Abstracts
OBJECTIVES: To describe the advances in research into the physiological role of white adipose tissue, with emphasis on its endocrinal role in inflammatory processes, feeding behavior, insulin sensitization and modulation of the atherogenetic process. To deal with the potential role of adipose tissue as a source of stem cells for regeneration of tissues, with special emphasis on adipogenesis and its consequences for development of obesity. SOURCES: Important information was compiled from the scientific literature in order that this analysis contains an explanatory synthesis of the aspects mentioned above. SUMMARY OF THE FINDINGS In addition to its classical functions as primary metabolic energy store, meeting energy requirements during periods of deprivation by means of lypolisis, adipose tissue also has the capacity to synthesize and secrete a variety of hormones - the adipokines. These are active in a range of processes, such as control of nutritional intake (leptin) and control of sensitivity to insulin and inflammatory processes (TNF-alpha, IL-6, resistin, visfatin, adiponectin). Furthermore, since adipose tissue also contains undifferentiated cells, it has the ability to generate new adipocytes, regenerating its own tissue (adipogenesis), and also the ability to give rise to other cells (myoblasts, chondroblasts, osteoblasts), which has great therapeutic potential in the not-too-distant future. CONCLUSIONS: The range of functional possibilities of adipose tissue has widened. An understanding of these potentials could make this tissue a great ally in the fight against conditions that are currently assuming epidemic proportions (obesity, diabetes mellitus, arterial hypertension and arteriosclerosis) and in which adipose tissue is still seen as the enemy.
Adipocyte; lipogenesis; lypolisis; adipokines adipogenesis
OBJETIVOS Mostrar os avanços na pesquisa sobre o papel fisiológico do tecido adiposo branco, ressaltando o seu papel endócrino em processos inflamatórios, no comportamento alimentar, na sensibilização à insulina e na modulação do processo de aterogênese. Abordar o potencial papel do tecido adiposo como fonte de células-tronco para regeneração de tecidos, com especial ênfase para a adipogênese e suas conseqüências para a geração de obesidade. FONTES DE DADOS: Informações importantes constantes da literatura científica foram compiladas de modo a que esta leitura contenha uma síntese esclarecedora dos aspectos mencionados acima. SÍNTESE DOS DADOS:O tecido adiposo possui, além das suas funções clássicas como principal estoque de energia metabólica, suprindo as necessidades energéticas em períodos de carência mediante a lipólise, a capacidade de sintetizar e secretar vários hormônios, as adipocinas. Estas agem em diversos processos, como o controle da ingestão alimentar (leptina) e o controle da sensibilidade à insulina e de processos inflamatórios (TNF-alfa, IL-6, resistina, visfatina, adiponectina). Além disso, como o tecido adiposo contém também células indiferenciadas, tem a habilidade de gerar novos adipócitos, regenerando o próprio tecido (adipogênese), bem como originar outras células (mioblastos, condroblastos, osteoblastos), fato este que tem grande potencial terapêutico em futuro não muito distante. CONCLUSÃO: Amplia-se o leque de possibilidades funcionais do tecido adiposo. A compreensão dessas potencialidades pode fazer deste tecido o grande aliado no combate de moléstias que atualmente vêm assumindo proporções epidêmicas (obesidade, diabetes melito, hipertensão arterial e arteriosclerose), nas quais o tecido adiposo ainda é tido como um grande vilão.
Adipócito; lipogênese; lipólise; adipocinas; adipogênese
REVIEW ARTICLE
Adipose tissue as an endocrine organ: from theory to practice
Miriam Helena Fonseca-AlanizI; Julie TakadaII; Maria Isabel Cardoso Alonso-ValeIII; Fabio Bessa LimaIV
IFarmacêutica. Doutora, Instituto de Ciências Biomédicas (ICB), Universidade de São Paulo (USP), São Paulo, SP, Brazil
IINutricionista. Mestra e Doutoranda, Programa de Fisiologia Humana, Instituto de Ciências Biomédicas (ICB), Universidade de São Paulo (USP), São Paulo, SP, Brazil
IIIFarmacêutica. Doutora e Pós-doutoranda, Laboratório de Fisiologia do Tecido Adiposo, Departamento de Fisiologia e Biofísica, Instituto de Ciências Biomédicas (ICB), Universidade de São Paulo (USP), São Paulo, SP, Brazil
IVProfessor associado, Departamento de Fisiologia e Biofísica, Instituto de Ciências Biomédicas (ICB), Universidade de São Paulo (USP), São Paulo, SP, Brazil
Correspondence Correspondence: Fabio B. Lima Depto. Fisiologia e Biofísica, Instituto de Ciências Biomédicas, Universidade de São Paulo Av. Prof. Lineu Prestes, 1524 CEP 05508-900 - São Paulo, SP - Brazil Tel.: +55 (11) 3091.7248 Email: fabio@icb.usp.br
ABSTRACT
OBJECTIVES: To describe the advances in research into the physiological role of white adipose tissue, with emphasis on its endocrinal role in inflammatory processes, feeding behavior, insulin sensitization and modulation of the atherogenetic process. To deal with the potential role of adipose tissue as a source of stem cells for regeneration of tissues, with special emphasis on adipogenesis and its consequences for development of obesity.
SOURCES: Important information was compiled from the scientific literature in order that this analysis contains an explanatory synthesis of the aspects mentioned above.
