Abstracts
Multidrug-resistant tuberculosis (MDR-TB) is a severe and feared problem, that is difficult to control and has shown a tendency to increase worldwide. OBJECTIVE: To analyze the risk factors for acquired MDR-TB. CASUISTIC AND METHODS: A retrospective population-based case-control study was conducted. A bacillus was considered multidrug-resistant whenever it was resistant at least to rifampin (RFP) + isoniazid (INH), and a case was considered as sensitive tuberculosis (TB) if it had undergone the first treatment during a similar period as the first treatment of an MDR-TB case, but was cured at the time of the interview. Case selection was made based on the list of Sensitivity Tests (ST) performed at the Central Public Health Laboratory of the State of Ceará, from 1990 through 1999. The Proportion Method was used to investigate resistance to the six antituberculosis drugs (isoniazid, rifampin, pyrazinamide, ethambutol, ethionamide, streptomycin) used as the standard treatment in Brazil. Controls were selected from the registry of the TB Control Program. Univariate and multivariate analysis were performed, with p < 0.05 considered significant. RESULTS: Out of the 1,500 STs performed during the studied period, 266 strains were multidrug-resistant; 153 patients were identified, 19 of which were excluded. The Group of Cases comprised 134 patients, and the Group of Controls comprised 185. Multivariate analysis helped to detect the following risk factors: lack of home sewer system, alcoholism + smoking, number of previous treatments, irregular treatment, and lung cavities. CONCLUSION: These five factors are important for the development of acquired MDR-TB, and an attempt to neutralize them might contribute to control TB.
Multidrug-resistant tuberculosis; Risk factors
A tuberculose multirresistente (TBMR) é um problema grave, temido, de difícil controle e vem apresentando tendência crescente em todo o mundo. OBJETIVO: Fazer uma análise de fatores de risco para TBMR adquirida. CASUÍSTICA E MÉTODOS: Foi feito um estudo caso-controle de base populacional de modo retrospectivo. Foi considerado multirresistente o bacilo resistente a pelo menos rifampicina (RFP) + isoniazida (INH) e tuberculose (TB) sensível o caso que tivesse feito o primeiro tratamento num período semelhante ao do primeiro tratamento do caso com TBMR, mas que estivesse curado no momento da entrevista. A seleção dos casos foi feita tomando como base a lista de testes de sensibilidade (TS) realizados no Laboratório Central de Saúde Pública do Estado do Ceará, no período de 1990 a 1999. Foi utilizado o método das proporções para pesquisar a resistência às seis drogas (isoniazida, rifampicina, pirazinamida, etambutol, etionamida, estreptomicina) antituberculose padronizadas no Brasil. Os controles foram selecionados do livro de registro do Programa de Controle da TB. Foi realizada análise univariada e multivariada e considerado significante um p < 0,05. RESULTADOS: Dos 1.500 TSs realizados no período, 266 cepas eram multirresistentes. Foram identificados 153 pacientes. Desses, 19 foram excluídos. O grupo dos casos ficou composto por 134 pacientes e o dos controles, por 185. Após a análise multivariada encontraram-se os seguintes fatores de risco: falta de esgoto no domicílio, alcoolismo + tabagismo, nº de tratamentos anteriores, tratamentos irregulares e cavidades pulmonares. CONCLUSÃO: Esses cinco fatores têm importância no desenvolvimento da TBMR adquirida e uma tentativa de neutralizá-los poderia contribuir para o controle da TB.
Tuberculose resistente a múltiplas drogas; Fatores de risco
ORIGINAL ARTICLE
Risk factors for acquired multidrug-resistant tuberculosis* * Master's Degree Dissertation, carried out with Prof. Dr. Jorge Luis Nobre Rodrigues as advisor, and presented on 10/09/01. Research performed at the Hospital of Maracanaú Ministry of Health and at the Hospital of Messejana Health Secretariat of the State of Ceará.
Fatores de risco para tuberculose multirresistente adquirida
Elizabeth Clara BarrosoI; Rosa Maria Salani MotaII; Raimunda Oliveira SantosIII;Ana Lúcia Oliveira SousaIV; Joana Brasileiro BarrosoV; Jorge Luís Nobre RodriguesVI
IMaster of Clinical Medicine. Pneumologist of the Ministry of Health, Hospital of Maracanaú, Health Secretariat of the State of Ceará, Hospital of Messejana
IIMaster of Statistics. Statistics Professor of the Federal University of Ceará
IIISocial Worker of the Health Ministry, Hospital of Maracanaú
IVNurse at the Health Secretariat of the State of Ceará, Hospital of Messejana
VUndergraduate student of Psychology, University of Fortaleza
VIMD, PhD in Infectious and Parasitic Diseases. Research Director of the Hospital Universitário Walter Cantídio, Federal University of Ceará
Correspondence Correspondence to Elizabeth Clara Barroso Rua Fonseca Lobo, 50, apto. 402 60175-020 Fortaleza, CE Tel./fax: 55 85 267-1557 E-mail: vbarroso@fortalnet.com.br
ABSTRACT
Multidrug-resistant tuberculosis (MDR-TB) is a severe and feared problem, that is difficult to control and has shown a tendency to increase worldwide.
