Abstracts
OBJECTIVE: To compare the prevalence of allergy to cockroaches (Blattella germanica and Periplaneta americana) in asthmatic and non-asthmatic children and to determine the degree of agreement between the skin prick test and serum specific IgE levels, as well as to establish the relationship between cockroach allergy and total IgE levels. METHODS: A case-control study involving 76 asthmatic and 42 non-asthmatic children aged between 6 and 14 years was conducted in Recife, Brazil. All individuals were submitted to the skin prick test and to the measurement of specific IgE for B. germanica and P. americana, as well as to the determination of total IgE concentration. RESULTS: Asthmatic children showed a higher frequency of positive skin reactions to B. germanica (27.6 vs. 4.8%) and P. americana (27.6 vs. 2.4%) than non-asthmatic ones. The agreement between the skin prick test and the specific IgE results was reasonable for B. germanica (kappa = 0.25 ) and weak for P. americana (kappa = 0.17). Those patients who tested positive for cockroaches had a significantly higher geometric mean for total IgE than those who tested negative. The agreement between specific IgE and the skin prick test increased as total IgE levels rose, although some skin tests were negative even when total IgE levels were as high as 5000 kU/l. All determinations of cockroach-specific IgE were positive for total IgE levels greater than 2500 kU/l, even among asymptomatic patients. CONCLUSION: Allergic sensitivity to cockroaches was a predictive factor for asthma severity. The skin prick test is more appropriate for the detection of clinically relevant sensitivity to cockroaches than specific IgE determination.
Asthma; cockroaches; hypersensivity
OBJETIVOS: Comparar a prevalência da sensibilização a baratas (Blattella germanica e Periplaneta americana) em crianças com e sem asma, verificando a concordância entre testes cutâneos e IgE específica bem como determinar a relação entre a sensibilização a baratas e níveis séricos de IgE total. MÉTODOS: Foi realizado um estudo caso-controle, envolvendo 76 crianças asmáticas e 42 não asmáticas, entre 6 e 14 anos de idade, em Recife, Brasil. Todas as crianças submeteram-se ao teste cutâneo e dosagem sérica de IgE específica para B. germanica e P. americana e determinação sérica da IgE total. RESULTADOS: As crianças asmáticas apresentaram maior positividade aos testes cutâneos para B. germanica (27,6 versus 4,8%) e P. americana (27,6 versus 2,4%) que aquelas do grupo controle. A concordância entre o teste cutâneo e a IgE específica foi razoável para B. germanica (Kappa = 0,25) e fraca para P. americana (Kappa = 0,17). A média geométrica da IgE total foi 591,70 kU/L entre pacientes asmáticos e 345,85 kU/L entre os controles, não havendo diferença estatisticamente significante. Em pacientes com testes positivos para baratas, a média geométrica da IgE total foi significativamente maior em comparação aos pacientes cujos exames foram negativos. CONCLUSÃO: A sensibilização a baratas foi associada à asma. O teste cutâneo pode refletir mais apropriadamente uma sensibilização a baratas clinicamente relevante que a IgE específica.
Asma; baratas; hipersensibilidade
ORIGINAL ARTICLE
Use of the skin prick test and specific immunoglobulin E for the diagnosis of cockroach allergy
Maria Isabella L. LopesI; Paulo J. MirandaII; Emanuel SarinhoIII
IMestre, Departamento de Pediatria, Universidade Federal de Pernambuco (UFPE), Recife, PE, Brasil
IIMestre, Departamento de Patologia e Imunopatologia, Laboratório Keizo Asami (LIKA), UFPE, Recife, PE, Brasil
IIIDoutor, Departamento de Pediatria, Divisão de Alergia e Imunologia, UFPE, Recife, PE, Brasil
Correspondence Correspondence: Emanuel Sarinho Av. Parnamirim, 327/202, Parnamirim CEP 52060-000 - Recife, PE - Brazil Tel.: +55 (81) 8881.4801 Fax: +55 (81) 3231.2122 E-mail: emanuel.sarinho@gmail.com
ABSTRACT
OBJECTIVE: To compare the prevalence of allergy to cockroaches (Blattella germanica and Periplaneta americana) in asthmatic and non-asthmatic children and to determine the degree of agreement between the skin prick test and serum specific IgE levels, as well as to establish the relationship between cockroach allergy and total IgE levels.