SUMMARY OF THE FINDINGS: In addition to its classical functions as primary metabolic energy store, meeting energy requirements during periods of deprivation by means of lypolisis, adipose tissue also has the capacity to synthesize and secrete a variety of hormones - the adipokines. These are active in a range of processes, such as control of nutritional intake (leptin) and control of sensitivity to insulin and inflammatory processes (TNF-a, IL-6, resistin, visfatin, adiponectin). Furthermore, since adipose tissue also contains undifferentiated cells, it has the ability to generate new adipocytes, regenerating its own tissue (adipogenesis), and also the ability to give rise to other cells (myoblasts, chondroblasts, osteoblasts), which has great therapeutic potential in the not-too-distant future.
CONCLUSIONS: The range of functional possibilities of adipose tissue has widened. An understanding of these potentials could make this tissue a great ally in the fight against conditions that are currently assuming epidemic proportions (obesity, diabetes mellitus, arterial hypertension and arteriosclerosis) and in which adipose tissue is still seen as the enemy.
Keywords: Adipocyte, lipogenesis, lypolisis, adipokines adipogenesis.
Introduction
General background
Animal species need to guarantee their survival under inhospitable or unfavorable conditions. Vertebrates in general, and mammals in particular, have adipose tissue (AT), which allows them to store excess calories as lipids (triacylglycerols - TAG). These are hydrophobic and can be stored in large quantities without water as a solvent and contain twice as much energy per unit mass than other nutrients.
Since it is the primary energy reserve, AT has always been known as an energy reserve and thermal insulator. For a long time, these two properties were applied to the study of energy metabolism regulation and little attention was given to analysis of its other capabilities. Research into AT dealt with it as though there was a single type with uniform responses, without taking into consideration characteristics such as location or participation in local demands, unconnected to more global metabolic control processes. For this reason, the majority of older studies analyzed metabolic responses in samples from specific locations, and took these as representative of the entire adipose mass. These studies undervalued localized aspects of metabolic regulation, distorting or hiding an important functional dimension.
It is also true that, depending on the origin of the AT (for example, whether periepididymal or retroperitoneal), differences have been detected in its lipolytic or lipogenic capacity. Periepididymal tissue, from rodents in general, was widely used as a standard to assess responses to the most varied normal or pathological conditions. Questions as to the representativity of this fatty cushion began to emerge, indicating that a detailed review of its physiological role was imperative.
Adipocytes are the only cells that are specialized and perfectly adapted to store lipids without this compromising their functional integrity. They have the enzymatic machinery necessary to synthesize fatty acids (a process known as lipogenesis) and to store TAG during periods of abundant energy supply and to mobilize them via lypolisis when there is a calorie deficit. The central nervous system takes part in regulation of these two processes by means of direct or indirect neural activity (for example, initiating behavior to seek and consume nutrition). Other regulatory systems (digestive and endocrine) participate by means of nutrients and hormones depending on requirements at any given moment.1
The autonomous nervous system acts directly on AT through its subdivisions, the sympathetic and parasympathetic systems. The sympathetic system promotes catabolic actions (lypolisis), via b-adrenergic stimulation, which activates the hormone-sensitive lipase enzyme (HSL).2 The parasympathetic system organizes anabolic actions by increasing insulin production and increasing glucose and fatty acid capture.3
In mammals, there are two types of AT: white adipose tissue (WAT) and brown adipose tissue (BAT). Their adipocytes exhibit important differences. Mature white adipocytes store TAG in a single large lipid droplet that occupies the center of the cell, accounts for 85-90% of the mass of the cell and dislocates the cytoplasm, nucleus and other organelles to the circumference, where they remain within a thin layer of cytosol. Curiously, during their development, young adipocytes contain multiple small lipid droplets, which coalesce to form a single lipid inclusion as the cell matures. Although they have variable volume, mature white adipocytes are large cells, hundreds to thousands of times larger than red blood cells, fibroblasts and immune system cells and their size can change greatly depending on the quantity of TAG accumulated.4
In addition to adipocytes, AT contains a matrix of conjunctive tissues (collagen and reticular fibers), nerve fibers, vascular stroma, lymph nodes, immune cells (leukocytes, macrophages), fibroblasts and preadipocytes (undifferentiated adipose cells).1
Brown adipose tissue
The BAT is specialized in heat production (thermogenesis) and is practically absent in adult humans, but is found in fetuses and newborn infants. Its adipocytes are on average 30-40 µm in diameter, smaller than those of white tissue (average diameter of 60-100 µm). They have many cytoplasmatic lipid droplets of varying sizes, relatively abundant cytoplasm, spherical and mildly eccentric nuclei and many mitochondria which release heat via oxidation of fatty acids.5 Calorigenesis is guaranteed by uncoupling protein-1(UCP-1 or thermogenin) which is located in the internal mitochondrial membrane and which acts as a proton channel, discharging the potential generated by the accumulation of protons in the intermembrane space during the Krebs cycle, diverting them from the F1F0 compound (ATP synthase), preventing synthesis of ATP and allowing it to be dissipated as heat.5 The high concentration of cytochrome oxidase in these mitochondria contributes to their darker color.6
White adipose tissue
While its participation in thermogenesis is negligible, its functional capacity is of much wider scope. It has a generalized distribution throughout the body, surrounding, or even infiltrating, throughout the subcutaneous region, hollow visceral organs of the abdominal cavity or mediastinum and a range of muscle groups where it offers mechanical protection, softening impacts and allowing muscle fiber bundles to slide over each other sufficiently, without compromising their functional integrity. Since it is an excellent thermal insulator and due to its wider distribution, including the dermis and subcutaneous tissues, it has an important role in conservation of body temperature. Due to its capacity to store energy (around 200,000-300,000 Kcal in adults who are not obese) and provide it when necessary, it is the most important buffer system for energy balance.
Over the last 15 years, with the discovery of its capacity to secrete hormones, great importance has been attributed to its endocrinal role. These hormones, known as adipokines, have revolutionized the conception of its biological function, consolidating the idea that it is not just a supplier and storer of energy, but a dynamic organ and central to metabolic regulation.
