Abstract
The objective of this study was to evaluate the efficacy of fibrin adhesive made up of snake venom and bubaline fibrinogen by rat colon anastomosis. Eighty rats were randomly assigned into 2 experimental groups: GI control (anastomosis with extramucous interrupted suture) and GII (repair suture + fibrin glue). The animals were studied at the following 4 times: T0 – preoperative, T1 – 7th day postoperative, T2 – 14th day postoperative, and T3 – 21 st day postoperative. The macroscopic characteristics of the intestinal segment open and closed anastomosis and the bursting strength of the anastomosed segments were observed at each of the above times. The results showed that the anastomosed segments coapted and there was no difference in the bursting strength values between the 2 groups. There was a decrease in the bursting strength values up until the 7 th day postoperative in both groups with its progressive increase at the other times. Although important experimental studies using large animals are needed for a better evaluation of tissue repair processes, this adhesive may become a valuable tool for use in anastomosis.
Fibrin glue; experimental healing; bursting strength; experimental anastomosis; snake venom; colon anastomosis
An evaluation by rat colon anastomosis of the efficacy of fibrin glue derived from snake venom
C.V.S. LEITE
 CORRESPONDENCE TO:
C. V. S. LEITE Depto. de Cirurgia e Ortopedia, Faculdade de Medicina de Botucatu, Distrito de Rubião Junior, S/N, CEP 18618-000, Botucatu, SP, Brazil.
, L.E. NARESSE
CORRESPONDENCE TO:
C. V. S. LEITE Depto. de Cirurgia e Ortopedia, Faculdade de Medicina de Botucatu, Distrito de Rubião Junior, S/N, CEP 18618-000, Botucatu, SP, Brazil.
, L.E. NARESSE , H.L. ARANTES
, H.L. ARANTES , A.F. LOPES
, A.F. LOPES , I.A. THOMAZINI-SANTOS
, I.A. THOMAZINI-SANTOS , M.J.S GIANNINI
, M.J.S GIANNINI , M.C. MERCADANTE
, M.C. MERCADANTE , B. BARRAVIERA
, B. BARRAVIERA , S. KOBAYASI
, S. KOBAYASI .
.
1 Department of Surgery and Orthopedics of Botucatu School of Medicine (FMB) São State University (UNESP), São Paulo, Brazil; 2 Undergraduate Students FMB/UNESP; 3 Laboratory of Surgical Technique and Experimental Surgery FMB/UNESP; 4 Laboratory of Hemostasis, Blood Center FMB/UNESP; 5 Department of Tropical Medicine and Imaging Diagnosis FMB/UNESP; 6 School of Pharmaceutical Sciences of Araraquara UNESP; 7 Center for the Study of Venoms and Venomous Animals CEVAP/UNESP.
ABSTRACT. The objective of this study was to evaluate the efficacy of fibrin adhesive made up of snake venom and bubaline fibrinogen by rat colon anastomosis. Eighty rats were randomly assigned into 2 experimental groups: GI control (anastomosis with extramucous interrupted suture) and GII (repair suture + fibrin glue). The animals were studied at the following 4 times: T0 preoperative, T1 7 th day postoperative, T2 14 th day postoperative, and T3 21 st day postoperative. The macroscopic characteristics of the intestinal segment open and closed anastomosis and the bursting strength of the anastomosed segments were observed at each of the above times.
The results showed that the anastomosed segments coapted and there was no difference in the bursting strength values between the 2 groups. There was a decrease in the bursting strength values up until the 7 th day postoperative in both groups with its progressive increase at the other times.
Although important experimental studies using large animals are needed for a better evaluation of tissue repair processes, this adhesive may become a valuable tool for use in anastomosis.