METHODS: A case-control study involving 76 asthmatic and 42 non-asthmatic children aged between 6 and 14 was conducted in Recife, Brazil. All individuals were submitted to the skin prick test and to the measurement of specific IgE for B. germanica and P. americana, as well as to the determination of total IgE concentration.
RESULTS: Asthmatic children showed a higher frequency of positive skin reactions to B. germanica (27.6 vs. 4.8%) and P. americana (27.6 vs. 2.4%) than non-asthmatic ones. The agreement between the skin prick test and the specific IgE results was reasonable for B. germanica (kappa = 0.25 ) and weak for P. americana (kappa = 0.17). Those patients who tested positive for cockroaches had a significantly higher geometric mean for total IgE than those who tested negative. The agreement between specific IgE and the skin prick test increased as total IgE levels rose, although some skin tests were negative even when total IgE levels were as high as 5000 kU/l. All determinations of cockroach-specific IgE were positive for total IgE levels greater than 2500 kU/l, even among asymptomatic patients.
CONCLUSION: Allergic sensitivity to cockroaches was a predictive factor for asthma severity. The skin prick test is more appropriate for the detection of clinically relevant sensitivity to cockroaches than specific IgE determination.
Key words: Asthma, cockroaches, hypersensivity.
Introduction
Cockroaches produce potent allergens that induce the formation of specific IgE (sIgE) antibodies and trigger asthma in individuals who are genetically susceptible to their exposure.1 Blattella germanica and Periplaneta americana are the most common domestic species of cockroaches, and are found all over the world under favorable conditions, such as a warm and humid climate and poor housing.1
The sensitivity of individuals with asthma or rhinitis to cockroach allergens varies widely. Sensitivity rates of up to 70% have been reported for some North American cities.2 However, cockroach allergy is less common in Europe.2-4 In Brazil, there have been few studies on the exposure and sensitivity to cockroaches. The frequency of positive skin prick tests (SPT) for B. germanica and/or P. americana is as high as 55%5 in some cities.
Cockroach allergens are found in the dust collecting on the surface of mattresses, bed linen, upholstered furniture, carpets, and mainly in the kitchen.6-8 Given the perennial nature of the exposure, which does not allow the establishment of a temporal relationship with the development of symptoms, the diagnosis of cockroach allergy based only on clinical data has become impractical, thus requiring ancillary tests.9
Allergen sensitivity is detected through in vivo tests, such as SPT for immediate hypersensitivity and in vitro techniques, represented by sIgE determination.10 Despite the progress made in this area, there is no gold standard for the detection of sIgE for all inhalant allergens.9-11 This is even more complex when it comes to the diagnosis of sensitivity to cockroach allergens, as for the time being, there are no standardized commercially available extracts for these allergens. The use of standardized extracts with a known potency would generate more easily reproducible responses and a lower number of false positives.11 In the future, the use of recombinant allergens will improve the accuracy of the diagnosis of specific allergies.11,12
The main objectives of this study are to compare the prevalence of cockroach allergy in asthmatic and non-asthmatic children and to evaluate the degree of agreement between the SPT and sIgE determination in the diagnosis of cockroach allergy. Additionally, we analyze the relationship between cockroach allergy and total IgE (tIgE) levels.
Methods
This study was performed in Recife, Brazil, a city whose tropical climate predisposes to the proliferation of cockroaches, especially in poverty-stricken households. The study group consisted of children aged between 6 and 14 years, selected from the Pediatric Allergy and Immunology Outpatient Clinic and from the General Pediatrics Division of the Hospital das Clínicas of Universidade Federal de Pernambuco, from April to August 2001. The study was approved by the Research Ethics Committee of the Health Sciences Center, Universidade Federal de Pernambuco, and a written informed consent was obtained from all subjects participating in the study.