OBJECTIVE: To analyze the risk factors for acquired MDR-TB.
CASUISTIC AND METHODS: A retrospective population-based case-control study was conducted. A bacillus was considered multidrug-resistant whenever it was resistant at least to rifampin (RFP) + isoniazid (INH), and a case was considered as sensitive tuberculosis (TB) if it had undergone the first treatment during a similar period as the first treatment of an MDR-TB case, but was cured at the time of the interview. Case selection was made based on the list of Sensitivity Tests (ST) performed at the Central Public Health Laboratory of the State of Ceará, from 1990 through 1999. The Proportion Method was used to investigate resistance to the six antituberculosis drugs (isoniazid, rifampin, pyrazinamide, ethambutol, ethionamide, streptomycin) used as the standard treatment in Brazil. Controls were selected from the registry of the TB Control Program. Univariate and multivariate analysis were performed, with p < 0.05 considered significant.
RESULTS: Out of the 1,500 STs performed during the studied period, 266 strains were multidrug-resistant; 153 patients were identified, 19 of which were excluded. The Group of Cases comprised 134 patients, and the Group of Controls comprised 185. Multivariate analysis helped to detect the following risk factors: lack of home sewer system, alcoholism + smoking, number of previous treatments, irregular treatment, and lung cavities.
CONCLUSION: These five factors are important for the development of acquired MDR-TB, and an attempt to neutralize them might contribute to control TB.
Key words: Multidrug-resistant tuberculosis. Risk factors.
RESUMO
A tuberculose multirresistente (TBMR) é um problema grave, temido, de difícil controle e vem apresentando tendência crescente em todo o mundo.
OBJETIVO: Fazer uma análise de fatores de risco para TBMR adquirida.
CASUÍSTICA E MÉTODOS: Foi feito um estudo caso-controle de base populacional de modo retrospectivo. Foi considerado multirresistente o bacilo resistente a pelo menos rifampicina (RFP) + isoniazida (INH) e tuberculose (TB) sensível o caso que tivesse feito o primeiro tratamento num período semelhante ao do primeiro tratamento do caso com TBMR, mas que estivesse curado no momento da entrevista. A seleção dos casos foi feita tomando como base a lista de testes de sensibilidade (TS) realizados no Laboratório Central de Saúde Pública do Estado do Ceará, no período de 1990 a 1999. Foi utilizado o método das proporções para pesquisar a resistência às seis drogas (isoniazida, rifampicina, pirazinamida, etambutol, etionamida, estreptomicina) antituberculose padronizadas no Brasil. Os controles foram selecionados do livro de registro do Programa de Controle da TB. Foi realizada análise univariada e multivariada e considerado significante um p < 0,05.
RESULTADOS: Dos 1.500 TSs realizados no período, 266 cepas eram multirresistentes. Foram identificados 153 pacientes. Desses, 19 foram excluídos. O grupo dos casos ficou composto por 134 pacientes e o dos controles, por 185. Após a análise multivariada encontraram-se os seguintes fatores de risco: falta de esgoto no domicílio, alcoolismo + tabagismo, nº de tratamentos anteriores, tratamentos irregulares e cavidades pulmonares.
CONCLUSÃO: Esses cinco fatores têm importância no desenvolvimento da TBMR adquirida e uma tentativa de neutralizá-los poderia contribuir para o controle da TB.
Descritores: Tuberculose resistente a múltiplas drogas. Fatores de risco.
Acronyms and abbreviations used in this paper
KB Koch's bacillus
CDC Centers for Disease Control
USA United States of America
HIV Human immunodeficiency virus
INH Isoniazid
Lacen Central Laboratory
WHO World Health Organization
OR Odds ratio
PZA Pyrazinamide
RFP Rifampin
AIDS Acquired immunodeficiency syndrome
TB Tuberculosis
MDR-TB Multidrug-resistant tuberculosis
STB Sensitive tuberculosis
ST Sensitivity test
INTRODUCTION
Over the last 50 years, the proliferation of antimicrobial agents for use in animals and humans has caused an unprecedented selective pressure on microorganisms, including M. tuberculosis. Soon after antituberculosis chemotherapy started, the first studies about resistance to the used drugs were published (1,2). During the 1980's, it was believed in the industrialized countries that the eradication of tuberculosis (TB) was imminent, and research in this field became scarce. Only in the early 1990's, when outbreaks of multidrug-resistant tuberculosis (MDR-TB) in HIV-positive subjects in the USA and Europe were published, worldwide attention was given to the problem(3). MDR-TB is defined as a case with a bacillus resistant to at least RFP + INH(4). The publication of those outbreaks raised such an uproar that, in 1993, the World Health Organization (WHO) declared TB a "global emergency", and started, in 1994, the Global Surveillance Project on Anti-tuberculosis Drug Resistance, to measure the prevalence of combined MDR-TB (primary + acquired)(4), thus identifying six critical regions. Brazil took part in that project, not qualifying as a "critical region". A prevalence of only 0.9% was found for primary MDR-TB, and the prevalence of combined MDR-TB was 1.3%, lower than the world mean of 2.2%(4). Ceará was one of the Brazilian states which took part in that study, and a 1.1% prevalence of combined MDR-TB was found in 1996, when the research came to an end. It should be pointed out that the prevalence of combined MDR-TB in Ceará was 0.82% in 1994, and 1.48% in 1999(5).