KEY WORDS: Fibrin glue, experimental healing, bursting strength, experimental anastomosis, snake venom, colon anastomosis.INTRODUCTION
The principle of performing an intestinal anastomosis consists of apposing the proximal and distal edges of the segments and suturing. Although there have been conflicting reports in literature about the techniques for intestinal suture, these are based on the number of suture layers (one or two), as well as the type of suture, such as continuous or interrupted. Initially, sutures were made using natural products (cotton, wood fiber, linen, animal sinews), and then synthetic materials (nylon, dacron, and polypropylene) also gained importance. Today, surgeons have shown a growing interest in testing biological adhesives to achieve a faster and more efficient tissue repair. These substances should establish hemostasis and produce a firm tissue adhesion, with no alteration in tissue repair and no adverse effects. Some substances have been used, such as cyano-acrylate (9,11,24), metyl-cyano-acrylate (8,22), but due to their irritating, toxic, and carcinogenic effects, they have been discontinued (12,24,29).
Recently, new adhesives have been tested, such as gelatin collagen mixed with formaldehyde, which produces immediate adhesion, but loses it within a few hours as well as being toxic. Gelatin collagen and formaldehyde were then mixed with resorcin (3,18), and later glutaraldehyde was added to this mixture (20). When this adhesive was used in our experimental intestinal anastomoses, tissue necrosis with dehiscences, fistulae, and peritonitis were observed. We then contraindicated its use (1,25).
More recently, another adhesive substance was developed made of fibrin and fibrinogen mixed with thrombin derived from human plasma, which may allow the transmission of infectious diseases (14,17,27).
A new biological adhesive was developed using snake venom and fibrinogen from large animals with a high adhesive capacity and no adverse effects.
The objective of this study was to evaluate the efficacy of this new fibrin glue by rat colon anastomosis.
MATERIALS AND METHODS
ANIMALS. Eighty Wistar rats weighing around 180 g were used. They were kept in plastic boxes (5 animals per box) in a closed room with artificial light for 12 hours at 23ºC, receiving water and food ad libitum.
EXPERIMENTAL DESIGN. The animals were randomly assigned into 2 experimental groups. The animals were randomly assigned into 2 experimental groups.
GI anastomosis interrupted suture 40 control rats
GII repair suture + fibrin glue 40 rats
These animals were evaluated at the following four experimental times:
To preoperative
T1 7 th day postoperative
T2 14 th day postoperative
T3 21 st day postoperative
ANESTHESIA. After 12-hour fasting, the rats were anesthetized using pentobarbital sodium 30 mg/kg diluted in distilled water and injected intraperitoneally. The anesthesia level was determined by observing the palpebral and respiratory reflexes and muscle tone. The animals were considered anesthetized when their respiration was regular and superficial and muscle tone was flaccid with absence of reflexes.
SURGERY. The animals were subjected to laparotomy after a midline abdominal incision under sterile conditions. After the identification of the distal colon, a transverse incision was made with scissors approximately 3 cm from the peritoneal reflexion.
Anastomosis was performed using 6-0 monofilamentar nylon with a gastrointestinal 1.3-cm needle. Anastomosis of the control animals (Group I) was extramucous with interrupted sutures approximately 1 mm apart. To achieve this, the two mesentery borders were aligned after performing an anterior and posterior suture. Group II was submitted to a similar procedure with one repair suture between the borders. Three drops of fibrin glue were applied on each side of the anastomosis for 5 minutes.
The abdominal wall was closed in a two-layer interrupted suture with 2-0 cotton. One layer consisted of the peritoneum and the musculature and the other the subcutaneous and skin.
POSTOPERATIVE CLINICAL OBSERVATION. The animals were placed in plastic boxes. Skin healing cosmetic aspect, food intake, abdominal distension, and evacuation were observed.
MACROSCOPIC EXAMINATION OF THE ABDOMINAL CAVITY. On the days set for sacrifice, the animals were anesthetized and alterations in the abdominal cavity were observed, such as peritoneal adherence, abscess, and dehiscence.
MATERIAL COLLECTION AND PROCESSING. Laparotomy was performed immediately after anesthetic induction. Adherences surrounding the anastomosis were isolated and the distal colon segment with anastomosis (measuring approximately 2cm in length) was removed for analysis. This segment was immersed in saline at 37ºC and 250 mg/l of papaverine hydrochloride for half an hour to study bursting strength.