Study design
A case-control study was conducted to compare the prevalence of cockroach allergy in both asthmatic and non-asthmatic children. Individuals were considered asthmatic (cases) if they had had at least three episodes of dyspnea in the past 12 months or if they had been diagnosed with asthma and were receiving prophylactic treatment. Children who did not have a history of dyspnea, wheezing, allergic rhinitis or atopic dermatitis were regarded as non-asthmatics (control group). Patients were randomly selected from the Pediatrics Outpatient Clinic and allocated to either of the groups based on the inclusion criteria. The sample size was calculated using Epi-Info 6.0 software, and the case-to-control ratio was 2:1, totaling 76 cases and 42 controls. All children were submitted to the SPT and to the determination of tIgE and sIgE for B. germanica and P. americana.
Skin prick tests
SPT were conducted with extracts from B. germanica (1:10 w/v) and P. americana (1:10 w/v), using the puncture technique. A histamine base solution (10 mg/ml) was used as positive control and a glycerin solution, as negative control. The extracts and controls were obtained from the Hollister-Stier laboratory, Spokane, Washington. To ensure uniformity, the same lots of cockroach extracts were used in all subjects, only one researcher performed all SPT, and the same skin puncture device was used in all subjects. The test was considered positive when the wheal was at least 3 mm larger than that of the negative control.11,12
Serum specific IgE and total IgE
Venous blood was collected from each patient. The samples were centrifuged at 3,500 rpm and 1 ml of serum was stored at -20ºC for later determination of tIgE and sIgE for B. germanica and P. americana. Both sIgE and tIgE were measured with UniCAP®/Pharmacia CAP System, according to the manufacturer's instructions. Sensitization was considered to be present when sIgE levels were greater than > 0.35 kUA/l (class 1).
Statistical analysis
The data obtained were analyzed using Epi-Info software, version 6.0. A bivariate analysis was carried out to identify a possible association between cockroach allergen sensitization and asthma severity. The strength of this association was assessed through the odds ratio, with a 95% confidence interval (95%CI). The chi-square test with Yates' correction, or Fisher's exact test whenever necessary, was used in order to evaluate statistical significance, where a p value of less than 0.05 was considered significant. The kappa coefficient was used to assess the degree of agreement between the SPT and the sIgE results. Serum levels of tIgE were compared using the geometric mean. To evaluate the variation in the proportion of patients with positive sIgE and SPT with respect to tIgE, a simple logistic regression model was used, in which the log-transformed tIgE value was the explanatory variable.
Results
Most study subjects, in both groups, come from the metropolitan area of Recife and have an average monthly per capita income of US$ 60 or less. Other demographic characteristics that might influence the level of exposure to cockroaches are shown in Table 1.
Skin prick tests
SPT were positive for B. germanica in 21 (27.6%) asthmatic children, compared to only two (4.8%) children in the control group (OR = 7.64; 95%CI 1.57-50.75; p = 0.006). SPT were positive for P. americana in 21 (27.6%) asthmatic children and in one (2.4%) child from the control group (OR = 15.65; 95%CI 2.05-330.51; p = 0.002). Twenty-six (34.2%) asthmatic children and two children (4.8%) from the control group were sensitized to at least one of the cockroach species, when submitted to the SPT (OR = 10.40; 95%CI 2.16-68.45; p < 0.001).
Cockroach-specific IgE
Serum-specific IgE antibodies against B. germanica at a concentration greater than 0.35 kUA/l were found in 32 (42.1%) asthmatic children and in 9 (21.4%) children from the control group (OR = 2.67; 95%CI 1.03-7.03; p = 0.04). As to P. americana, sIgE was positive in 22 (28.9%) asthmatic children and in 7 (16.7%) children from the control group (OR = 2.04; 95%CI 0.72-5.96; p = 0.21). Thirty-three asthmatic children (43.3%) and 10 (23.8%) non-asthmatic children presented positive sIgE to at least one of the cockroach species (OR = 2.46; 95%CI 0.98-6.29; p = 0.06).
Agreement between the skin prick test and cockroach-specific IgE
As far as the sensitivity to B. germanica is concerned, both tests agreed (regardless of their results) in 70% (82 out of 118) of the cases. Of these, 12% (14 out of 118) were positive and 58% (68 out of 118) were negative. With regard to P. americana, there was an overall agreement of 72% (85 out of 118), with positive results in 8% (9 out of 118) and negative results in 64% (76 out of 118).
