Abstracts
OBJECTIVE: To evaluate growth and body composition of patients with the salt wasting form of classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency and to compare them with healthy children. METHODS: Twenty-one prepubertal patients (eight boys and 13 girls) between 2.1 and 10.2 years and 67 prepubertal healthy controls (36 boys and 31 girls) between 1.2 and 11.7 years were included. Weight, height, upper-arm circumference, skinfolds, body composition determined by bioimpedance, and bone age were measured. The following data were obtained from the medical records: parents' height, serum levels of 17-hydroxyprogesterone and Δ4-androstenedione, prescribed hydrocortisone doses, weight and length at birth, in the beginning of the treatment, and at 2 years. RESULTS: Patients had lower weight and length z scores at the first appointment compared with the same data at birth, showing recovery after the beginning of the treatment without advanced bone age. Mean height z score was higher in controls (0.28±0.86) than in patients (-0.61±0.99, p < 0.001); this difference disappeared when the patients' height was adjusted to their bone age (0.33±1.68, p = 0.912). Patients had higher body mass index (p < 0.001), fat mass (p < 0.001), and fat mass index (p < 0.001) than controls. There was no difference in the skinfolds between the two groups (p = 0.157). CONCLUSIONS: Patients had growth recovery with mean height similar to the general population; however, they had higher body fat, which seems to be visceral, since there was no difference between the skinfolds of both groups.
Congenital adrenal hyperplasia; adrenal; body composition; growth; child
OBJETIVO: Avaliar crescimento e composição corporal de portadores da forma clássica perdedora de sal da hiperplasia adrenal congênita por deficiência da 21-hidroxilase, comparando-os com crianças saudáveis. MÉTODOS: Foram incluídos 21 pacientes (oito meninos e 13 meninas), entre 2,1 e 10,2 anos, e 67 controles pré-púberes (36 meninos e 31 meninas), entre 1,2 e 11,7 anos. Avaliou-se peso, estatura, perímetro braquial, dobras cutâneas, composição corporal por bioimpedância e idade óssea. Foram obtidas dos prontuários dos pacientes as seguintes informações: estatura dos pais, valores de 17-OH progesterona e Δ4-androstenediona, dose de hidrocortisona prescrita, dados de peso e estatura ao nascimento, no início do tratamento e aos 2 anos de idade. RESULTADOS: Os pacientes apresentaram menor escore z de peso e de altura na primeira consulta em relação à situação de nascimento, com posterior recuperação após o início do tratamento, sem apresentar avanço da idade óssea. A média do escore z da altura dos controles (0,28±0,86) foi maior que a dos casos (-0,61±0,99, p < 0,001). Essa diferença desaparece quando se ajusta a altura dos pacientes para a idade óssea (0,33±1,68, p = 0,912). Os pacientes apresentaram maiores índices de massa corporal (p < 0,001), massa gorda (p < 0,001) e índice de massa gorda (p < 0,001) do que os controles. Não houve diferença entre as dobras cutâneas dos 2 grupos (p = 0,157). CONCLUSÕES: Os pacientes apresentaram recuperação do crescimento com média de estatura semelhante à da população geral, porém com maior adiposidade corporal, que parece ser visceral, já que não houve diferença entre as dobras cutâneas.
Hiperplasia supra-renal congênita; glândulas adrenais; composição corporal; crescimento; criança
ORIGINAL ARTICLE
IMestre, Saúde da Criança e do Adolescente, Faculdade de Ciências Médicas (FCM), Universidade Estadual de Campinas (UNICAMP), Campinas, SP, Brazil
IIDoutora, Pediatria, Unidade de Endocrinologia Pediátrica, Departamento de Pediatria, FCM, UNICAMP, Campinas, SP, Brazil
IIIDoutora, Ciências Médicas, Disciplina de Endocrinologia, Departamento de Clínica Médica, FCM, UNICAMP, Campinas, SP, Brazil
IVLivre-Docente, Pediatria, Unidade de Endocrinologia Pediátrica, Departamento de Pediatria, FCM, UNICAMP, Campinas, SP, Brazil
VLivre-Docente, Centro de Biologia Molecular e Engenharia Genética (CBMeG), UNICAMP, Campinas, SP, Brazil
VIProfessor associado, Pediatria, Unidade de Endocrinologia Pediátrica, Departamento de Pediatria, FCM, UNICAMP, Campinas, SP, Brazil
Correspondence
ABSTRACT
OBJECTIVE: To evaluate growth and body composition of patients with the salt wasting form of classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency and to compare them with healthy children.