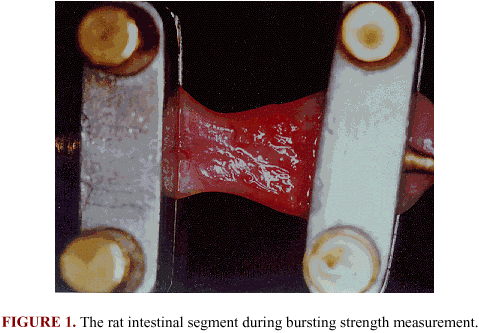
BURSTING STRENGTH. The bursting strength of intestinal segments was measured using a tensometer connected to a computer (Figure 1).
FIBRIN GLUE. Fibrin glue derived from snake venom is a biological adhesive made up of two components. Component I consists of bubaline cryoprecipitate, which includes fibrinogen, factor VIII, factor XIII, von Willebrants factor, fibronectin, and plasminogen. Component II consists of thrombin-like fraction of the venom from added with calcium chloride. The glue must be prepared at the time of use. Component 2 must be applied on the chosen area before component 1 or simultaneously with a Y syringe.
RESULTS
During the clinical evolution of the control group (GI), two rats developed infection in the abdominal wall. This was resolved by daily cleaning with saline and antiseptics. At T1, one animal showed partial dehiscence of anastomosis with adherence. Two animals died immediately after surgery due to anesthesia and one rat died on the 12 day postoperative from unknown causes. The remaining animals evolved well and had the stitches removed on the 6
day postoperative from unknown causes. The remaining animals evolved well and had the stitches removed on the 6 or 7
or 7 day postoperative.
day postoperative.
The animals in the fibrin glue group (GII) showed the following results: one animal died immediately after surgery, one rat showed partial dehiscence of anastomosis with adherence at T2, one died on the 6 day postoperative, and two had infections in the abdominal wall, which were resolved by daily cleaning with saline and antiseptics.
day postoperative, and two had infections in the abdominal wall, which were resolved by daily cleaning with saline and antiseptics.
The animals in the control group showed adherences surrounding the anastomosis on the 7 day postoperative. The open anastomosed intestinal segments were coapted and fibrin was seen on the anastomosis. The animals in GII showed a more intense adherence surrounding the anastomosis than in the control group. The open anastomosed intestinal segments were coapted with the presence of fibrin on the anastomosis (Figure 2 and Figure 3).
day postoperative. The open anastomosed intestinal segments were coapted and fibrin was seen on the anastomosis. The animals in GII showed a more intense adherence surrounding the anastomosis than in the control group. The open anastomosed intestinal segments were coapted with the presence of fibrin on the anastomosis (Figure 2 and Figure 3).
On the 14 day postoperative, a good coaptation of the anastomosed segments was observed with the presence of residual fibrin in animals of the control group. The animals in GII showed moderate adherence surrounding the anastomosis. The open anastomosed intestinal segments showed a good coaptation with the presence of residual fibrin (Figure 4 and Figure 5).
day postoperative, a good coaptation of the anastomosed segments was observed with the presence of residual fibrin in animals of the control group. The animals in GII showed moderate adherence surrounding the anastomosis. The open anastomosed intestinal segments showed a good coaptation with the presence of residual fibrin (Figure 4 and Figure 5).
On the 21 day postoperative, the animals in the control group (GI) exhibited a firm adherence surrounding the anastomosis and a perfect coaptation of the intestinal segments. The animals in GII showed coaptation with residual fibrin. The macroscopic alterations were apparently more intense in the animals of GII than in the control group.
day postoperative, the animals in the control group (GI) exhibited a firm adherence surrounding the anastomosis and a perfect coaptation of the intestinal segments. The animals in GII showed coaptation with residual fibrin. The macroscopic alterations were apparently more intense in the animals of GII than in the control group.