A study by Becerra et al.(6) attempted to redefine the "critical points" of MDR-TB transmission identified by the WHO, and, in addition to the indicator adopted by the WHO in the Global Project, in this new approach, two other indicators were used: an estimate of the incidence of MDR-TB/100,000 inhabitants/year, and an estimate of the expected absolute number of new MDR-TB cases/year. Other critical regions were detected, needing intervention to curb the dissemination of MDR-TB, and Brazil was one of them.
Drug resistance is a threat to TB control programs worldwide. Patients infected with multiple-drug resistant strains are less likely to become cured (7), particularly if they are infected by HIV or suffer from another immune disease. The treatment is much more toxic and much more expensive (about 700 times) than the one of patients with sensitive organisms (8).
There are few reports in the international literature about a decrease in MDR-TB cases over the last ten years (9,10). The great majority of countries still report an increase in their rates, including industrialized countries like Germany(11), England(12), Denmark(13), and Korea(14).
As mentioned earlier, in Brazil, primary MDR-TB has not presented concerning figures; so, the larger figures are due to acquired MDR-TB, which is mainly originated by human error (8). MDR-TB is a growing problem almost all over the world, including the Brazilian State of Ceará. The objective of this study was to make an analysis of the risk factors which might be associated with the development of acquired MDR-TB.
CASUISTIC AND METHODS
A case-control study was made, nested in a population-based cohort study.
The group of cases comprised patients with acquired MDR-TB, i.e., patients with a history of previous treatment for TB upon MDR-TB diagnosis. Primary MDR-TB was considered, if, upon MDR-TB diagnosis, the patient had not undergone previous TB treatment of any kind. The international definition of MDR-TB was adopted, according to which the bacillus has to be resistant at least to RFP + INH. The control group was composed of patients with sensitive tuberculosis (STB), considered as such if the patient had undergone first treatment during a period similar to that of the first treatment of an MDR-TB case. The patient should have been bacillipherous at the beginning of treatment, to have become cured with medication program I (2RFP + INH + PZA/4RFP + INH), and to be cured at the time of the interview.
The cases were selected based on the list of 1,500 STs performed from 1990 through 1999 at the Central Laboratory (Lacen), the only one performing ST in the State of Ceará, and supervised by the Professor Hélio Fraga Reference Center (a nationwide reference center for ST in Brazil). Controls were selected so as to be matched by gender, age, and year of first treatment. The time elapsed between the first treatment and the diagnosis of MDR-TB was calculated for each case, and the mean found was 6.5 ± 3 (years ± standard deviation). The research was conducted in the year 2000. Drawing the mean back another year, we started selecting from the TB treatment registry (Hospitals of Maracanaú and Messejana) all bacillipherous cases from 1993 on. We did this taking into account that patients had to have had time to develop MDR-TB. We intended our control group to be larger then the group of cases, to increase the statistical power of the study. For each age bracket (10-19, 20-29, etc.) and gender we selected four times the number of cases, because we knew that many of them might have changed addresses, some might have died, and others might not show interest in responding to the call for clinical evaluation and interview. When we finished this selection phase, it was not necessary to make a draft, because the number of selected subjects was the predetermined one. Information was mailed to make the patients aware of the study. When they came to the Phthisiology Service, they signed the consent form of the Research Ethics Committee of the Federal University of Ceará, underwent simple chest X-ray, routine blood tests (including HIV, if they agreed), and sputum BAAR tests (if they had any expectoration). If the patient was cured, a questionnaire was filled out for the control database.