METHODS: Twenty-one prepubertal patients (eight boys and 13 girls) between 2.1 and 10.2 years and 67 prepubertal healthy controls (36 boys and 31 girls) between 1.2 and 11.7 years were included. Weight, height, upper-arm circumference, skinfolds, body composition determined by bioimpedance, and bone age were measured. The following data were obtained from the medical records: parents' height, serum levels of 17-hydroxyprogesterone and Δ4-androstenedione, prescribed hydrocortisone doses, weight and length at birth, in the beginning of the treatment, and at 2 years.
RESULTS: Patients had lower weight and length z scores at the first appointment compared with the same data at birth, showing recovery after the beginning of the treatment without advanced bone age. Mean height z score was higher in controls (0.28±0.86) than in patients (-0.61±0.99, p < 0.001); this difference disappeared when the patients' height was adjusted to their bone age (0.33±1.68, p = 0.912). Patients had higher body mass index (p < 0.001), fat mass (p < 0.001), and fat mass index (p < 0.001) than controls. There was no difference in the skinfolds between the two groups (p = 0.157).
CONCLUSIONS: Patients had growth recovery with mean height similar to the general population; however, they had higher body fat, which seems to be visceral, since there was no difference between the skinfolds of both groups.
Keywords: Congenital adrenal hyperplasia, adrenal glands, body composition, growth, child.
Introduction
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency (21-OHD CAH) is an autosomal recessive disease. It is characterized by a reduction of the synthesis of glucocorticoids and an increase in the production of androgens. The incidence of the classic form ranges from 1:12,000 to 1:15,000 births, with the salt-wasting form1 being responsible for 75% of the cases.
Treatment consists of replacement of glucocorticoids and, whenever necessary, mineralocorticoid.1,2 The major difficulty posed by this treatment is the administration of physiological doses that can promote adequate growth and allow final height as close as possible to normal height.3,4
The use of supraphysiological doses of glucocorticoids causes a decrease in growth, whilst the use of insufficient doses does not block the excessive production of sexual steroids, resulting in bone age advancement.1,2 The excess of androgens can also change body composition because of an increase in muscle mass,4 whilst the use of high doses of glucocorticoids can lead to obesity and increased body mass index (BMI).5-9
Several studies have evaluated the final height of patients with 21-CAH and most of them have shown loss of height, with final height below the parental target.3,10-13
In addition to growth and final height, some studies have evaluated body composition based on BMI4,6,7 and dual energy x-ray absorptiometry (DEXA),4 and their results showed an increase in the fat mass of these patients.4,6,7
The objective of the present study was to investigate the growth and body composition of prepubertal patients with salt-wasting 21-OHD CAH, comparing them with a control group, and to evaluate in which period of life height recovery occurred.
Patients and methods
This is a cross-sectional, retrospective, descriptive and analytic study conducted with prepubertal patients (Tanner stage I) with salt-wasting classic form of 21-OHD CAH who were being followed up at the outpatient clinic of pediatric endocrinology of the Hospital das Clínicas/Universidade Estadual de Campinas (UNICAMP). The diagnosis was established based on the serum levels of 17-hydroxyprogesterone (17OH) using solid phase radioimmunoassay (kit manufacturer: Diagnostic System Laboratories INC/USA - DSL) and confirmed by molecular study.14,15
With the purpose of having a more homogeneous group of patients and allowing enough time to evaluate the effect of the treatment, inclusion criteria were as follows: beginning of follow-up until the age of 4 months and follow-up for at least 18 months. Twenty-one out of the thirty-four patients being monitored met these criteria. Six patients were excluded because of the age at the beginning of follow-up and seven for insufficient follow-up time. Hormonal replacement was always done with hydrocortisone acetate at three daily oral doses and fludrocortisone at one or two daily doses. The whole treatment period was supervised by only one physician (SHVLM). During the patients' follow-up at least four yearly appointments were conducted to evaluate the anthropometric parameters (weight and height), to determine the values of 17OH and Δ4-androstenedione (Δ4) - solid phase radioimmunoassay, using the DSL kit, and to measure bone age.