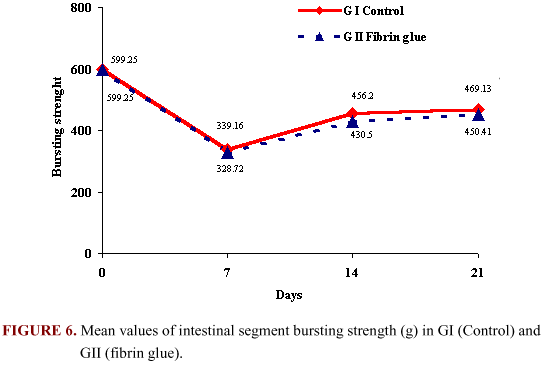
The bursting strength varied from a higher mean value to a decrease on the 7 day postoperative in both groups and then progressive increase at the other times. However, no statistical difference in the values was observed for both groups. The bursting strength mean values for both groups are shown in Figure 6.
day postoperative in both groups and then progressive increase at the other times. However, no statistical difference in the values was observed for both groups. The bursting strength mean values for both groups are shown in Figure 6.
DISCUSSION
This fibrin adhesive is made up of fibrinogen, factor XIII, aprotinin, fibronectin, plasminogen, thrombin, and calcium chloride (6). Over the past decade, this adhesive has been used in clinical and experimental studies (4,7,19,27).
Most fibrin adhesives derive from human plasma, and therefore, their use has been the object of criticism due to the risk of transmitting blood-borne diseases. Some authors have proposed the use of the patients own plasma to avoid viral transmission (5,27).
The use of fibrinogen derived from pooled human blood sources has been banned by the FDA due to the high risk of transmissible diseases. As a consequence, the single donor or autologous fibrinogen has been used to produce fibrin adhesives in the United States (23,28).
The fibrin glue made up of bovine fibrinogen and thrombin has been used as an efficient hemostatic substance (2).
In 1996, Carrol et al. (2) treated 21 patients with fibrin glue as a topical hemostatic substance without any hemorrhagic, allergic, or thrombotic reactions.
The fibrin adhesive used in this study is made up with a fraction venom and bubaline fibrinogen. This adhesive was produced at the Center for the Study of Venoms and Venomous Animals CEVAP, of São Paulo State University, UNESP , on the Campus of Botucatu.
CEVAP-formulated fibrin glue was tested in the reparation of the peripheral nerve of Wistar rats and produced an efficient tissue repair and firm adhesion of the segments. The results obtained were similar to those of other researchers with the conventional fibrin glue (15).
In this study, the difficulties encountered apposing the proximal and distal segments in the left colon of rats during the application of fibrin glue were resolved by using repair suture between the borders of the anastomosis. This glue allowed the coaptation of intestinal segments and efficient hemostatic effect.
Our results showed that there was no difference in the bursting strength for the anastomoses between the animals with one-layer suture and extramucous interrupted suture and those submitted to non-suture fibrin adhesive. These results confirm the observations of other investigators (14,17). A decrease was seen in bursting strength values of GI and GII on the 7 day postoperative with a progressive elevation in the other moments, as previously reported (13,16,21,26).
day postoperative with a progressive elevation in the other moments, as previously reported (13,16,21,26).
The use of fibrin adhesive in anastomosis, however, requires further investigations in larger animals to learn how this adhesive behaves in larger organs. In addition, for a better understanding of the processes involved in tissue repair, histopathological studies as well as collagen quantification and qualification are needed. This adhesive may become a valuable tool for use in anastomoses and as an impermeable agent, especially when associated with risk factors which cause dehiscences, such as infection, hypoxia, hematoma, diabetes, and malnutrition (10,20,25).
In conclusion, the biological adhesive derived from snake venom proved to be efficient in achieving rat colon anastomosis.
REFERENCES
01 BIONDO-SIMÕES MLP., KOPPE GL., HANSEL H., DO ROSÁRIO MAK., MALAFAIA O. Uso de adesivo biológico em anastomoses intestinais: estudo experimental em cães. Acta Cir. Bras., 1992, 7, 151-3.
02 CARROL JF., MOSKOWITZ KA., EDWARDS NM., HIECKEY TJ., ROSE EA., BUDZYNSKI AZ. Immunological assessment of patient treated with bovine fibrin as hemostatic agent. Thromb. Haemost., 1996, 76, 925-31.