The observed agreement between the SPT and sIgE using the kappa coefficient was reasonable for B. germanica (kappa = 0.25) and weak for P. americana (kappa = 0.17).
Total IgE
The geometric mean of tIgE was 591.70 kU/l in asthmatic patients and 345.85 kU/l in non-asthmatic ones, with no statistical difference between the groups (Student's t-test without Welch's correction, p = 0.08).
Relationship between the skin prick test and total IgE
The geometric mean for tIgE was significantly higher (989.4 kU/l) among patients with positive SPT results for B. germanica and/or P. americana than among those whose SPT was negative (392.4 kU/l) (p = 0.002; Student's t-test for independent samples).
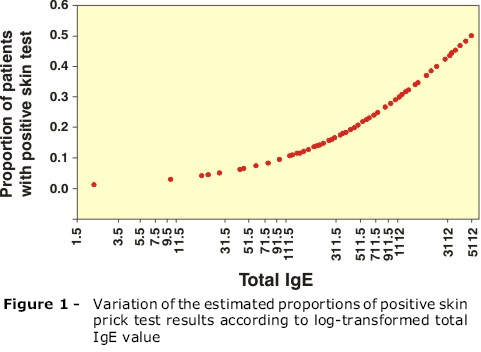
The proportion of patients with positive SPT results for B. germanica and/or P. americana grew with the increase in tIgE. This was evidenced through the adjustment of a simple logistic regression model, in which the log-transformed tIgE value was the explanatory variable. Figure 1 shows the variation of the estimated proportions of SPT results according to the log-transformed tIgE value.
Relationship between total IgE and cockroach-specific IgE
The geometric mean of tIgE was also significantly higher (1255.0 kU/l) in the group of patients with positive sIgE results for B. germanica and/or P. americana when compared to the group with negative sIgE results (284.6 kU/l) (p < 0.001; Student's t-test for independent samples).
The number of children with positive sIgE results for B. germanica and/or P. americana grew proportionally to the increase in tIgE levels, similarly to what occurred on the SPT. Again, a simple adjusted logistic regression model was used, in which the log-transformed tIgE value was the explanatory variable. Figure 2 shows the variation of the estimated proportions of positive sIgE results according to the log-transformed tIgE value.
Discussion
There is no gold standard to evaluate the accuracy of the clinical diagnosis of cockroach allergy. In our study, skin prick tests for allergy to B. germanica and P. americana were positive in 27.6% of the asthmatic children, similar to the results of another Brazilian study.13 There was a significant association between the sensitivity to both cockroach species and asthma severity, however, given the small size of the sample, the odds ratio showed wide confidence intervals, which did not allow a precise estimate of the strength of this association.
When cockroach sensitivity was determined by the measurement of sIgE levels, we found that the sensitivity to B. germanica was higher in asthmatics (42.1%) and controls (21.4%) than when it was determined by the SPT. The sensitivity to P. americana in the asthmatic group was equivalent when determined either by the SPT or by the measurement of sIgE levels (27.6 vs. 28.9%, respectively), but more patients in the control group tested positive in the sIgE measurement (16.7%) than on the SPT (2.4%). Given the large number of control patients who tested positive for P. americana, the association between cockroach allergy and asthma severity in this group was significant only for B. germanica. The lack of association between positive sIgE results for P. americana and asthma severity in this study may have resulted from differences in the sensitivity and specificity of the immunoassay to this antigen, and/or from the fact that the sample was not large enough to detect a significant difference between cases and controls.
The high percentage of positive sIgE results observed in the controls of this study is consistent with that found in recently published articles. In a Taiwanese study, for instance, 43% of asthmatic and 20% of non-asthmatic children were found to be sensitive to B. germanica.14 In another case-control study conducted in Ghana, the SPT was positive in 30% of the asthmatics and in 10% of the controls, whereas sIgE was positive in 76% of the asthmatics and in 28% of the controls.15 Traditionally, tests for the determination of sIgE levels are considered less sensitive than the SPT, but recent progress in immunoassay techniques16 may have improved the performance of these tests, which may therefore justify their greater sensitivity. However, the loss of specificity persists.