Data on parents' height, values of 17-OH and Δ4, dose of hydrocortisone prescribed, weight and height at birth, in the beginning of the treatment, and at the age of 2 years were obtained from medical records.
The anthropometric evaluation representing the age of 2 years was consistent with the weight and height measured at the closest appointment to the second birthday date, ranging from 20.4 to 25.4, with a mean value of 23.9 (standard deviation [SD] = 1.3) months.
Mean hydrocortisone doses were determined. We used the medians for the values of 17OH and Δ4 because of the difficulties with the values of lower (< 0.01 ng/mL) and upper (> 20 or > 50 ng/mL) thresholds of some results.
The control group comprised 67 healthy prepubertal children selected among patients' siblings and friends and hospital employees' children.
Weight, height, upper-arm circumference, (biceps, triceps, subscapular and suprailiac) skinfolds, and bone age were measured in the last appointment. Based on these measures, BMI, arm muscle area and arm fat area were calculated.16 During the same appointment, body composition was evaluated using bioimpedance (BIA 101-Q RJL Systems, Detroit, USA), and lean mass was determined.17 Fat mass was obtained subtracting the lean mass from the total weight. Fat mass index (FMI) was determined as the quotient between fat mass and height squared to better evaluate body composition. Lean mass index was calculated using the same formula. Patients were classified as obese (> 95th percentiles), overweight (between 85th and 95th percentiles), or normal according to their BMI.
Bone age was evaluated by only one observer using the TW20 method based on a wrist X ray taken on the closest date to the anthropometric evaluation.18
Midparental target height was calculated as suggested by Tanner19: (father's height + mother's height)/2±6.5 cm, for boys and girls, respectively. The mean value of the lower and upper thresholds was ±8.5 cm.
Weight, height, arm muscle area, and arm fat area z scores were determined. Data from CDC 2000 were used to calculate weight and height z scores and BMI percentiles.20 Data provided by Marcondes21 were used to determine birth weight and height z scores, and Frisancho's16 data were used to calculate arm muscle area and arm fat area.
Chi-square test or Fisher's exact test was employed for the qualitative variable analysis. Student's t test or Mann-Whitney's test was employed for the evaluation of two means. Repeated measures analysis of variance and Bonferroni test were used in the comparison of weight and height in the beginning of the treatment, at 2 years, and at the end of the study. Stepwise multiple linear regression was employed for the multivariate analysis. The significance level was set at 5%.
Data were processed using SPSS 16.0 (SPSS Inc, Chicago, USA), which automatically produced the graphs of the study.
The participants and their guardians signed a written consent form before the beginning of the study. The study protocol was approved by the Research Ethics Committee of the School of Medicine of Universidade Estadual de Campinas (UNICAMP).
Results
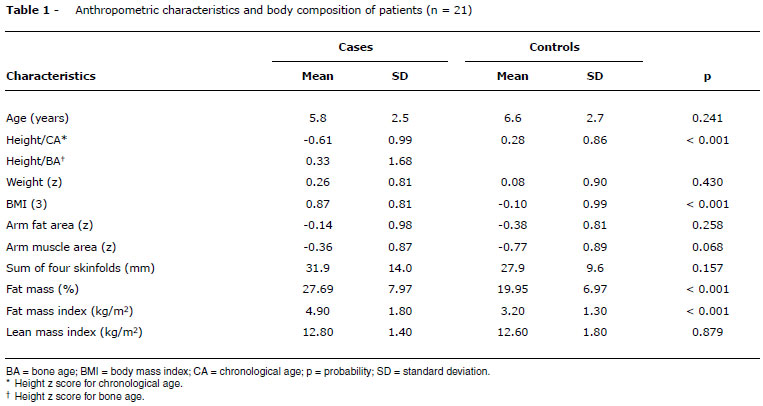
Twenty-one patients (eight boys and 13 girls) were assessed. Their mean age was 5.8 years (minimum-maximum [min-max] = 2.1 to 10.2), mean follow-up was 5.7 years (min-max = 1.9 to 9.9), and mean age at first appointment was 36.7 days (min-max = 3 to 123) (Table 1).