Given the structural diversity of adipokines and the variety of functions so far identified, it can be stated that they include everything from proteins related to the immune system - tumor necrosis factor-a (TNF-a) and interleukin-6 (IL-6) -, to growth factors - transforming growth factor-b (TGF-b) - and proteins of the alternative complement pathway (adipsin). There are even adipokines involved in the regulation of pressure (angiotensinogen), of blood coagulation (plasminogen activator inhibitor-1, PAI-1), of glycemic homeostasis (adiponectin, resistin, visfatin, leptin) and of angiogenesis (vascular endothelial growth factor - VEGF),7 in addition to many others (Table 1).
In this review we will provide a brief report on the adipokines that have been most studied, with special emphasis on leptin (LEP), adiponectin (ADP), TNF-a and resistin, although comments will also be made on others.
Leptin
Leptin was identified in 1994 as the product of the ob gene that had been described in mice. A strain of obese mice (ob/ob) exhibited a genetic defect that resulted in non-production of this protein.8 Among other signs and symptoms, these animals exhibited the behavior and physiology of animals in a constant state of fasting, with elevated levels of corticosterone, lack of capacity to keep themselves warm, growth deficits, accentuated hypogonadism and exacerbated appetite, resulting in obesity, insulin resistance and diabetes mellitus.
The gene responsible, with three exons and two introns, is located on chromosome 7q31.3. Its promoter region has sites such as a TATA box and CCAAT/enhancer binding protein (C/EBP), glucocorticoid response element (GRE) and AMPc response element (CRE). The protein has 167 amino acids (16 KDa). Many types of tissue, in addition to adipose tissues, express LEP, such as the placenta, adeno-pituitary, gastric fundus mucosa, skeletal musculature and mammary epithelium, although, in global terms, greater or lesser production is directly related to AT mass, because levels in circulation are more directly related to the quantity of its RNAm in these tissues. Other metabolic and endocrinal factors contribute to regulate its transcription: insulin exhibits a directly proportional relationship with LEP levels. Glucocorticoids, estrogens, inflammatory cytokines and acute infectious states increase them, while low temperatures, adrenergic stimulation, growth hormone (GH), thyroid hormones, androgens, melatonin and smoking appear to reduce levels. The levels also exhibit circadian oscillation, with higher plasma concentrations at night.
Leptin receptors, OB-R, belong to the class I cytokine receptor family, which includes several interleukins (IL2 to IL7), GH, prolactin and erythropoietin. In the presence of LEP, the receptors dimerize, undergo conformational changes and activate Janus kinase (JAK) and signal transduction and activation of transcription (STAT) proteins.7 During this process, each monomer in the receptor phosphorylates into tyrosine under action of JAK2, locking in three STAT proteins (3, 5 and 6). These STAT are then phosphorylated into tyrosine by the JAK, become dissociated from the receptor and form homodimers or heterodimers which migrate to the nucleus, where they bind with specific DNA sequences, stimulating expression of specific genes. Other signal pathways in addition to this one have also been demonstrated, such as JNK (NH2-TERMINAL C-Jun kinase), p38 (p38 MAP kinase), extracellularly regulated kinase (ERK), phospholipase C (PLC), prostaglandins E2/F2 (PGE2/PGF2) and others.9
Leptin has an important role in regulation of energy balance, having two effects: 1) in a population of parvocellular hypothalamic arcuate nucleus (ARC) neurons, it stimulates expression of neuropeptides, which induce inhibition of nutritional intake (proopiomelanocortin [POMC] and cocaine- and amphetamine-related transcript [CART]) and increase overall energy consumption; with this last involving a population of neurons similar to the paraventricular (PV) nucleus which promote an increase in sympathetic tone; and 2) in another population of ARC neurons, it inhibits expression of neuropeptide Y (NPY) and the agouti peptide (AgRP), which are involved in increasing nutritional intake and reducing energy consumption.10
In addition to this important lipostatic function (as a measure of lipid deposits in the body), LEP modulates reproduction, angiogenesis, immunoresponse, blood pressure control and osteogenesis.7
Leptin is also necessary to the maturation of the reproductive axis, as is demonstrated by its ability to restore puberty and fertility in ob/ob rats, to accelerate puberty in wild rats and to facilitate reproductive behavior in rodents. Deficiencies of or insensitivity to LEP are associated with hypothalamic hypogonadism both in humans and in rodents. Mutations to the LEP gene seriously compromise menstrual cycles in women. If, on one hand, LEP is essential for puberty and the reproductive cycle, on the other hand it does not appear to affect gestation or lactation.
In the immune system, LEP increases cytokine production, macrophage adhesion and phagocytosis, and proliferation of T cells, increasing immune system competence.7
The angiogenic effect consists of the formation of capillaries in in vitro cultures of endothelial cells, prolonging and increasing proliferation of these cells.
Leptin provokes a pressure response attributed to activation of the central sympathetic system and to a depressive response attributed to local synthesis of NO, indicating a dual action, simultaneously producing a neurogenic pressurizing action and a humoral depressive action.
With relation to lipid metabolism, it activates adenylcyclase, increases lipid oxidation in the skeletal muscles and reduces TAG synthesis in the liver.
Tumor necrosis factor-a (TNF-a±)
Tumor necrosis factor-a is an immunomodulatory and proinflammatory cytokine. It has been described as a factor that induces cachexia in animals and inhibits lipogenesis in adipocytes. It is a multifunctional cytokine, involved in inflammation, apoptosis, cytotoxicity, the production of other cytokines, such as IL-1 and IL-6, and induces insulin resistance. With relation to adipocytes, it acts directly in insulin-dependent processes, including homeostasis of carbohydrate and lipid metabolism.11 It inhibits lipogenesis and stimulates lypolisis.
