatic pulmonary edema, allowing for a higher water intake with the aim of increasing energy intake. An intravenous or oral dose of 1 to 2 mg/kg is recommended twice a day. The use of diuretics should be discontinued when the patient shows improvement of pulmonary symptoms. In the cases in which diuretics have to be maintained for long periods, we recommend combining hydrochlorothiazide and spironolactone in the dose of 2 to 4 mg/kg/day or the use of furosemide on alternate days, in order to minimize adverse effects. The use of diuretics does not bring any evident benefits to patients older than six months, except if there are additional indications (nephrocalcinosis, cor pulmonale).
Corticosteroids: once BPD is established, inhaled corticosteroids are recommended (pressurized metered-dose inhaler with spacer device) for the management of bronchial hyperresponsiveness.1,2,10 We recommend the use of inhaled corticosteroids in patients with a more pronounced involvement of respiratory dynamics and with clearer clinical and radiological signs of lower airway obstruction. In this case, we recommend metered-dose inhalers coupled to valved spacers with an attached face mask. Low doses should be used for relatively long periods (at least six months of continuous use).
Bronchodilators: inhaled bronchodilators (administered via metered-dose spacers) are recommended for the management of respiratory symptoms (cough, wheezing) when there is clinical and/or functional evidence of good response. It is widely known that BPD causes airway smooth muscle hypertrophy; therefore, most patients may benefit from the use of these drugs.1,2,10,24,47 The use of oral bronchodilators should be avoided, due to the high risks of side effects.
Nutrition: several factors contribute to malnutrition in BPD patients. Among these factors, some of the most important include high energy expenditure, due to the increase in breathing effort, disorders related to glucose and lipid metabolisms, due to chronic hypoxemia, and lower food intake. We recommend the use of a hypercaloric diet (around 110 to 150 kcal/kg/day) with supplements rich in medium-chain triglycerides and some carbohydrate restriction for those patients who retain carbon dioxide. In our setting, we may use supplementation with foods containing canola oil or sunflower oil, which are inexpensive. It should be underscored that other causes of poor weight gain may be present, such as hypoxemia, anemia, gastroesophageal reflux, congenital heart disease, and swallowing incoordination, among others. These problems should be investigated and treated as early as possible.1,2,10,24,42,48
Immunization strategies: BPD patients are affected by recurrent respiratory tract infections. In addition to conventional vaccines, these patients should receive antipneumococcal vaccine and be vaccinated against influenza once a year. Also, there exists a great risk of infection by the respiratory syncytial virus (RSV) in preterm infants compared to full-term newborns. Several studies have shown that prophylaxis with immunoglobulin against RSV reduces the risk of hospitalization and the severity of RSV infections. Currently, the American Academy of Pediatrics49 recommends the use of intravenous anti-RSV immunoglobulin or preferably intramuscular anti-RSV monoclonal antibody (palivizumab) during the fall and winter seasons (when the incidence of RSV is higher) in BPD patients younger than two years in the following cases:
- patients on oxygen therapy; and
- patients who have required treatment for BPD within six months before the RSV season.
These patients should receive palivizumab during the fall and winter months in five intramuscular doses of 15 mg/kg, once a month. The use of palivizumab is contraindicated in infants with cyanogenic congenital heart disease.1,2,10,50 Nowadays, prophylaxis against RSV is not possible in public institutions, as this treatment is not covered by the Brazilian Ministry of Health. Despite the results obtained in the USA, there are no studies in Brazil that confirm the reduction in treatment costs when this kind of prophylaxis is used.
Management of pulmonary hypertension and cor pulmonale: oxygen therapy should be used for relief of pulmonary hypertension, since cardiovascular disorders are secondary to pulmonary disorders. As additional treatment, the following medications may be used: diuretics, calcium channel blockers, nitric oxide, among others.1
Criteria for hospital discharge
The following criteria have been suggested for the hospital discharge of infants with BPD:
- oxyhemoglobin saturation around 95% with a stable oxygen flow or reduced flow at least in the last two weeks;
- satisfactory weight gain;
- absence of apneic episodes at least in the last two weeks;
- no need to change drug therapy in the last week;
- possibility of returning for outpatient care in 48 to 72 hours;
- parents feel confident and comfortable in performing infant care tasks, including the use of oxygen and/or handling of gastric tube, in specific cases, in addition to showing qualification for the detection of signs of symptom deterioration and on how to proceed in case of emergency.