At the time of evaluation, there was no difference between age, sex, mother's educational level, and per capita family income of patients and controls.
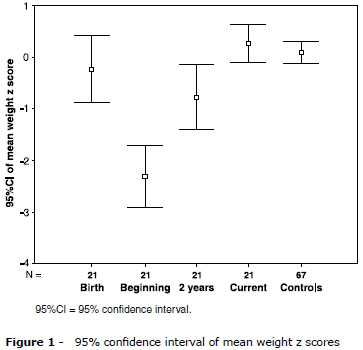
Mean weight z scores at birth was -0.23 (SD = 1.4), -2.31 (SD = 1.3) in the beginning of the treatment, -0.77 (SD = 1.3) at 2 years, and 0.26 (SD = 0.8) at the end of the study (Figure 1). There was a significant difference when all periods were compared (p < 0.001). Mean weight z scores of controls was 0.08 (SD = 0.90), with no difference between the two groups (p = 0.430) (Table 1).
Mean height z scores at birth was -0.69 (SD = 2.30), -1.87 (SD = 1.7) in the beginning of the treatment, -1.07 (SD = 1.0) at 2 years, and -0.61 (DP = 0.99) at the end of the study. Mean height z scores of controls was 0.28 (SD = 0.86), which was higher than the patients' mean at the end of the study (p < 0.001) (Figure 2 and Table 1).
Patients' mean bone age was 5.3 years (min-max = 0.9 to 12.1) and the mean of the differences between bone age and chronological age was -0.56 years (min-max = -3.5 to 3.4).
Patients' mean height z score corrected for bone age was 0.33 (SD = 1.6), and there was no difference in the comparison with the controls' height regarding their chronological age (p = 0.912) (Figure 2 and Table 1).
Five patients (one boy and four girls) had delayed bone age higher than 2 years compared with their chronological age, and only one girl, whose height z score was within the parental target range, had advanced bone age higher than 2 years.
In only two patients (one boy and one girl) the height was lower than the lower threshold of the parental target; 15 patients (six boys and nine girls) showed adequate height; and in four patients (one boy and three girls) the height was higher than the upper threshold without advanced bone age.
Cases and controls had no difference in terms of arm fat area, arm muscle area, sum of four skinfolds, and lean mass index (Table 1). Patients had higher BMI than controls (z = 0.87 vs. z = -0.10), as well as higher fat mass (27.69 vs. 19.95%), and this difference was also found after adjusting the fat mass for height (4.9 vs. 3.2) (Table 1).
Five patients (23.8%) were obese and five (23.8%) were overweight. None of the controls was obese and nine (13.8%) were overweight (p < 0.001).
The mean prescribed hydrocortisone doses were 17.8 (SD = 1.4, min-max = 12.9 to 23.4) mg/m2BS/day. The mean value of 17OH was 1.8 (SD = 3.19, min-max < 0.1 to 41.57) ng/mL (nL 0.07 to 1.53) and mean Δ4 was 0.13 (SD = 0.04, min-max < 0.1 to 3.07) ng/mL (nL male < 0.53, female < 0.37).
We used multiple linear regression to determine the models that best adjusted to the difference (delta) between the weight and height z scores at the end and in the beginning of the treatment. The independent variables assessed were sex, birth weight, family income, mother's educational level, follow-up duration, bone age, hydrocortisone dose, and serum levels of 17OH and Δ4. The model adjusted to the difference of height z scores included the variables hydrocortisone dose and serum level of 17OH (R2 = 47.8%). Higher hydrocortisone doses and lower values of 17OH were associated with lower height delta.
The model adjusted to the difference between weight z scores included birth weight and serum level of 17OH during follow-up (R2 = 36.4%). We found higher weight gain in patients with lower birth weight and higher values of 17OH. There was no association between the hydrocortisone doses and weight delta.
We used multiple linear regression to try to determined the models that best adjusted to the current anthropometric variables: height and weight z scores, BMI, arm fat area, arm muscle area, sum of four skinfolds, fat mass and lean mass, fat mass index and lean mass index. The following independent variables were assessed: age, sex, mother's educational level, family income, and case (case = 1; control = 0). There was difference between the anthropometric indicators of cases and controls in the models adjusted for height z scores (R2 = 18.1%), BMI z scores (R2 = 15.6%), fat mass (R2 = 36.8%), fat mass index (R2 = 34.0%): patients had lower height, while BMI z scores, fat mass z scores, and fat mass index were higher in patients.