The questionnaire used contained the following definitions: family income was expressed in minimum wages; persons were considered as uneducated if they were either illiterate (unable to write their name or, if they were, unable to read and write) or literate (able to read and write, but having concluded less than the first four grades); persons were considered as educated on the elementary level (if they had concluded more than the first four grades), on the high school level (if they had concluded more than half of the three high school grades), and on the college level (if they had concluded more than half of a university study); tap water and home sewer considered as such if connected to the water and sewer system network. Cured TB and treatment dropout were defined according to the First Brazilian Consensus on Tuberculosis(15). Tuberculosis in the home was defined as any TB case in the home. Number (nº) of previous treatments: number of treatments undergone until diagnosis of MDR-TB for the cases, and number of treatments undergone until interview for controls. Treatment was considered as being on a regular basis if the medication was used as prescribed, or if a failure occurred during less than five consecutive days or less than 10 alternate days per month. Treatment was considered irregular if there was failure in the use of medication for five or more consecutive days, or 10 or more alternate days, provided it did not reach 30 days a month. To analyze the quality of the treatment, we studied the first three treatments (there were patients with up to 12 treatments), because we had reached the conclusion that this would be sufficient to obtain a true profile of treatment quality. Lung cavities were considered large, if cavities of more than 4 cm were found on simple chest X-ray. Classification was made based on X-rays of patients already diagnosed with MDR-TB. Regarding controls, we considered their X-rays upon first discharge from treatment or at the time of the interview. Drinking (risk alcoholism) and smoking were defined according to O'Connor and Schottenfeld(16) and Fahn et al.(17), respectively. For chronic obstructive pulmonary disease (COPD), the definition given by Andreoli et al.(18) was used. Cases were considered as HIV-positive if their serum tests were positive (+) for HIV. Diabetes: the patient's own report regarding his diabetic condition or glycemia > 127mg/dl. Illegal drug use: patient's or a relative's report, or information from patient's record on illegal drug use. Psychiatric diseases: patient's or a relative's report, or information from patient's record on psychiatric treatment or use of controlled drugs.
Löwenstein-Jensen's culture medium was used. For ST, the proportions method with solid medium was used, and resistance was defined as the growth of at least 1% of colonies under critical concentrations of the following drugs: 0.2µg/mL for isoniazid, 2µg/mL for ethambutol, and 40µg/mL for rifampin; and the growth of at least 10% of colonies under critical concentrations of the following drugs: 20µg/mL for ethionamide, 100µg/mL for pyrazinamide, and 4µg/mL for streptomycin).
For univariate analysis, the generalized likeliness ratio test, Pearson's chi-square test, and Fisher's exact test were used. For the continuous variables, logistic regression was used. For the joint analysis of risk factors, multiple linear regression was used, according to the logistic regression model. Values of p < 0.05 were considered significant. The statistical analyses were performed with the help of computer softwares SPSS, Excel for Windows and Word for Windows.
RESULTS
A total of 41,073 TB cases were notified in Ceará during the period between 1990 and 1999(5). The population of this state amounts to 6,809,290 inhabitants(19), and its capital, Fortaleza, has a population of 1,965,513 inhabitants(19) and accounts for 50% of the TB notifications in the State. The Dona Libânia Health Unit and the Hospital of Messejana, two reference state services for outpatient care for TB cases, contributed to the study with a small number of patients. The Hospital of Maracanaú provided most patients of the study; it is a state reference center for hospitalization of TB cases, and is located in the city of Maracanaú, near Fortaleza, with a the population of 160,065 inhabitants(19).
Of the 1,500 STs performed at the Lacen during the 1990's, 264 strains (by eliminating the repeats) were resistant at least to RFP + INH. We were able to identify 151 of these patients, and had the standard questionnaire filled out for them. Five of them were excluded because they had atypical mycobacteria, and 12 because they had no history of previous treatment (primary MDR-TB). So, the group of cases was composed of 134 patients.
To select the controls, 615 patient records were examined, 504 letters were mailed, and the remaining 111 were excluded because they had died, moved away, etc. Of the 504 letters sent, 114 were returned, and only 188 responded to the call. Out of these 188 patients, one had a MDR-TB diagnosis and was transferred to the case group, two were symptomatic and were excluded from the research because, although they were abacillipherous, there was no time to wait for the results of their KB + ST cultures. Thus, the final control group comprised 185 patients.
Regarding the time elapsed between the first treatment and the MDR-TB diagnosis (for the cases), we found a mean of 6.5 ± 3 (years ± standard deviation) and, between the first treatment and the date of the interview (for the controls), we found a mean of 6.5 ± 4, showing that the intended match had been achieved.
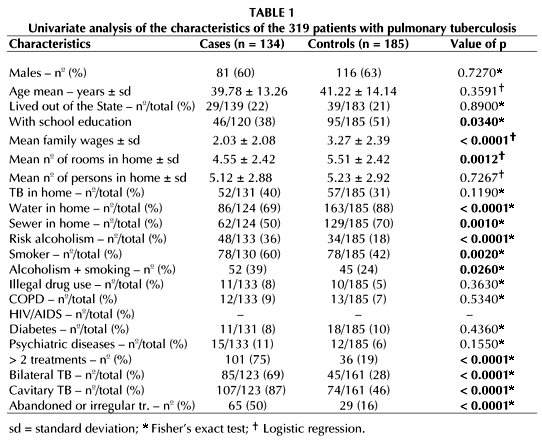
The variables studied and the results of the univariate analysis can be found in Table 1, showing that that the match for gender and age occurred as planned.
A study was carried out on the association of three degrees of smoking with MDR-TB, and another one only on the presence or absence of smoking and MDR-TB. The second study showed a more important association with MDR-TB (Table 1), therefore being included in the multivariate analysis.