A home visit should be made by the medical team in order to check the necessary equipment and to know the people who will be in charge of patient care. It is also important to assess the family's socioeconomic conditions, housing conditions, access to telephone, transportation and proximity to referral hospitals.1,3, 24
Prognosis and outcome
The prognosis varies according to the severity of the disease. Morbidity and mortality rates are higher in the first year of life, decreasing in subsequent years.1,10,22-24
Lungs: BPD patients show higher pulmonary morbidity in their lives, especially in the first two years of life. This characteristic may persist into adulthood. Approximately 50% of extremely preterm infants have recurrent wheezing episodes and 33% still show these symptoms at preschool age. It has been reported that the risk of severe wheezing is minimized by 10% with the advance of gestational age, week after week. The risk for recurrent symptoms increases if the patient has siblings, family history of atopic disease or tobacco exposure, which is why smoking among family members is strongly discouraged. In addition to a greater risk for the development of respiratory symptoms, extremely preterm infants with BPD have a 50% hospital readmission rate, twenty times higher than that observed in full-term infants. The main reason for hospital admission is lower respiratory tract infection, especially a viral one; hence, the importance of the previously described anti-infectious strategies. With regard to the pulmonary disorders that were previously mentioned, patients have airway obstruction disease with increased airway resistance and air trapping, mainly in the first two years of life. These disorders are observed even in asymptomatic patients, but to a lesser degree. We may also observe chest deformity with more or less intensity. The functional residual capacity tends to return to normal between the 12th and 24th months of life. Lung compliance which, at 2 to 4 months of life amounts to only 35-50%, reaches 60% at 10 months, returning to baseline values around the third year of life. Residual volume improves slowly and may return to normal only between the 8th and 10th years of life, whereas airway resistance may persist at high levels into adulthood. It is estimated that around 50% of these patients have exercise-induced respiratory symptoms. Lung growth, with the development of new gas exchange units, is believed to occur up to the age of eight years. Oxygen dependence is therefore reversible in most patients. Apparently, the functional impact of these disorders is not severe.1,10,22-24,51
Heart: electrocardiographic evidence of right ventricular hypertrophy has been reported in 50% of school-aged children with BPD. In adolescents and in young adults, cardiovascular disorders are rarely observed.22
Growth: in general, infants reach the height growth curve at around 18 months of life, but the so-called catch-up growth occurs up to the 10th month of life. According to short-term follow-up studies, there has been weight and height deficiency in infants with BPD in comparison to infants without BPD. Long-term developmental studies have not confirmed this difference in children aged between 8 and 10 years. Currently, growth delay is more frequently associated with preterm birth and low birthweight than with the fact that these patients have BPD.22-24
Development: the frequency of neurological disorders in children who had BPD ranges from 0 to 60% at 24 months of life. This high variability shows the discrepancies related to the definition used, to different socioeconomic backgrounds, and to different perinatal care practices, in addition to the variable severity of the disease. It is widely known that patients with severe BPD, especially those who require prolonged mechanical ventilation and systemic corticosteroid therapy, have a poor outcome. Despite cognitive, sensory, motor and speech disorders, infants often improve with age. Therefore, the neurological follow-up of BPD patients is of paramount importance.23,52
One should recall that most long-term follow-up studies include BPD patients from the pre-surfactant era, whose disease severity is greater. So far, a few data exist on the late prognosis of patients belonging to the "new BPD" group.
Conclusion
BPD has been extensively investigated in an attempt to identify its causes and possibilities of prevention and treatment. There are, however, controversies over these issues and also over the prognosis of these patients, especially with regard to the late development of .new BPD.. The identification of strategies for the prevention and treatment of BPD is a major priority. It constitutes a promising and wide field to future studies.
Finally, the importance of a follow-up of BPD patients by a multidisciplinary team should be highlighted. In our setting, the treatment of patients from underprivileged socioeconomic backgrounds is extremely difficult, due to their precarious housing conditions, large number of people sharing the same household, poor access to health centers and hospitals, lack of money to buy food and medications, among other factors. In this case, it is important that healthcare professionals from different fields work together so as to improve growth and development and provide a better quality of life to these patients and their families.