Its expression and secretion are increased in obesity and correlate positively with body mass index (BMI), the content of its RNAm and adipocyte volume. Neutralizing TNF-a in obese rats improves their response to insulin, revealing its relationship with insulin resistance. In obese humans there is a robust inverse correlation between TNF-a and glucose metabolism, due to suppression of insulin signaling, reducing phosphorylation of insulin receptor substrate-1 (IRS-1) and of phosphatidylinositol-3-kinase (PI3K), with reduced synthesis and translocation of the glucose transporter GLUT-4 to the membrane.12
This cytokine is also involved in induction of atherogenesis, participating in monocyte migration and their conversion into macrophages at the endothelial wall by means of nuclear factor k-B (NFkB) activation, triggering inflammatory changes in the vascular wall.
It binds to two types of membrane receptors: TNFR I and II, which mediate transduction of the signal triggered by TNF-a, forming compounds with cytoplasmatic adaptor proteins.
For more than 20 years it has been known that AT expresses TNF-a. Despite the variety of AT cell types, all of which are capable of producing cytokines, adipocytes are the principal sources and they express both receptors.
TNF-a is associated to insulin resistance in obesity. A positive correlation is observed between levels of its RNAm in subcutaneous AT and insulinemia in women, and obese patients with insulin resistance (especially women) have increased TNF-a secretion. The mechanisms of TNF-a-induced insulin resistance include: acceleration of lypolisis, increasing free fatty acids (FFA) in circulation; reduction of GLUT4 synthesis, and expression of insulin and IRS-1.
Adiponectin
The gene for ADP (AdipoQ, apM1, ACRP30) was first described in 1995 and is located at chromosome 3q27. It is the most abundant of the proteins produced by AT and it varies in concentration from 2 to 10 µg/mL, which is much higher than the concentration of other known hormones. It is a 30kDa protein and, in its primary molecular structure it has a globular domain (gADP), a collagenous domain and a variable region.
Several effects have been attributed to ADP, such as increased insulin sensitivity, NFkB modulating effects and TNF-a inhibition. There is an inverse correlation between levels of the hormone in circulation and the risk of obesity, insulin resistance and cardiovascular diseases.
The hormone forms trimers which can circulate in oligomers of four to six trimers each. Investigations into the bioactivity of whole ADP (fADP) or its gADP in isolation have shown that it is the second of these that is responsible for practically all of its biological activity.
Adiponectin receptors 1 and 2 have been identified. They contain seven transmembrane domains, but differ both structurally and functionally from G protein-coupled receptors. Receptor 1 (ADP-R1) is primarily expressed in muscle and functions with a high level of affinity for gADP and low affinity for fADP. Receptor 2 (ADP-R2) is primarily expressed in the liver and works as an intermediate affinity receptor for the gADP and fADP forms. The biological effects do not only depend on blood concentrations, but also on tissue specificity. Adiponectin in circulation does not fluctuate to a great degree, suggesting that its liberation is not acute, but regulated by longer term metabolic changes.13 Differences between men and women have also been observed, with levels being higher in women, which constitutes a sexual dimorphism.
A negative correlation has been clearly demonstrated between degree of obesity and levels of ADP in circulation, as have an increase in its concentration with reductions in weight and the association between hypoadiponectinemia with insulin resistance and hyperinsulinemia. The increased oxidation of fatty acids, capture and utilization of glucose in AT and skeletal muscle, and the reduction of hepatic glucose production, leading to better control of glycemia, FFA and TAG, are all testaments to the improved insulin sensitivity. In rat adipocytes, in vitro, a 60% reduction in ADP expression resulted in a significant increase in insulin resistance. Thiazolidinediones (TZD), which are insulin sensitizing drugs, induce increased secretion of ADP.
Several vascular effects of ADP have been described: 1) increased endothelium-dependent vasodilation; 2) increased endothelium-independent vasodilation; 3) antiatherosclerotic effect; 4) suppression of the expression of receptors known as scavengers of vascular adhesion molecules; 5) reduced expression of TNF-a and reduction of the effects of this adipokine on the endothelial inflammatory response; 6) amelioration of the effect of growth factors on vascular smooth musculature; 7) inhibition of the effects of oxidized low density lipoproteins (oxLDL) on the endothelium, with suppression of cell proliferation, of generation of superoxides and of activation of mytogen activated protein kinase (MAP); 8) increased production of NO; 9) stimulation of angiogenesis; 10) reduction of the thickness of the tunica intima and smooth musculature that is secondary to artery wall injury; and 11) inhibition of migration and proliferation of endothelial cells.
Adiponectin and atherosclerosis
High sensitivity C-reactive protein (hs-CRP), a marker of risk for coronary atherosclerotic disease, is expressed by AT. A negative correlation between ADP and CRP has been described in humans with atherosclerosis. This negative association supports the hypothesis that ADP is an antagonist of the development of atherosclerosis and vascular inflammation.
Adhesion of the monocytes to the vascular endothelium and the consequent transformation into foam cells is considered crucial to the development of vascular diseases. Adiponectin inhibits adhesion of monocytes to endothelium and reduces myeloid differentiation, the production of cytokines by macrophages and phagocytosis. It also inhibits the production and action of TNF-a and suppresses transformation of macrophages into foam cells, i.e., the link between vascular inflammation and atherosclerosis. A relationship has been recorded between the capacity to inhibit growth factors in smooth vascular musculature and reduction of macrophage migration by ADP. It has, therefore, direct cellular anti-atherosclerotic effects.