In this study the observed agreement between the SPT and sIgE, according to the kappa coefficient, was weak for P. americana (0.17) and reasonable for B. germanica (0.25). These results were not due to the cutoff value used to define sensitization, since the agreement was even worse when such value was changed to class 2 (> 0.7 kUA/l): weak for B. germanica (0.15) and for P. americana (0.10). Maia et al. also did not observe an agreement between the two tests in the sensitivity to B. germanica.17 Sastre et al. observed only a 21% agreement between the SPT and the sIgE to B. germanica.5
Discordance between SPT and sIgE results might also arise from the use of non-standardized extracts, which have variable potency and less reproducible results. Non-standardized extracts may be contaminated with other proteins, allergens, and enzymes.11 Studies using standardized inhalant allergen extracts demonstrate better agreement between the SPT and sIgE determination.18,19
Recombinant allergens contain well-defined concentrations of major allergens, having greater specificity and presenting fewer problems with spurious cross-reactions.20 After such reagents become available for the assessment of cockroach sensitization, there will probably be a greater agreement between the SPT and sIgE determination. Other factors influencing the results of the SPT include the potency of the extract, the type of device used for puncturing the skin, the skills of the professional responsible for performing the test, accuracy of interpretation, and the possibility of cross-reactions. Despite the limitations related to the use of medication, skin problems, and patient age, the SPT is still the method of choice for the study of inhalant allergens.21
Errors in the seric determination of sIgE may result from the type of allergen used, insufficient amount of allergens available, destruction of the epitope during attachment in the solid phase, poor IgE binding, levels of IgG (that could compete with IgE, leading to false negatives), elevated levels of tIgE that could lead to false positives due to nonspecific binding,12,16 presence of sIgE for cross-reactive carbohydrate determinants22 and other cross-reactions.23 When analyzing all samples for seric sIgE it is recommended that tIgE levels be also determined, as the higher the tIgE levels, the higher the degree of nonspecific binding. It is suggested that the results of serum positive sIgE in patients with extremely high tIgE levels be investigated by allergen inhibition studies.12
In a study comparing the performance of the SPT and sIgE with nasal allergen provocation using recombinant pollen allergens, Niederberger et al. observed that SPT was more useful in predicting the allergenic potential for induction of respiratory symptoms than quantitative serology, as there was not a good correlation between the levels of sIgE and the biological tests.24
Total serum IgE levels are frequently used to diagnose atopy, but their clinical value is limited due to the remarkable overlapping of IgE levels in both atopic and non-atopic individuals, in addition to the existence of a series of disorders that cause the elevation of tIgE, including intestinal helminthiasis.25 In the current study, there was no statistically significant difference between the geometric mean of tIgE levels in asthmatics (591.7 kU/l) and in the controls (345.85 kU/l). Special attention should be given to the increased tIgE levels in both groups. Approximately 34% of the asthmatics and controls presented total IgE levels equal to or greater than 1000 kU/l. Total IgE levels equal to or greater than 3000 kU/l were more often found among controls (12%) than among asthmatics (9%).
Increased tIgE levels might be caused by helminthic infections, which complicate the relationship between asthma, atopy, and intestinal parasitism.26 In a research study conducted with 150 preschoolers from Natal, Brazil, Sales et al. found a high rate (88.6%) of ascariasis, with tIgE levels significantly higher among the infected children (geometric mean = 715 IU/ml), when compared to the non-infected ones (geometric mean = 72.8 IU/ml).27 In these children, Ascaris infection and positive SPT were strongly associated with wheezing.
The high levels of tIgE in asthmatics and controls in our study may have been caused by intestinal helminthiasis, since the low socioeconomic status of our patients makes them susceptible to this kind of infection. Alternative explanations for the diagnosis of asymptomatic sensitivity in the control group also include the inhibition of allergic manifestations due to the saturation of mast cells,28 false positives due to nonspecific binding caused by the high levels of IgE12 or cross-reactions with parasitic allergens in the intestine, especially Ascaris, which is very prevalent in the study area.