Acknowledgments
We express our thanks to Suely Dornellas do Nascimento, from Universidade Federal de São Paulo (UNIFESP/EPM), for helping with the final revision of this paper.
Referências
Manuscript received Feb 06 2004, accepted for publication Apr 14 2004
- 1. Silva Filho LVF. Doença pulmonar crônica neonatal. J Pediatr (Rio J). 1998;74:265-74.
- 2. Barrington KJ, Finer NN. Treatment of bronchopulmonary dysplasia. A review. Clin Perinatol. 1998;25:177-202.
- 3. Hazinski TA. Bronchopulmonary dysplasia. In: Chernik, V, editor. Disorders of the respiratory tract in children. Philadelphia: WB Saunders; 1990. p. 300-20.
- 4. Northway Jr WH, Rosan RG, Porter DY. Pulmonary disease following respiratory therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357-68.
- 5. Pursey VA, Macpherson RI, Chernick V. Pulmonary fibroplasia following prolonged artificial ventilation of the newborn infant. Can Med Assoc J. 1969;100:451-7.
- 6. Bancalari E, Abdenour GE, Feller R, Gannon J. Bronchopulmonary dysplasia: clinical presentation. J Pediatr. 1979;95:819-23.
- 7. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527-32.
- 8. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723-9.
- 9. Lee SK, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU network 1996-1997. Pediatrics. 2000;106:1070-9.
- 10. Nievas FF, Chernick V. Bronchopulmonary dysplasia: an update for the pediatrician. Clin Pediatr. 2002;41:77-85.
- 11. Rojas MA, Gonzales A, Bancalari E, Claure N, Poole C, Silva-Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr. 1995;126:605-10.
- 12. Sinkin RA, Cox C, Phelps DL. Predicting risk for bronchopulmonary dysplasia: selection criteria for clinical trials. Pediatrics. 1990;86:728-36.
- 13. Cunha GS, Mezzacappa FF, Ribeiro JD. Fatores maternos e neonatais na incidência de displasia broncopulmonar em recém-nascidos de muito baixo peso. J Pediatr (Rio J). 2003;79:550-6.
- 14. Clark RH, Gerstmann DR, Jobe AH, Moffitt ST, Slutsky AS, Yoder BA. Lung injury in neonates: causes, strategies for prevention, and long-term consequences. J Pediatr. 2001;139:478-86.
- 15. Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998;53:81-94.
- 16. van Waarde WM, Brus F, Okken A, Kimpen JL. Ureaplasma urealyticum colonization, prematurity and bronchopulmonary dysplasia. Eur Respir J. 1997;10:886-90.
- 17. Lyon A. Chronic lung disease of prematurity. The role of intra-uterine infection. Eur J Pediatr. 2000;159:789-802.
- 18. Darlow BA, Graham PJ. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. Cochrane Database Syst Rev. 2002;(4):CD000501.
- 19. Verma RP, McCulloch KM, Worrell L, Vidyasagar D. Vitamin A deficiency and severe bronchopulmonary dysplasia in very low birthweight infants. Am J Perinatol. 1996;13:389-93.
- 20. De Dooy JJ, Mahieu LM, van Bever HP. The role of inflammation in the development of chronic lung disease in neonates. Eur J Pediatr. 2001;160:457-63.
- 21. Papoff P. Infection, neutrophils, and hematopoietic growth factors in the pathogenesis of neonatal chronic lung disease. Clin Perinatol. 2000;27:717-31.
- 22. Eber E, Zach MS. Paediatric origins of adult lung disease. Thorax. 2001;56:317-23.
- 23. Greenough A. Measuring respiratory outcome. Semin Neonatol. 2000;5:119-26.
- 24. Primhak RA. Discharge and aftercare in chronic lung disease of the newborn. Semin Neonatol. 2003;8:117-25.
- 25. Truffert P, Empana JP, Breart G, Saugstad OD, Goelz R, Halliday HL, et al. Treatment strategies for bronchopulmonary dysplasia with postnatal corticosteroids in Europe: the EURAIL survey. Acta Paediatr. 2003;92:948-51.
- 26. Halliday HL. Clinical trials of postnatal corticosteroids: inhaled and systemic. Biol Neonate. 1999;76(Suppl 1):S29-40.