Adiponectin intracellular signaling
In the liver, skeletal muscle and AT, ADP activates AMP kinase (AMPK). This enzyme is activated by a variety of conditions, which lead to accumulation of AMP generated from ATP. It has been identified that AMPK is involved in the action of metformin in the liver and TZD in sensitization to insulin, suggesting a mediatory effect of the antidiabetic medications mentioned and reinforcing the effects of ADP. It also appears that AMPK mediates signaling in endothelial cells. Activation of AMPK in endothelium increases oxidation and synthesis of ATP. Since AMPK activates eNOS, this enzyme system appears to be an important link between ADP and production of NO.
Resistin
This hormone was described in 2001,14 when a relationship was demonstrated between resistin and insulin resistance induced by obesity (hence the name). Resistin has 12.5 KDa and belongs to a family of proteins generically known as resistin-like molecules (RELM), which are characterized by the consistent presence of a segment rich in cysteine (11 cysteines) at the C-terminal end. The principal representatives of this family are: 1) RELM-a (also known as FIZZ1 or found-in-inflammatory-zone), which was discovered in bronchoalveolar exudate triggered by allergic processes; 2) RELM-b (FIZZ2), which is abundantly expressed, especially in tumors of the colon, being related to the process of tumorigenesis; 3) resistin (FIZZ3) described in AT. Because of their cysteine-rich segment, resistin and RELM-b dimerize, forming homo or heterodimers.
Apparently, its secretion is stimulated by insulin, but experimental data are inconsistent. Inflammatory processes, glucocorticoids and lipopolysaccharides (LPS) and C/EBP-a all increase expression, whereas TNF-a and b-adrenergic stimulation and peroxisome proliferator-activated receptor-gamma (PPARy) inhibit it.
Many studies corroborate the hypothesis that there is an association between resistin and insulin resistance: in experiments with obese mice, where resistin was neutralized using antibodies, there was improved glucose tolerance and insulin sensitivity; intraperitoneal injections of resistin provoked glucose intolerance and hyperinsulinemia in normal mice; in 3T3-L1 adipocytes, the use of an anti-resistin saline improved glucose capture stimulated by insulin, whereas resistin antagonized this response.
In contrast, studies with ob/ob and db/db mice revealed that, despite the hyperesistinemia of these animals, treatment with TZD, which induced further increase of the resistinemia, improved their symptoms of resistance.
States associated with insulin resistance, such as lactation, exposure to the cold and cachexia due to cancer, did not exhibit increased resistin expression. In contrast, removal of the visceral fat of obese rats attenuated or impeded the development of resistance. Visceral fat proved to be the location where there is greatest resistin expression, 15 times more intense than in subcutaneous fat. Treatments with prolactin or testosterone lead to increased insulin resistance and an increase in the expression of resistin, and also to pathological conditions, such as hyperthyroidism, and physiological ones, such as gestation to half term, or use of steroids, which progress as resistin expression increases.
In human beings, the Resistin gene is located on chromosome 19, and its expression, which has been determined in population studies, does not correlate very strongly with obesity, except in one study performed in China.
Studies of human AT have shown that resistin is expressed more in preadipocytes than in mature adipocytes, in which it is negligible. Nevertheless, there is not a clear relationship between obesity and Resistin, although, even on this question, there is intense controversy, indicating that further studies are needed to elucidate the physiological role of resistin.15
As with TNF-a and IL-6, resistin is a protein with proinflammatory properties secreted by monocytes and adipocytes. Although it is expressed and secreted in lean individuals, levels are generally more elevated with obesity.
Some words about other adipokines
Several other products secreted by adipocytes merit mention (see Table 1). For example, the acylation stimulating protein (ASP), which has an important effect on lipogenesis, since it increases translocation of GLUT-4, production of glycerol-3-phosphate and the activity of diacylglycerol acyltransferase (DGAT), a TAG synthesis catalyst enzyme. At the same time, it inhibits lypolisis by means of inhibition of HSL.
Another adipokine with proinflammatory effects and actions on the metabolism of carbohydrates and lipids is IL-6. If it is infused into healthy humans at doses close to physiological levels it provokes lypolisis, irrespective of modulation by catecholamines, glucagon and insulin. This effect is the result of inhibition of lipoprotein lipase (LLP) and of the increase in liberation of FFA and glycerol. IL-6 is secreted by macrophages and adipocytes, and its expression can be stimulated by catecholamines via b 2 and b 3 adrenergic receptors in WAT, when at elevated concentrations.
PAI-1 provokes formation of thrombi and rupture of unstable atherogenic plaques and, by means of inhibition of plasmin production, it is capable of altering the balance between fibrinolysis and fibrinogenesis, contributing to vascular architecture remodeling and the atherosclerotic process. Several different studies have appeared demonstrating a strong correlation in obese individuals between elevated levels of PAI-1 and other conditions related to the metabolic syndrome (hyperglycemia, hyperinsulinemia and fasting hypertriglyceridemia and high concentrations of LDL-cholesterol).16,17
The association between adiposity and the renin-angiotensin system has been suggested in some pathological models. White adipose tissue is capable of secreting angiotensinogen and renin and of synthesizing angiotensin II AT1 and AT2 receptors and angiotensin-converting enzyme (ECA), proteins which take part in the differentiation of adipocytes and in lipogenesis, indicating their involvement with the process of accumulating body fat. Furthermore, the strong atherogenic role of angiotensin II, directly stimulating production of adhesion molecule-1 (ICAM-1) and granulocyte-macrophage colony-stimulating factor (GM-CSF) on the endothelial wall, which increase generation of NO and free radicals, platelet activity and expression of PAI-1, indicates an intense link with obesity, hypertension and cardiovascular diseases. Nevertheless, the adipocyte renin-angiotensin system does not appear to be regulated in a similar manner to the renal system, since changes to the level of NaCl in the diet to not change the genic expression of its components.