The geometric mean of tIgE levels in patients with positive SPT results for B. germanica and/or P. americana was around 2.5 times greater than that of the non-sensitized group, and it should be observed that the proportion of positive tests increased as tIgE levels rose. Huss et al. verified that tIgE levels in asthmatic children represented a risk factor for positive SPT for cockroaches and dust mites, particularly when the levels were greater than 869 ng/ml (362 IU/ml).29
A similar trend was observed in the relationship between sIgE and tIgE levels. The geometric mean of tIgE levels was around 4.5 times greater among sensitized patients when compared to non-sensitized ones. We noted that the proportion of positive sIgE results also increased as tIgE levels rose, and that all the determinations of cockroach-specific IgE were positive for tIgE levels greater than 2500 kU/l, even among asymptomatic patients. According to our data, a study in Tanzanian women demonstrated a positive correlation between tIgE and sIgE to dust mites and cockroaches, which is neither associated with current asthma, nor with levels of allergen exposure.30 One of the explanations for these findings was the saturation of IgE receptors caused by elevated tIgE levels, blocking the access of sIgE to the mast cells, thereby suppressing the allergic response. The increased tIgE levels probably resulted from intestinal helminthiasis. A cross-reaction among parasites, dust mites, and cockroaches could be another explanation for the observed results.30 In our study the positive results for sIgE found in patients with very high tIgE levels are possibly related to the polyclonal activation of B cells due to intestinal helminthiasis or cross-reaction among several allergens.
This comparative study between the SPT and serum-specific IgE for the diagnosis of cockroach allergy suggests that the SPT may be more appropriate for the detection of clinically relevant sensitivity to cockroaches. However, studies with a larger sample size are needed to look further into this issue.
Acknowledgements
We thank Dr. Joao Bosco de Oliveira Filho for his critical reading of the manuscript.
References
Manuscript received Oct 18 2005, accepted for publication Mar 08 2006.
Financial support: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
- 1. Chapman MD, Vailes LD, Hayden ML, Benjamin DC, Platts-Mills TA, Arruda LK. Structural and antigenic studies of cockroach allergens and their relevance to asthma. Adv Exp Med Biol. 1996;409:95-101.
- 2. Hirsch T, Stappenbeck C, Neumeister V, Weiland SK, Von Mutius E, Keil U, et al. Exposure and allergic sensitization to cockroach allergen in East Germany. Clin Exp Allergy. 2000;30:529-37.
- 3. Peruzzi M, Luca M, Novembre E, Martino M, Vierucci A. Incidence of cockroach allergy in atopic Italian children. Ann Allergy Asthma Immunol. 1999;83:167-71.
- 4. Sastre J, Ibañez MD, Lombardero M, Laso MT, Lehrer S. Allergy to cockroaches in patients with asthma and rhinitis in an urban area (Madri). Allergy. 1996;51:582-6.
- 5. Santos ABS, Chapman MD, Aalberse RC, Vailes LD, Ferriani VPL, Oliver C, et al. Cockroach allergens and asthma in Brazil: identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J Allergy Clin Immunol. 1999;104:329-37.
- 6. De Lucca SD, Taylor DJ, O'Meara TJ, Jones AS, Tovey ER. Measurement and characterization of cockroach allergens detected during normal domestic activity. J Allergy Clin Immunol. 1999;104:672-80.
- 7. Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102:563-70.
- 8. de Blay F, Sanchez J, Hedelin G, Perez-Infante A, Verot A, Chapman M, et al. Dust and airborne exposure to allergens derived from cockroach (Blattella germanica) in low-cost public housing in Strasbourg (France). J Allergy Clin Immunol. 1997;99:107-12.
- 9. Current issues relating to in vitro testing for allergen-specific IgE: a workshop report. Ann Allergy Asthma Immunol. 1999;82:407-12.
- 10. Homburguer HA. Methods in laboratory immunology. In: Middleton E, Reed CE, editors. Allergy: principles & practice. 5th ed. St. Louis: Mosby-Year Book; 1998. p. 417-29.
- 11. The use of standardized allergen extracts. American Academy of Allergy, Asthma and Immunology (AAAAI). J Allergy Clin Immunol. 1997;99:583-6.
- 12. Bernstein IL, Storms WW. Practice parameters for allergy diagnostic testing. Joint Task Force on Practice Parameters for the Diagnosis and Treatment of Asthma. The American Academy of Allergy, Asthma and Immunology and the American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1995;75:543-625.