03 COOPER CW., FALB RD. Surgical adhesives. Ann. N. Y. Acad. Sci., 1968, 146, 214-24.
04 DARGENIO R., RANELLETTI FO., CIMINO C., RAGUSA G., PANETTA V., GARCCA N. Fibrin glue versus nylon anastomosis of fallopian tubes. J. Reprod. Med., 1986, 31, 961-5.
05 DURHAM LH., WILLATT DJ., YUNG MW., JONES I., STEVENSON PA. A method for preparation of fibrin glue. J. Laryngol. Otol., 1987, 101, 1182-6.
06 ELLIS DA., PELAUSA ED. Fibrin glue in facial plastic and reconstructive surgery. J. Otholaryngol., 1988, 17, 74-7.
07 FABRICIUS PG., JOCHAM D., PERMANETTER V., UNSOLD E. Application of fibrin adhesive in the urinary bladder. Urol. Res., 1987, 15, 307-10.
08 FERLIC DC., GLODNER L. Evaluation of the methyl - 2 - cyanoacrylote (Eastman 910 monomer) on peripheral nerves. South. Med. J., 1965, 58, 679-85.
09 FISCHL RA. An adhesive for primary closure of skin incisions. Plast. Reconstr. Surg., 1962, 30, 607-10.
10 HAWLEY PR. Causes and prevention of colonic anastomotic breakdown. Dis. Colon Rectum, 1973, 16, 272-7.
11 HEALEY JR JE., CLARK L., GALLAGER HS., ONEILL P., SHEENA KS. Non-suture repair of blood vessels. Ann. Surg., 1962, 155, 817-26.
12 HERRMANN JB., WOODWARD SC. The affect of cyenoacrylate tissue adhesives upon granulation tissue formation in ivalon sponge implants in rat. Surgery, 1966, 59, 559-64.
13 HERRMANN JB., WOODWARD SC., PULASKI EJ. Healing of colonic anastomosis in the rat. Surg. Gynecol. Obstet., 1964, 119, 269-75.
14 HJORTRUP A., NORDKILD P., KJERGAARD J., STONTOFT E., OLESEN HP. Fibrin adhesive versus sutured anastomosis: a comparative intra-individual study in the small intestine pigs. Br. J. Surg., 1986, 73, 760-1.
15 IUAN FC., THOMAZINI IA., GIANNINI MJSM., VITERBO S., TOSCANO E., MORAES RA., BARRAVIERA B. Preparation of peripheral nerves with fibrin glue prepared from snakes venous. Preliminary results. Rev. Paul. Med., 1995, 113, 1000-2.
16 JIBORN H., AHONEN J., ZEDERFELDT B. Healing of experimental colonic anastomoses. II Breaking strength of the colon after left colon resection and anastomosis. Am. J. Surg., 1978, 136, 595-9.
17 KJAERGAARD J., NORDKILD P., SJONTOFT E., HJORTRUP A. Non-sutured fibrin adhesive versus sutured anastomosis. A comparative intra-individual study in dog colon. Acta Chir. Scand., 1987, 153, 599-601.
18 KOEHNLEIN HE., LEMPERLE G. Experimental studies with a new gelatin - resorcin - formaldehyde glue. Surgery, 1969, 66, 377-82.
19 KRAM HB., REUBEN BI., FLEMING AW., SHOEMAKER WG. Use of fibrin glue in hepatic trauma. J. Trauma, 1988, 28, 1195-201.
20 LAURIAN C., GIGOU F., BICAL O., BARBAGELATA M., BACHET J., GOUDOT B., GUILMET D. Traitement chirurgical des dissetions oortiques argés por utilisation dunc colle biologique. J. Chir., 1979, 166, 143-8.
21 LEITE CVS., NARESSE LE., KOBAYASI S., MINOSSI JG., BURINI RC., CURI PR., HOSSNE WS. Efeito da desnutrição protéica na anastomose do cólon distal no rato. Efeito da força ruptura e do colágeno tecidual. Acta Cir. Bras., 1993, 8, 145-50.
22 MALAMENT M. Experimental bladder closure with a tissue adhesive. Investig. Urol., 1966, 3, 429-38.
23 MARTINOVITZ V., SCHULMAN S. Fibrin sealant in surgery of patient with hemorrhage diathesis. Thromb. Haemost., 1995, 74, 486-92.