Discussion
Most authors who studied patients with the classic form of 21-CAH, who were treated with glucocorticoids, found final height lower than expected3,10-13,22 and increased fat mass5-9,22 when compared with the general population, control groups, or parental target.
In the present sample, we found a more significant impairment of body composition than height, since the patients' mean height was within the normal range and our patients showed increased BMI and fat mass compared with controls.
In a previous study with the same group of patients, we demonstrated progressive nutritional recovery after the beginning of the treatment and, at 2 years, weight and height were within normal limits, though below the mean for the general population.23 Height recovery was also demonstrated in the present study, and there was no difference between patients and controls after adjusting height for bone age. Most children did not have advanced bone maturation; therefore, we may conclude that this height gain was the result of efficient disease control.
Whereas a previous study conducted in the same hospital showed impairment of the final height of patients with the classic form of 21-CAH,13 we are not sure whether the patients in this group will be able to maintain adequate growth, since there may be height loss during puberty, a period when adherence to treatment of chronic diseases tends to be lower. It should be taken into account, however, that the previously studied group was more heterogeneous: some patients received late diagnosis, several had been treated with prednisone or dexamethasone, which have a greater growth blocking effect than hydrocortisone, in addition to the fact that their diagnosis and treatment were established in various departments or under the supervision of different professionals. The fact that the current group of patients has a more homogeneous follow-up since an early age and hormone replacement therapy is being performed with hydrocortisone in all patients should contribute to a better result.
Genetical factors are also important to determine the final height of patients with 21-CAH. Thus, parental target should be taken into consideration while assessing growth.22 Most of the cases studied have adequate height or their height is above the parental target.
Similarly to the study by Stikkelbroek et al.8, we also did not find a difference in the mean weight of patients in comparison with the control group.
In agreement with most studies, in the present study, there was increased BMI in patients with 21-CAH.5,6,8,9. Whether the increased BMI found in patients with 21-CAH is a result of the increased fat mass, which could be caused by excess of glucocorticoids, or increased lean mass, which could occur because of excess of androgens,8 has not been well established yet.
Cornean et al.5 found increased BMI z score in 22 prepubertal children with 21-CAH at 5 and 10 years compared with the first year of life. These patients had increased BMI because they gained weight, since their height z score remained constant. Such weight gain was caused by increased fat mass because there was a significant increase in the skinfold z score at 5.5 years when compared with the skinfold z score measured at 2.5 years. In the present study, there was no difference in the skinfolds and the arm fat area between patients and controls. Patients had increased fat mass when assessed using bioimpedance, a method that is considered a good indicator of body fat,24 and the difference persisted after we adjusted fat mass for height (FMI).
The mean prescribed hydrocortisone dose was higher than the physiological dose.25 Although we did not find an association between the hydrocortisone dose and weight gain in our patients, when we compared our data with those provided by Völkl et al.9, a study where only one patient had been treated with inappropriate high doses of glucocorticoids, we found that our proportion of obese patients was larger (16.8 and 23.8%, respectively). On the other hand, we also found that those patients who received higher hydrocortisone doses and those who had lower values of 17OH achieved poorer height recovery. This finding supports the hypothesis that the lowest possible dose of glucocorticoid should be used to avoid growth impairment and that low values of 17OH may suggest excessive use of medication. Our findings are similar to those by Stikkelbroeck et al.8, who found higher fat mass in their patients: these authors demonstrated that patients with 21-CAH are at higher risk for obesity and that glucocorticoids, even at replacement doses, may lead to weight gain. Arguments against the idea that obesity is only related to the dose of glucocorticoid are the fact that Völkl et al.9 found increased obesity rate in patients with 21-CAH who did not use glucocorticoid doses above the recommended dose and the fact that we did not find, in the present study, an association between hydrocortisone dose and patients' weight. However, it should be taken into consideration that often the dose of glucocorticoid prescribed does not correspond to the real dose received by the patients. Our patients are instructed to increase the hydrocortisone dose in stressful situations and, mainly during the first years of live, when the frequency of infectious processes with fever is high, which may have caused the use of higher doses of glucocorticoid than expected. Another factor that should be considered is that if there is no appropriate adherence to treatment, the dose received by the patient may be lower than the dose reported.