- 13. Rosário Filho NA, Faria L, Riedi CA, Zulato S. Sensibilização a baratas em crianças asmáticas: relação com a gravidade da doença. Rev Bras Alerg Imunopatol. 1999;22:151-5.
- 14. Lin YC, Su HJ, Hsiue TR, Lee CH, Chen CW, Guo YL. Levels of house dust mite-specific IgE and cockroach-specific IgE and their association with lower pulmonary function in Taiwanese children. Chest. 2002;121:347-53.
- 15. Addo-Yobo EO, Custovic A, Taggart SC, Craven M, Bonnie B, Woodcock A. Risk factors for asthma in urban Ghana. J Allergy Clin Immunol. 2001;108:363-8.
- 16. Yunginger JW, Ahlstedt S, Eggleston PA, Homburguer HA, Nelson HS, Ownby DR, et al. Quantitative IgE antibody assays in allergic diseases. J Allergy Clin Immunol. 2000;105:1077-84.
- 17. Maia AAM, Croce J, Guimarães JH, Lopez M. Sensibilização alérgica à Blattella germanica (Insecta: Dyctyoptera) em pacientes com asma e rinite na cidade de São Paulo, Brasil. Rev Bras Alerg Imunopatol. 1996;19:47-50.
- 18. Sarinho E, Rizzo MC, Just E, Fernandez-Caldas E, Solé D. Sensibilização aos ácaros domésticos em crianças atópicas e não atópicas do Recife, PE, Brasil. Rev Bras Alerg Imunopatol. 2000;23:105-10.
- 19. Wood RA, Phipatanakul W, Hamilton RG, Eggleston PA. A comparison of skin prick tests, intradermal tests, and RASTs in the diagnosis of cat allergy. J Allergy Clin Immunol. 1999;103:773-9.
- 20. Chapman MD, Smith AM, Vailes MD, Arruda LK, Dhanaraj V, Pomés A. Recombinant allergens for diagnosis and therapy of allergic disease. J Allergy Clin Immunol. 2000;106:409-18.
- 21. Wood RA. The diagnosis of allergy: why is it so difficult? Ann Allergy Asthma Immunol. 2003;91:1-2.
- 22. Mari A, Iacovacci P, Afferni C, Barletta B, Tinghino R, Di Felice G, et al. Specific IgE to cross-reactive carbohydrate determinants strongly affect the in vitro diagnosis of allergic diseases. J Allergy Clin Immunol. 1999;103:1005-11.
- 23. Aalberse RC. Allergens from mites: implications of cross-reactivity between invertebrate antigens. Allergy. 1998;53:47-8.
- 24. Niederberger V, Stübner P, Spitzauer S, Kraft D, Valenta R, Ehrenberger K, et al. Skin test results but not serology reflect immediate type respiratory sensitivity: a study performed with recombinant allergen molecules. J Invest Dermatol. 2001;117:848-51.
- 25. Ownby DR. Clinical significance of immunoglobulin E. In: Middleton E, Reed CE, editors. Allergy: principles & practice. 5th ed. St. Louis: Mosby-Year Book; 1998. p. 770-82.
- 26. Lynch NR, Goldblatt J, Le Souëf PN. Parasite infections and the risk of asthma and atopy. Thorax. 1999;54:659-60.
- 27. Sales VS, Rodrigues CE, Cavalcanti GB, Trombone APF, Lima RC, Santos ABR, et al. Infection with Ascaris lumbricoides in pre-school children: role in wheezing and IgE responses to inhalant allergens. J Allergy Clin Immunol. 2002;109:S27.
- 28. Weiss ST. Parasites and asthma/allergy: what is the relationship? J Allergy Clin Immunol. 2000;105:205-10.
- 29. Huss K, Adkinson NF Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol. 2001;107:48-54.
- 30. Sunyer J, Torregrosa J, Anto JM, Menedez C, Acosta C, Schellenberg D, et al. The association between atopy and asthma in a semirural area of Tanzania (East Africa). Allergy. 2000;55:762-6.
Correspondence:
Publication Dates
-
Publication in this collection
10 July 2006 -
Date of issue
June 2006
History
-
Accepted
08 Mar 2006 -
Received
18 Oct 2005