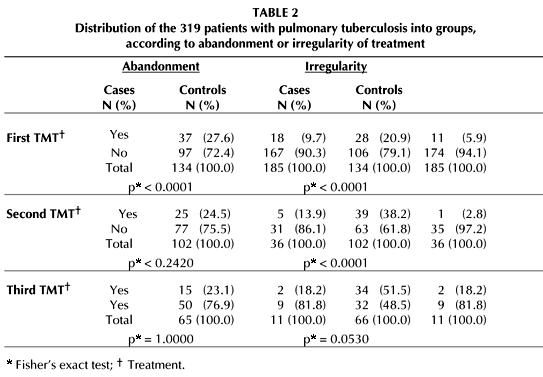
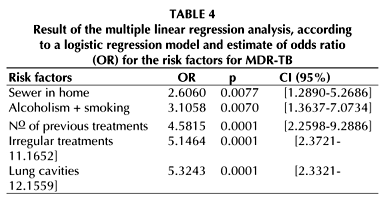
A separate analysis of the abandoned and irregular treatments showed a higher risk of developing MDR-TB for irregular treatments (Tables 2 and 3). This is confirmed by multivariate analysis (Table 4). The adjusted model has a predictive value of 84.41%, a sensitivity of 73.53%, and a specificity of 91.30%.
Chart 1 shows the risk degrees by associating the risk factors found.
DISCUSSION
Notification of TB cases in the State of Ceará has decreased from 43,508 in the 1980's to 41,073 in the 1990's, showing a downward tendency along this decade (20). This might reflect failure in notifying such cases, since the number of MDR-TB diagnoses has shown a growing tendency in the 1990's(5).
Before commenting on our results, we must mention several limitations of the study. The first one is the fact that we were only able to identify the patient records of 146 (56%) out of the 259 patients with MDR-TB of the Lacen list, as far as the group of cases is concerned. As for the control group, out of the 400 letters which probably reached their destination, 212 remained unanswered, and 188 answered the call. Here, the limitations inherent to studies with volunteers have to be considered. Answering the call may have been influenced by the degree of concern about the disease, by the presence of complaints, or by time availability, but we have no elements to either accept or reject these possibilities.
The characteristic limitations of retrospective studies should also be considered. Only 25% of the patients in the case group had their data collected from the patient records, without the patient being present, but, as they were all chronic cases, they had a great number of hospitalizations, and the records contained a great amount of information. In order to prevent any bias due to lack of data or of credibility, information was also collected from nurses and social workers, who had not only been responsible for great part of the information in the records, but also knew the patients very well, due to their many visits and admissions, just like the authors of this research. The other 75% had been followed-up by the authors of this study over the last decade and were present when the questionnaires were filled out. As for the control group, data were collected by the authors in the patients' presence, using the records concomitantly, thus being able to confront the data. For both groups, in addition to questioning the patients, the appointment and visit dates were strictly checked, searching for any failure in taking the medication.
The radiological lesions of the controls were classified based on the tests made upon hospital discharge or at the interview. This may be considered late, but, just like it was done for the cases, the tests performed on the controls were also different from those made at the beginning of the treatment.
A further limitation of the study was the fact that, in our services, the sensitivity test (ST) to antituberculosis drugs is not routinely performed upon the first TB diagnosis. This may have minimized the percentage of primary MDR-TB found (only 8% against 92% of acquired MDR-TB). On the other hand, it may have had no influence at all, for, when the State of Ceará took part in the Global Project for Antituberculosis Drug Surveillance, conducted by the Ministry of Health in 1995 and 1996 to study primary and acquired resistance (5), a proportion of 0.6% of primary MDR-TB was found, against 3.3% of acquired MDR-TB (20). By these percentages, if STs had been carried out in all first-treatment cases, the percentages of primary and acquired MDR-TB should have been 15% and 85%, respectively. These percentages are not very different from those found.
We matched cases and controls for gender and age bracket, considering that those variables could act as confusing factors, since the results found in the literature are rather discrepant regarding those two characteristics(21,22).
We made no racial study, because there is profound miscegenation in the State of Ceará, making a satisfactory racial classification of patients impossible.
Few authors worldwide had the concern of investigating the patients' educational level as a risk factor for MDR-TB(23,24). Those who did investigate it found no association with MDR-TB. We could not have left this out here, since the illiteracy rate in the population over 15 years of age in the Brazilian North-East is 26.6%. As shown in Table 1, univariate analysis revealed an association between MDR-TB and lack of school education. Another Brazilian study also found this association (26).
Our work showed that the association known for centuries between TB and poverty also applies to MDR-TB, since we found a rather significant inverse association between MDR-TB and family income, and between MDR-TB and the number of rooms in the home (Table 1). The lack of a hydro-sanitary infrastructure (tap water and sewer) in the homes also showed to be associated with MDR-TB.
In our research, alcoholism and smoking appeared as risk factors for MDR-TB, whether associated or not. Although we found smoking to be a risk factor for MDR-TB, there is no report about this fact in the literature searched. Yet, there are reports on association between alcoholism and MDR-TB (21,27,28).
As for illegal drug use, many authors have investigated this problem(10,27), but only a few found a significant association with MDR-TB(27). We did not find such an association.