Recently, other adipokines have been discovered: 1) visfatin, an adipokine predominant in visceral AT, appears to play an important role in regulation of glycemic homoeostasis when it bonds with the insulin receptor, "mimetizing" its intracellular signaling; 2) apelin, the function of which appears to be related to regulation of nutritional intake.
Given the great diversity of proteins that are secreted by WAT, and also the diversity of their effects, which affect the adipocytes themselves and other tissues of the body, it becomes ever clearer that WAT has a direct link with pathologies associated with obesity, especially insulin resistance and the metabolic syndrome.
White adipose tissue is distributed across a large number of different deposits in the body, classified anatomically as subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). The SAT is primarily accounted for by the deposits below the skin in the abdominal, gluteus and femoral areas. The VAT includes tissues deposited close to or even inside the viscera of the abdominal cavity, and good examples are mesenteric, omental and retroperitoneal fat. There is sexual dimorphism in the regional distribution of WAT, with women generally having a greater degree of adiposity than men and having a greater SAT/VAT ratio.18
In addition to the differences in anatomic location, the functionality and metabolism of VAT and SAT also vary from region to region, exhibiting a certain specificity and, possibly, specialization. In the visceral adipocytes, the lipolytic effect of catecholamines is more intense and the antilipolytic effect of insulin is weaker, which results in greater mobilization of FFA by lypolisis of intraabdominal fat deposits than of gluteal and femoral subcutaneous deposits.19 The accentuated response to catecholamines in VAT may explain the increased quantity of b1 and b2 adrenergic receptors in the cellular surface and its increased genic expression in abdominal and omental adipocytes, compared with subcutaneous ones.
Production and secretion of proteins are metabolic activities of WAT that are also subject to regional variations. Thus, while the ASP protein is predominantly expressed in SAT, ADP, angiotensinogen, IL-6, PAI-1 and the cholesterol ester transfer protein (CETP) are all factors which are principally secreted by visceral adipocytes. Another example of these differences can be seen in the activity of the 11 beta-hydroxysteroid dehydrogenase type 1 enzyme (11bHSD1), which is responsible for generating active cortisol from cortisone, and which is elevated in visceral adipocytes when compared with subcutaneous ones.20,21
In a study that determined levels of LEP RNAm in adipocytes isolated from obese individuals, it was reported that there was a greater quantity of RNAm in subcutaneous adipocytes than in omental ones. Secretion and genic expression of LEP were assessed in the SAT and VAT of obese and not obese women, and the results demonstrated that the rate of secretion and RNAm levels were approximately two to three times greater in subcutaneous deposits than in omental deposits in both groups of women.
Metabolism of adipocytes
As a result of the prominent activity of WAT, together with the importance it has acquired in a recent years, it has come to be considered a central organ of metabolic control. This impression is further reinforced by the immense list of hormones which act on this tissue, whether on metabolism or on hormone production and adipogenesis (Table 2).
Its principal metabolic actions can be divided into: lipogenic actions (biosynthesis, incorporation and storage of TAG) and lipolytic actions (liberation of FFA and glycerol).
For TAG biosynthesis, adipocytes require glycerol-3-phosphate (glycerol-3-P) and FFA esterized with coenzyme A (acylCoA). The first comes via the glycolytic pathway and the second is biosynthesized from acetylCoA or captured from lipoproteins (chylomicron and VLDL).
In order to produce glycerol-3-P, glucose is required, which involves specific transporter proteins, the GLUT proteins (GLUT1 and GLUT4), and is a process controlled by insulin. Thus, the insulin secreted during the prandial period stimulates translocation of GLUT4 to the cell membrane, increasing glucose transport. Furthermore, the rhythm of metabolism of hexose is accelerated by insulin, creating more glycerol-3-P.
A proportion of the metabolites from the glycolytic pathway are diverted to form pyruvate, which, inside the mitochondria, is transformed into acetylCoA by pyruvate dehydrogenase (PDH). This then couples with oxalacetate by citrate synthase (CS), creating citrate. A portion of the site trait returns to the cytoplasm, where it undergoes the action of ATP-citrate lyase (ATP-CL), reconstructing acetylCoA. This, under the action of acetylCoA carboxylase (ACC), is transformed into malonylCoA. This last enters into fatty acid synthesis, catalyzed by fatty acid synthase (FAS), and culminates in the formation of acylCoA, which is esterized with glycerol-3-P to form TAG. This is finally incorporated into the cytoplasm the fat droplet. In order for FAS to act, it requires NADPH2 which is provided via the pentose pathway (parallel to the glycolytic pathway) or by the malic enzyme (ME).
In addition to FFA being synthesized, they are also provided, in greater quantities, by lipoproteins. They undergo the action of LPL within the AT microcirculation. Thus, FFA liberated from particles are captured by adipocytes. Diffusion of FFA through the adipocyte membranes is a process whose capture is by diffusion, facilitated by FFA transporters, including CD36, found in countless biological membranes, where it acts as an acceptor for several types of molecules, including FFA. The CD36 presents the FFA molecule to another protein, FATP (FFA transporter protein), which, like CD36, is an integral membrane protein which facilitates transport to the cell interior. In cytosol, which is aqueous, FFA binds to a different protein, FABP, which transports it to be esterized with coenzyme A. This process is performed by another protein integral to the membrane, acylCoA synthase (ACS). Once this stage is completed, the acylCoA is transported by ACBP (acylCoA binding protein) to glycerol-3-P esterization locations. Triacylglycerols are formed and transferred to the lipid droplet.