Although these patients have higher fat mass than controls, both in terms of absolute values and in relation to height, we could not find a difference between arm fat area, arm muscle area, and mean sum of skinfolds, which is not in agreement with the findings of other authors.5,26
Wajchenberg27emonstrated that hypercortisolemic subjects had increased total body fat and, especially increased subcutaneous and visceral abdominal fat. While comparing hypercortisolemic subjects with obese individuals, this author also found increased visceral fat, but not subcutaneous fat in the hypercortisolemic subjects. Fraser et al.28suggest that even a small chronic excess of cortisol may lead to central fat deposition. Our findings suggest that increased BMI is caused by increased fat mass and that this fat may not be peripheral, since there is no difference in the arm fat area or in the skinfolds. This fat should thus be visceral.
There is evidence that the diseases related to obesity may have their onset during childhood. Increased visceral fat is a risk factor for the development of the metabolic syndrome27,28 and the adverse effects caused by the increase in the intra-abdominal fat include insulin resistance, type 2 diabetes, dyslipidemia, and cardiovascular disease.29-31
We showed a higher impairment of the body composition, since the patients' height was not different after being adjusted for bone age.
The group of patients investigated showed increased BMI and higher fat mass when compared with controls, and this fat seems to be visceral.
Therefore, we recommend careful monitoring of weight gain and its comorbidities during the follow-up of patients with 21-CAH. The body composition of these patients should also be further investigated.
References
- 1. Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133-60.
- 2. Forest MG. Recent advances in the diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Reprod Update. 2004;10:469-85.
- 3. Eugster EA, Dimeglio LA, Wright JC, Freidenberg GR, Seshadri R, Pescovitz OH. Height outcome in congenital adrenal hyperplasia caused by 21-hydroxylase deficiency: a meta-analysis. J Pediatr. 2001;138:26-32.
- 4. Cameron FJ, Kaymakci B, Byrt EA, Ebeling PR, Warne GL, Walk JD. Bone mineral density and body composition in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1995;80:2238-43.
- 5. Cornean RE, Hindmarsh PC, Brook CG. Obesity in 21-hydroxylase deficient patients. Arch Dis Child. 1998;78:261-3.
- 6. Hagenfeldt K, Martin Ritzén E, Ringertz H, Helleday J, Carlström K. Bone mass and body composition of adult women with congenital virilizing 21-hydroxylase deficiency after glucocorticoid treatment since infancy. Eur J Endocrinol. 2000;143:667-71.
- 7. Manoli I, Kanaka-Gantenbein Ch, Voutetakis A, Maniati-Christidi M, Dacou-Voutetakis A. Early growth, pubertal development, body mass index and final height of patients with congenital adrenal hyperplasia: factors influencing the outcome. Clin Endocrinol (Oxf). 2002;57:669-76.
- 8. Stikkelbroeck NM, Oyen WJ, van der Wilt GJ, Hermus AR, Otten BJ. Normal bone mineral density and lean body mass, but increased fat mass, in young adult patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2003;88:1036-42.
- 9. Völkl TM, Simm D, Beier C, Dörr HG. Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 2006;117:e98-105.
- 10. Muthusamy K, Elamin MB, Smushkin G, Murad MH, Lampropulos JF, Elamin KB, et al. Clinical review: Adult height in patients with congenital adrenal hyperplasia: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2010;95:4161-72.
- 11. Hargitai G, Sólyom J, Battelino T, Lebl J, Pribilincová Z, Hauspie R, et al. Growth patterns and final height in congenital adrenal hyperplasia due to classical 21-hydroxylase deficiency. Results of a multicenter study. Horm Res. 2001;55:161-71.
- 12. Van der Kamp HJ, Otten BJ, Buitenweg N, De Munick Keiser-Schrama SM, Oostdijk W, Jansen M, et al. Longitudinal analysis of growth and puberty in 21-hydroxylase deficiency patients. Arch Dis Child. 2002;87:139-44.
- 13. Lemos-Marini SH, Guerra-Júnior G, Morcillo AM, Baptista MT, Silva LO, Maciel-Guerra AT. Hiperplasia congênita das supra-renais por deficiência da 21-hidroxilase: altura final de 27 pacientes com a forma clássica. Arq Bras Endocrinol Metab. 2005;49:902-7.