In agreement with the literature, we did not find any association either between MDR-TB and chronic obstructive pulmonary disease, diabetes, or psychiatric diseases.
The issue of HIV infection being a risk factor for MDR-TB has been discussed for several years. The controversy started with the CDC publication on the MDR-TB outbreak among HIV-infected subjects in New York and Miami. Several investigators associated MDR-TB with HIV(22), others showed that there was no association whatsoever (29,30). In 1998, Spellman et al. published a study conducted with the purpose of comparing the prevalence of MDR-TB among patients with and without HIV infection, to settle the matter; they concluded that HIV infection was not a risk factor for MDR-TB(31). The present study confirms the lack of association between MDR-TB and HIV. It may seem unbelievable, but we had no HIV (+) case either in the case group or in the control group, in spite of having requested the test in 68% of the cases and in 55% of the controls. Our HIV prevalence in TB in hospitalized patients is 2.03%(32), and in outpatients 0.44%(33). These low percentages might justify the absence of HIV infection in our study groups.
There is virtually a consensus among MDR-TB researchers regarding the fact that the number of previous treatments is a risk factor for MDR-TB(10,23,27,34), and our study confirmed this association. By reviewing the literature that mentions univariate analysis, we found the smallest association with an odds ratio (OR) = 3.14; CI 95%: 1.22-8.04(10), and the biggest, with an OR = 109.40; CI 95%: 15.02-796.43(27).
The professionals are still in doubt about whether the lung cavities are a cause or a consequence of MDR-TB. They may be both. A cause, because the cavity lesions hold larger populations of bacilli, among which there is a higher probability of existence of initially resistant mutants. The selection of mutants is facilitated by the fast multiplication to up to 108 or 109 bacilli inside the cavities, due to the high level of oxygenation and to the protection granted to the bacilli by thick walls which keep drugs from reaching adequate inhibitory concentrations (2). Concomitantly with inadequate treatments, this is a favorable environment for the development of MDR-TB. A consequence, because the existence of MDR-TB leads to a much more prolonged state of active disease, with consequent bigger lung damage. The possibility of less severe forms developing into cavities as a consequence of inadequate use of the medication cannot actually be ruled out. The development of MDR-TB is multifactorial and occurs mainly as a result of human action, which can be related to the patient, to the health care service and/or the drugs. The fact is that, ever since the first decades of antituberculosis chemotherapy, cavities have been associated with the resistant forms. A patient presenting lung cavities before or after treatment gives reason for concern, for the percentage of cavitary forms is significantly higher in those groups which do not become cured.
Extensive and/or cavitary TB have already been mentioned in the literature as risk factors for tuberculosis reactivation (35), as they have been appointed as predisposing factors for the development of MDR-TB. Some authors presented this factor quantified (7,23,36). In our study, a significant correlation between MDR-TB and bilateral and cavitary TB was found. Rosemberg et al. published in 1967 in Brazil a large research about acquired resistance, comprising 1,759 patients, whose bacteriological tests were performed at the Clemente Ferreira Research Institute. Of those patients, 751 (42.7%) had moderately advanced forms, 1,008 (57.3%) had very advanced forms, and 1,532 (87.0%) had cavities (37). Fiúza de Melo et al. published in 2000 a study where they report on the presence of lung cavities in 100% of a series of 182 patients with MDR-TB(28).
In addition to studying the association of MDR-TB with previous treatments, it is equally important to investigate the quality of those treatments. If a patient has had previous treatment, even an adequate one, it still represents a minimal risk for MDR-TB(34). A treatment can be considered inadequate because it was discontinued too early (abandoned), or because it was made on an irregular basis (irregular), but continued. The selection of resistant mutants occurs due to inadequate treatment, resulting in acquired mono-resistant TB. Novel mutations in a growing population will eventually lead to MDR-TB, if the inadequate treatment continues (irregular). Inadequate treatment can be defined as direct or indirect monotherapy, and this can be related to the health professional, to the drug, or to the patient himself(38,39).
Some publications (40) mention treatments with insufficient duration as a cause of drug resistance; this is important, but irregular treatment is even worse (41). If the treatment is irregular, the number of bacterial death and bacterial growth cycles will be greater, giving more opportunities for individual mutations of different independent genes to accumulate. This is in accordance with the findings of our research, with a 7.01 risk of developing MDR-TB for cases with irregular treatments, versus a 2.73 risk for cases which abandoned treatment.
Table 4 summarizes the risk factors which remained significant after multiple linear regression. It contains the number of previous treatments, the factor most frequently mentioned in the literature. We emphasize the need of a sewer in the home, which is not found in the literature, and can be considered here as representing the influence of socioeconomic conditions on MDR-TB. Smoking associated with alcoholism is not mentioned in the literature either in connection with MDR-TB, only with TB. Irregular treatment showed to be actually more important than abandoned treatment. The picture is completed by the lung cavities, which had proven important in the development of resistance ever since the first years of antituberculosis chemotherapy. These cavities had been forgotten for decades, probably because most epidemiological studies were based on microbiological data, without considering the X-rays (36).