Biosynthesis of TAG is not the exclusive domain of adipocytes. This process has been described in other tissues, where accumulation of TAG also leads to the formation of intracytoplasmic droplets. The occurrence of these inclusions in other tissues is abnormal. The accumulation of TAG in cells that are not adipocytes facilitates the formation of ceramides, which activate inducible nitric oxide synthase (iNOS), with consequent formation of NO and induction of apoptosis by means of NFkB activation.22 This is lipotoxicity. The manifestations of lipotoxicity include hepatic and muscular steatosis, which are common in a series of conditions. Pancreatic beta cells are also subject to steatosis, compromising their capacity to secrete insulin, the adipocyte least susceptible to lipotoxicity.
Another important capability of adipocytes is lypolisis of TAG, liberating FFA and glycerol. This process depends on HSL activation. This is by phosphorylation in serine, by means of protein kinase A (PKA). This process is primarily stimulated by catecholamines and occurs during fasting or when energy demand is high, such as during physical exercise and stressful situations, due to intense sympathetic solicitation. Cyclic AMP (AMPc) is thereby generated intracellularly, with consequent activation of PKA, which also acts on perilipins (proteins that surround the fat droplet) in a similar manner to HSL. The phosphorylated perilipins leave the surface of the droplets, disperse through the cytosol and open spaces for HSL to reach their substrate, the TAG. The liberated FFA bond with FABP, are taken to the cell membrane and transported to the extracellular environment by means of FATP. Glycerol is transported to the outside by means of specific transporters, proteins belonging to the aquaglyceroporin family (aquaporin 7 or AQP7) (Figure 1).
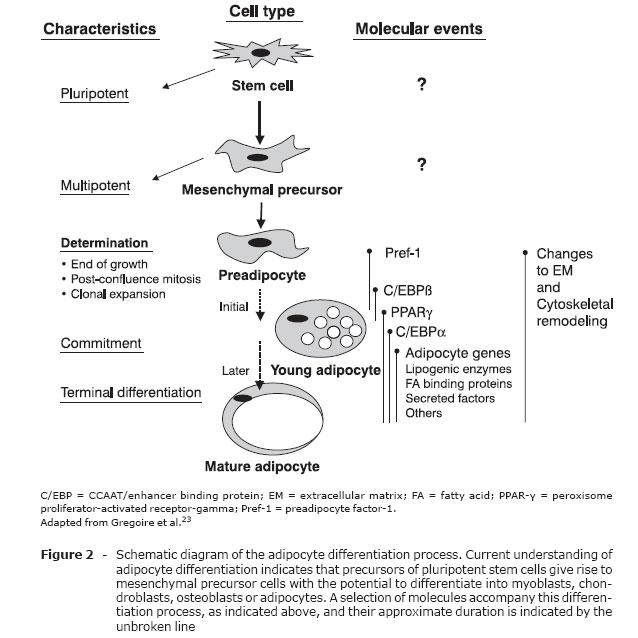
Adipogenesis
Adipose tissue differentiation, known as adipogenesis, has been extensively studied in vitro, with the aim of unmasking the molecular and cellular basis of the development of this tissue and of its involvement in physiological and pathological states, in such a way as to permit the formation of therapeutic and preventative strategies for excess AT (obesity) and also for lack of it (lipodystrophies and lipoatrophies).
Adipogenesis begins before birth. The chronology of the emergence of AT depends on the species and the adipose deposit in question.
After birth, there is rapid expansion of adipose tissue, as a result of the increasing size and number of cells. The potential to generate new adipocytes remains even in adulthood. Dietary factors influence this process, since rats fed a diet rich in carbohydrate or fats will exhibit adipocyte hyperplasia. The differentiation of preadipocytes into adipocytes is in an extremely controlled process. Adipogenic transcription factors, including PPARy, sterol regulatory element binding protein 1c (SREBP-1c) and C/EBP, carry out a key role in the complex transcriptional cascade of adipogenesis. Hormonal and nutritional signals affect adipocyte differentiation positively or negatively, and the components involved in cell-cell interaction or cellular matrix are also important to regulation of the process.23
Preadipocytes are a cell line derived from multipotent embryonic stem cells of mesodermal origin and with the capacity to differentiate into adipocytes, chondrocytes, osteoblasts or myocytes (Figure 2).
The SREBP protein is a transcription factor that was originally cloned from rat AT and which has the following characteristics: it is a basic helix-loop-helix (bHLH) type, it contains a leucine "zipper" with an important role in adipogenesis, insulin sensitivity and fatty acid homeostasis.24 The SREBP family has two members: SREBP-1 and SREBP-2. There are two isoforms of SREBP-1 (SREBP-1a and SREBP-1c), derived from alternative splicing of the first exon within the same primary transcription. Adipocyte determination- and differentiation-dependent factor (ADD1) is homologous to isoform SREBP-1c from humans.25 Expression of SREBP-1c/ADD1 is predominantly in the liver, adrenal gland, AT and skeletal muscle, while SREBP-1a is expressed in the spleen. The SREBP-2 protein regulates biosynthesis of cholesterol. In vitro, ADD1/SREBP-1c increases the transcriptional activity of PPARy, raising the proportion of cells in the process of differentiation.
PPARy is part of a superfamily of nuclear receptors. It is highly expressed in AT and stimulates transcription of many specific adipocyte genes, and also the critical initial steps of adipogenesis.26 There are two isoforms of PPARy (PPARy-1 and -2) generated by distinct promoter regions and alternative splicing mechanisms. PPARy-1 is highly expressed in AT and several other cells in lower proportions (macrophages, pneumocytes, epithelium of the colon, etc.) PPARy-2 is exclusively found in AT. It has 30 amino acids more than PPARy-1 in its N-terminal region. PPARy is activated by compounds known as TZD, which are used as antidiabetic agents.