- 14. Paulino LC, Araujo M, Guerra G Jr, Marini SH, De Mello MP. Mutation distribution and CYP21/C4 locus variability in Brazilian families with the classical form of the 21-hydroxylase deficiency. Acta Paediatr. 1999;88:275-83.
- 15. Soardi FC, Barbaro M, Lau IF, Lemos-Marini SH, Baptista MT, Guerra-Junior G, et al. Inhibition of CYP21A2 enzyme activity caused by novel missense mutations identified in Brazilian and Scandinavian patients. J Clin Endocrinol Metab. 2008;93:2416-20.
- 16. Frisancho AR. Anthropometric standards. In: Frisancho AR. Anthropometric standards for the assessment of growth and nutritional status. Michigan: The University of Michigan Press; 1993. p.37-118.
- 17. Goran MI, Kaskoun MC, Carpenter WH, Poehlman ET, Ravussin E, Fontvieille AM. Estimating body composition of young children by using bioelectrical resistance. J Appl Physiol. 1993;75:1776-80.
- 18. Tanner JM, Whitehouse RH, Cameron N, Marshall WA, Healy MJ, Goldstein H. The rating system. In: Tanner JM, Whitehouse RH, Cameron N, Marshall WA, Healy MJ, Goldstein H. Assessment of skeletal maturity and prediction of adult height (TW2 Method). 2nd ed. London: Academic Press; 1983. p.50-103.
- 19. Tanner JM, Goldstein H, Whitehouse RH. Standards for children's height at ages 2-9 years allowing for heights of parents. Arch Dis Child. 1970;45:755-62.
- 20. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1-27.
- 21. Marcondes E. Curvas de crescimento e normalidade do crescimento. In: Marcondes E. Desenvolvimento da criança: desenvolvimento biológico, crescimento. Rio de Janeiro: Sociedade Brasileira de Pediatria; 1994. p.39-56.
- 22. Yu AC, Grant DB. Adult height in women with early-treated congenital adrenal hyperplasia (21-hydroxylase type): relation to body mass index in earlier childhood. Acta Paediatr. 1995;84:899-903.
- 23. Mendes-dos-Santos CT, Lemos-Marini SH, Baptista MT, Guerra-Junior G, De-Mello MP, Morcillo AM. Crescimento de pacientes com hiperplasia congênita das supra-renais, forma perdedora de sal, nos primeiros dois anos de vida. Rev Bras Saude Matern Infant. 2009;9:415-21.
- 24. Fernandes RA, Rosa CS, Buonani C, Oliveira AR, Freitas Júnior IF. The use of bioelectrical impedance to detect excess visceral and subcutaneous fat. J Pediatr (Rio J). 2007;83:529-34.
- 25. Linder BL, Esteban NV, Yergey AL, Winterer JC, Loriaux DL, Cassorla F. Cortisol production rate in childhood and adolescence. J Pediatr. 1990;117:892-6.
- 26. Isguven P, Arslanoglu I, Mesutoglu N, Yildiz M, Erguven M. Bioelectrical impedance analysis of body fatness in childhood congenital adrenal hyperplasia and its metabolic correlates. Eur J Pediatr. 2008;167:1263-8.
- 27. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697-738.
- 28. Fraser R, Ingram MC, Anderson NH, Morrison C, Davies E, Connell JM. Cortisol effects on body mass, blood pressure, and cholesterol in general population. Hypertension. 1999;33:1364-8.
- 29. Goran MI, Gower BA. Relation between visceral fat and disease risk in children and adolescents. Am J Clin Nutr. 1999;70:149S-56S.
- 30. Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367-73.
- 31. Madeira IR, Carvalho CN, Gazolla FM, Pinto LW, Borges MA, Bordallo MA. Impact of obesity on metabolic syndrome components and adipokines in prepubertal children. J Pediatr (Rio J). 2009;85:261-8.
Normalization of height and excess body fat in children with salt-wasting 21-hydroxylase deficiency
Publication Dates
-
Publication in this collection
27 Sept 2011 -
Date of issue
June 2011
History
-
Accepted
16 Feb 2011 -
Received
25 Dec 2010