Considering R as the risk of a patient developing MDR-TB in the absence of the risk factors: sewer in the home, alcoholism + smoking, lung cavities, nº of treatments undergone, and irregular treatments, then the Risk of the patient having MDR-TB grows as one of these factors appears, growing geometrically as two, three, four or five of these factors become associated, up to a value 1,000 times bigger than R (the minimal risk occurring in the absence of the risk factors) (Chart 1).
Based on the presented data, we concluded firstly that the most important risk factors for acquired MDR-TB were, in increasing order of importance: lack of sewer in the home, alcoholism + smoking, nº of previous treatments, irregular treatments, and lung cavities. Secondly, we concluded that the risk of developing MDR-TB increased markedly as the risk factors became associated, making it necessary to identify such factors early, in order to bypass them and thus minimize their effects.
ACKNOWLEDGEMENTS
The authors thank Prof. Dr. José Rosemberg, Prof. Dr. Marta Maria das Chagas Medeiros and Prof. Dr. Maria Grasiela Teixeira for advice regarding the research project and during other phases of the study. They also thank the librarians Leonilha Maria Brasileiro Lessa and Norma de Carvalho Linhares for help with the references.
25. Instituto Brasileiro de Geografia e Estatística. Censo Demográfico 2000. Disponível em: \\www.ibge.net/ibge/estatistica/populacao/censo2000/sinopse.php?tipo=&uf=23. Acesso em: 12 jun. 2000.
Received for publication on 08/27/02
Approved, after revision, on 01/13/03
- 1. Crofton J, Mitchinson DA. Streptomycin resistance in pulmonary tuberculosis. BMJ 1948;2:1009-15.
- 2. Canetti G. Present aspects of bacterial resistance in tuberculosis. Am Rev Respir Dis 1965;92:687-703.
- 3. Alland D, Kalkut GE, Moss AR, McAdam RA, Hahan JA, Bosworth MAW, et al. Transmission of tuberculosis in New York City: an analysis by DNA fingerprint and conventional epidemiologic methods. N Engl J Med 1994;30:1710-6.
- 4. Pablos-Mendez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, et al. Global surveillance for antituberculosis-drug resistance, 1994-1997. World Health Organization International Union against Tuberculosis and Lung Disease Working Group on Anti-tuberculosis Drug Resistance Surveillance. N Engl J Med 1998;338:1641-9.
- 5. Barroso EC, Rodrigues JLN, Pinheiro VGF, Campelo CL. Prevalência da tuberculose multirresistente no Estado do Ceará, 1990-1999. J Pneumol 2001;27:310-4.
- 6. Becerra MC, Bayona J, Freeman J, Farmer PE, Kim JY. Redefining MDR-TB transmission 'hot spots'. Int J Tuberc Lung Dis 2000;4:387-94.
- 7. Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh Jr CR. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med 1993;328:527-32.
- 8. Mahmoud AMD, Michael D, Iseman MD. Pitfalls in the care of patients with tuberculosis: common errors and their association with the acquisition of drug resistance. JAMA 1993;270:65-8.
- 9. Tuberculosis morbidity United States, 1997. MMWR Morb Mortal Wkly Rep 1998;47:253-7.
- 10. Marin Royo M, González Moran F, Moreno Munôz R, Pardo Serrano F, Alfaro Prada P, Arnedo Pena A, et al. Evolution of drug-resistant Mycobacterium tuberculosis in the province of Castellon. 1992-1998. Arch Bronconeumol 2000;36:551-6.
- 11. Schaberg T, Glocer G, Reichert B, Mauch H, Lode H. Resistant lung tuberculosis in Berlin 1987-1993. Pneumologie 1996;50:21-7.
- 12. Glaissberg V. The threat of multidrug resistance: is tuberculosis ever untreatable or uncontrollable? Lancet 1999;353:998-9.
- 13. Horsburgh CR Jr. The global problem of multidrug-resistant tuberculosis: the genie is out of the bottle. JAMA 2000;283:2575-6.
- 14. Schraufnagel D, Abubaker J. Global action against multidrug-resistant tuberculosis. JAMA 2000;283:54-5.
- 15. Coordenação Nacional de Pneumologia Sanitária. I Consenso Brasileiro de Tuberculose-1997. J Pneumol 1997;23:294-319.
- 16. O'Connor PG, Schottenfeld RS. Patients with alcohol problems. N Engl J Med 1998;338:592-600.
- 17. Fahn HJ, Wang LS, Kao SH, et al. Smoking-associated mitochondrial DNA mutations and lipid peroxidation in human lung tissues. Am J Respir Cell Mol Biol 1998;19:901-9.
- 18. Andreoli TE, Bennett JC, Carpenter CJC, Plum F, Editores. Cecil Medicina interna básica. 4ª ed. Rio de Janeiro: Guanabara Koogan, 1998; Cap.16:127-34.