- 27. Williams O, Greenough A. Post-natal corticosteroid use. Eur J Pediatr. 2003;162:613-15.
- 28. American Academy of Pediatrics Committee on Fetus and Newborn Canadian Paediatric Society Fetus and Newborn Committee. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330-8.
- 29. Doyle L, Davis P. Postnatal corticosteroids in preterm infants: systematic review of effects on mortality and motor function. J Paediatr Child Health. 2000;36:101-7.
- 30. Halliday HL, Ehrenkranz RA, Doyle LW. Early postnatal (< 96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2003;(1):CD001146.
- 31. Grier DG, Halliday HL. Corticosteroids in the prevention and management of bronchopulmonary dysplasia. Semin Neonatol. 2003;8:83-91.
- 32. Cole CH. Postnatal glucocorticosteroid therapy for treatment and prevention of neonatal chronic lung disease. Expert Opin Investig Drugs. 2000;9:53-67.
- 33. Jobe AH. Postnatal corticosteroids for preterm infantsdo what we say, not what we do. N Engl J Med. 2004;350:1349-51.
- 34. Cole CH, Colton T, Shah BL, Abbasi S, MacKinnon BL, Demissie S, et al. Early inhaled glucocorticoid therapy to prevent bronchopulmonary dysplasia. N Engl J Med. 1999;340:1005-10.
- 35. Shah V, Ohlsson A, Halliday HL, Dunn MS. Early administration of inhaled corticosteroids for preventing chronic lung disease in ventilated very low birth weight preterm neonates. Cochrane Database Syst Rev. 2000;(2):CD001969.
- 36. Shah SS, Ohlsson A, Halliday H, Shah VS. Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants. Cochrane Database Syst Rev. 2003;(2):CD002057.
- 37. Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatrics. 2001;1:1-14.
- 38. Cole CH. Postnatal glucocorticoid therapy for prevention of bronchopulmonary dysplasia: routes of administration compared. Semin Neonatol. 2001;6:343-50.
- 39. Cole CH. Inhaled glucocorticoid therapy in infants at risk for neonatal chronic lung disease. J Asthma. 2000;37:533-43.
- 40. Auten RL, Vozzelli M, Clark RH. Volutrauma. What is it, and how do we avoid it? Clin Perinatol. 2001;28:505-15.
- 41. Jobe AH, Ikegami M. Prevention of bronchopulmonary dysplasia. Curr Opin Pediatr. 2001;13:124-9.
- 42. Shah PS. Current perspectives on the prevention and management of chronic lung disease in preterm infants. Paediatr Drugs. 2003;5:463-80.
- 43. Suresh GK, Davis JM, Soll RF. Superoxide dismutase for preventing chronic lung disease in mechanically ventilated preterm infants. Cochrane Database Syst Rev. 2001;(1):CD001968.
- 44. Ho LY. Bronchopulmonary dysplasia and chronic lung disease of infancy: strategies for prevention and management. Ann Acad Med Singapore. 2002;31:119-30.
- 45. Brion LP, Primhak RA. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev. 2002;(1):CD001453.
- 46. Brion LP, Primhak RA, Yong W. Aerosolized diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev. 2000;(2):CD001694. Review. Update in: Cochrane Database Syst Rev. 2001;(2):CD001694.
- 47. Ng GY, Ohlsson A. Bronchodilators for the prevention and treatment of chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2001;(3):CD003214.
- 48. Atkinson SA. Special nutritional needs of infants for prevention and recovery from bronchopulmonary dysplasia. J Nutr. 2001;131:S942-6.
- 49. American Academy of Pediatrics. Prevention of respiratory syncytial virus infections: indications for the use of palivizumab and update on the use of RSV-IGIV. Pediatrics. 1998;102:1211-16.
- 50. The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531-6.
- 51. Kennedy JD. Lung function outcome in children of premature birth. J Paediatr Child Health. 1999;35:526-1.
- 52. Böhm B, Katz-Salamon M. Cognitive development at 5.5 years of children with chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 2003;88:F101-5.
Correspondence to
Publication Dates
-
Publication in this collection
30 July 2005 -
Date of issue
Apr 2005
History
-
Accepted
14 Apr 2004 -
Received
06 Feb 2004