The C/EBP are part of the b-zip family (basic DNA bonding domain), which contains the leucine zipper necessary for dimerization. The isoforms of C/EBP (a, b and Î' ) are highly expressed in adipocytes and are induced during adipogenesis. C/EBPa has an important role in differentiation of preadipocytes into adipocytes. C/EBPb also induces adipogenesis, possibly by stimulating expression of PPARy, whose gene contains sites for C/EBP in its promoter region. It has been demonstrated that PPARy acts synergetically with C/EBPa to promote adipogenesis27 or induce differentiation of fibroblasts into adipocytes.26 C/EBPa and PPARy bind with the promoter region and activate genes specific to WAT, such as the fatty acid binding protein aP2 and phosphoenolpyruvate carboxykinase (PEPCK).
It is known that aging is a process characterized by functional decline in many processes, including adipogenesis.28 Molecular studies have shown that expression of C/EBPa was substantially reduced in preadipocytes in the process of differentiation as age advanced. This drop, in function of age, affected several adipose groupings (periepididymal, inguinal and perirenal).
In addition to differentiation, changes in the expression of transcription factors also influence the metabolic functions of adipocytes. A reduction in cell size and lower expression of C/EBP prejudice glucose tolerance by compromising expression of GLUT4, among other mechanisms.
Final comments
Evidently, the advances made during recent years in research into the biology of adipose tissue have changed the understanding of its true role within the body, making an in-depth conceptual review imperative. It is also clear that obesity, associated or not with diabetes mellitus, systemic arterial hypertension or the metabolic syndrome, is a high risk factor for potentially lethal atherosclerotic lesions. Given the range of functional abilities and the immense arsenal of mechanisms that adipose tissue has at its disposal to correct metabolic disorders, it is important that consideration be given to the possibility of utilizing these abilities in health promotion and in prevention or treatment of disease. A better understanding of the potential of adipose tissue would, without doubt, make it a great ally in the fight against, not just the afflictions mentioned above, but against many others too, and it could become a major internal agent of health promotion.
References
- 1. Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327-332.
- 2. Pénicaud L, Cousin B, Leloup C, Lorsignol A, Casteilla L. The autonomic nervous system, adipose tissue plasticity, and energy balance. Nutrition. 2000;16:903-8.
- 3. Kreier F, Fliers E, Voshol PJ, Eden CG, Havekes LM, Kalsbeek A, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat - functional implications. J Clin Invest. 2002:110:1243-50.
- 4. Pond C. Ecology of storage and allocation of resources: animals. In: Encyclopedia of Life Sciences. Chichester, UK: John Wiley & Sons; 2001. p. 1-5.
- 5. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277-359.
- 6. Curi R, Pompéia C, Miyasaka CK, Procópio J. Entendendo a gordura: os ácidos graxos. São Paulo: Manole; 2002.
- 7. Fruhbeck G, Gómez-Ambrosi J, Muruzabal J, Burrel MA. The adipocyte: a model for integration of endocrine and metabolic signalling in energy metabolism regulation. Am J Physiol Endocrinol Metabol. 2001;280:E827-47.
- 8. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-32.
- 9. Frübeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7-20.
- 10. Schwartz MW, Woods SC, Porter D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661-71.
- 11. Sethi JK, Hotamisligil GS. The role of TNF alpha in adipocyte metabolism. Semin Cell Dev Biol. 1999;10:19-29.
- 12. Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med. 1995;27:435-8.
- 13. Park KG, Park KS, Kim MJ, Kim HS, Suh YS, Ahn JD, et al. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res Clin Pract. 2004;63:135-42.
- 14. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerje RR, Wright CM, al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-12.
- 15. McTernan PG, McTernan CL, Chetty R, Jenner K, Fisher FM, Lauer MN, et al. Increased resistin gene and protein expression in human abdominal adipose tissue. J Clin Endocrinol Metab. 2002;87:2407-10.
- 16. Lyon CJ, Law RE, Hsueh W. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195-200.
- 17. Wajchenberg B L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697-738.
- 18. Rosenbaum M, Pietrobelli A, Vasseli JR, Heymsfield SB, Leibel RL. Sexual dimorphism in circulating leptin concentration is not accounted for by differences in adipose tissue distribution. Int J Obes Relat Metab Disord. 2001;25:1365-71.
- 19. Arner P, Hellström L, Wahrenberg H, Brönnegard M. Beta-adrenoceptor expression in human fat cells from different regions. J Clin Invest. 1990;86:1595-600.
- 20. Wajchenberg BL, Gianella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616-21.
- 21. Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276-83.
- 22. Unger RH, Zhou YT, Orci L. Regulation of fatty acid homeostasis in cells: novel role of leptin. Proc Natl Acad Sci USA. 1999;96:2327-32.
- 23. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783-809.
- 24. Osborne TF. Sterol regulatory element- binding proteins (SREBPS): key regulations of nutritional homeostasis and insulin action. J Biol Chem. 2000;275:32379-82.
- 25. Tontonoz P, Kim JB, Graves RA, Spiegelman BM. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol. 1993;13:4753-59.
- 26. Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev. 1995;5:571-6.
- 27. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147-56.
- 28. Kirkland JL, Hollenberg CH, Kindler S, Gillon WS. Effects of age and anatomic site on preadipocyte number in rat fat depots. J Gerontol. 1994;49:B31-35.
Publication Dates
-
Publication in this collection
17 Dec 2007 -
Date of issue
Nov 2007