-
1919. Instituto Brasileiro de Geografia e Estatística. Ceará û Contagem da populacão de 1996. Disponível em: URL: http:\\www.ibge.gov.br/ibge/estatistica/populaçao/contagem/cecont96.shtm. Acesso em: 12 jun. 2000.
- 20. Barroso EC. Fatores de risco para tuberculose multirresistente [dissertação]. Fortaleza: Faculdade de Medicina, Universidade Federal do Ceará, 2001;107p.
- 21. Garcia Rodriguez JF, Marino Callejo A, Lorenzo Garcia MV, Rodríguez Mayo M, Domínguez Gómez D, Sesma Sánchez P. Resistance of Mycobacterium tuberculosis in Ferrol, Spain. Associated factors. Med Clin (Barc) 1999;113:572-4.
- 22. Weltman AC, Rose DN. Tuberculosis susceptibility patterns, predictors of multidrug resistance, and implications for initial therapeutic regimens at New York City Hospital. Arch Intern Med 1994;154:2161-7.
- 23. Al Jarad N, Parastatides S, Paul EA, Sheldon CD, Gaya H, Rudd RM, et al. Characteristics of patients with drug resistant and drug sensitive tuberculosis in East London between 1984 and 1992. Thorax 1994; 49:808-10.
- 24. Murray J, Sonnenberg P, Shearer S, Godfrey-Faussett P. Drug-resistant pulmonary tuberculosis in a cohort of southern African goldminers with high prevalence of HIV infection. S Afr Med J 2000;90:381-6.
- 26. Natal S, Toledo A, Penha MLF, Valente J. Modelo de predição para resistência aos tuberculostáticos [abstract]. J Pneumol 2000;26:S23.
- 27. Torres L. Resistance of Mycobacterium tuberculosis in Zaragoza, Spain (1993-1997) and related factors. Med Clin (Barc) 2000;115:605-9.
- 28. Fiúza de Melo FA, Afiune JB, Ide Neto J, Spada DTA, De Felice EAA, Antelmo ANL, et al. Características da TBMR num serviço de referência: influência sobre o controle do tratamento. J Pneumol 2000;26: S94-5.
- 29. Wilkinson D, Pillay M, Davies GR, Sturm AW. Resistance to antituberculosis drugs in rural South Africa: rates, patterns, risks, and transmission dynamics. Trans R Soc Trop Med Hyg 1996;90:692-5.
- 30. Boudville IC, Wong SY, Snodgrass I. Drug resistant tuberculosis in Singapore. Ann Acad Med Singapore 1997;26:549-56.
- 31. Spellman CW, Matty JK, Weis SE. A survey of drug-resistant Mycobacterium tuberculosis and its relationship with HIV infection. AIDS 1998;12:191-5.
- 32. Barroso EC, Oliveira TRB, Azevedo HC, Sales SMC, Santos RO. Incidência do HIV na tuberculose [abstract]. J Pneumol 1996;22:S59.
- 33. Broutet N, Queiroz SA, Basilio FP, As HL, Simon F, Dabis F. Prevalence of HIV1, HIV2 and HTLV antibody in Fortaleza, Ceará, Brazil, 1993-1994. Int J STD AIDS 1996;7:365-9.
- 34. Garcia Vazquez E, Esteban J, de Gorgolas, Fernández Guerrero ML. Infection by resistant Mycobacterium tuberculosis in a hospital population. A longitudinal study of incidental cases at the Fundation Jimenez Diaz. Rev Clin Esp 1999;199:564-8.
- 35. Edsall J, Collins JG, Gray JAC. The reactivation of tuberculosis in New York City in 1967. Am Rev Respir Dis 1970;102:725-36.
- 36. Ben-Dov I, Mason GR. Drug-resistant tuberculosis in Southern California Hospital: trends from 1969 to 1984. Am Rev Respir Dis 1987; 135:1307-10.
- 37. Rosemberg J, Waisbich E, Passos Filho MCR. Resistência às drogas standard em 1759 casos de tuberculose pulmonar, bacilíferos, submetidos à quimioterapia por tempos diversos. Rev Serv Nac Tuberc 1967; 11:449-66.
- 38. Lambregts-van Weezembeek CSB, Veen J. Control of drug-resistant tuberculosis. Tuber Lung Dis 1995;76:455-9.
- 39. González Saldanha N, Neme Diaz GA, Macias M, Palau M, Levy A, de la Roz R. Resistencia a los fármacos antituberculosos en América Latina. Rev Enfermedades Infec Ped 1999;11:256-9.
- 40. Nunn P, Felten M. Surveillance of resistance to antituberculosis drugs in developing countries. Tuber Lung Dis 1994;75:163-7.
- 41. Snider DE Jr, Long MW, Cross FS, Farer LS. Six-months isoniazid-rifampin therapy for pulmonary tuberculosis. Am Rev Respir 1984; 129:573.
Publication Dates
-
Publication in this collection
23 June 2003 -
Date of issue
Apr 2003
History
-
Received
27 Aug 2002 -
Accepted
13 Jan 2003