24 MATHES GL., TERRY JJW. Non-suture closure of nephrotomy. J. Urol., 1963, 89,122-4.
25 MEDEIROS AC., RAMOS CCF. FREIRE TMGL., PINTO JR FEL., MEDEIROS PJ., MELLO LEB., AZEVEDO FC. Uso de adesivo cirúrgico em anastomose do cólon. Estudo experimental em ratos. Acta Cir. Bras., 1990, 5, 136-4.
26 NARESSE LE, LEITE CVS., RODRIGUES MAM., ANGELELI AYO., MINOSSI JG., KOBAYASI S. Efeito da peritonite fecal na cicatrização do cólon distal no rato. Acta Cir. Bras., 1993, 8, 48-53.
27 SILBERSTEIN LE., WILLIAMS JJ., HUGHLETT MA., MAGEE DA., WEISMAN RA. An autologous fibrinogen: based adhesive for use in othologic surgery. Transfusion, 1988, 28, 319-21.
28 SPOTNITZ WB. Fibrin sealant in the United States: clinical use at the University of Virginia. Thromb. Haemost., 1995, 74, 482-5.
29 YOHO AV., DRACH G., KOLETSKY S., PERSKY L. Experimental evaluation of tissue adhesives in urogenital surgery. J. Urol., 1964, 92, 56-9.
Received 27 August 1998
Accepted 23 August 1999
- 01 BIONDO-SIMÕES MLP., KOPPE GL., HANSEL H., DO ROSÁRIO MAK., MALAFAIA O. Uso de adesivo biológico em anastomoses intestinais: estudo experimental em cães. Acta Cir. Bras., 1992, 7, 151-3.
- 02 CARROL JF., MOSKOWITZ KA., EDWARDS NM., HIECKEY TJ., ROSE EA., BUDZYNSKI AZ. Immunological assessment of patient treated with bovine fibrin as hemostatic agent. Thromb. Haemost, 1996, 76, 925-31.
- 03 COOPER CW., FALB RD. Surgical adhesives. Ann. N. Y. Acad. Sci, 1968, 146, 214-24.
- 04 DARGENIO R., RANELLETTI FO., CIMINO C., RAGUSA G., PANETTA V., GARCCA N. Fibrin glue versus nylon anastomosis of fallopian tubes. J. Reprod. Med., 1986, 31, 961-5.
- 05 DURHAM LH., WILLATT DJ., YUNG MW., JONES I., STEVENSON PA. A method for preparation of fibrin glue. J. Laryngol. Otol, 1987, 101, 1182-6.
- 06 ELLIS DA., PELAUSA ED. Fibrin glue in facial plastic and reconstructive surgery. J. Otholaryngol., 1988, 17, 74-7.
- 07 FABRICIUS PG., JOCHAM D., PERMANETTER V., UNSOLD E. Application of fibrin adhesive in the urinary bladder. Urol. Res., 1987, 15, 307-10.
- 08 FERLIC DC., GLODNER L. Evaluation of the methyl - 2 - cyanoacrylote (Eastman 910 monomer) on peripheral nerves. South. Med. J., 1965, 58, 679-85.
- 09 FISCHL RA. An adhesive for primary closure of skin incisions. Plast. Reconstr. Surg., 1962, 30, 607-10.
-
10HAWLEY PR. Causes and prevention of colonic anastomotic breakdown. Dis. Colon Rectum, 1973, 16, 272-7.
-
11HEALEY JR JE., CLARK L., GALLAGER HS., O’NEILL P., SHEENA KS. Non-suture repair of blood vessels. Ann. Surg., 1962, 155, 817-26.
-
12HERRMANN JB., WOODWARD SC. The affect of cyenoacrylate tissue adhesives upon granulation tissue formation in ivalon sponge implants in rat. Surgery, 1966, 59, 559-64.
-
13HERRMANN JB., WOODWARD SC., PULASKI EJ. Healing of colonic anastomosis in the rat. Surg. Gynecol. Obstet, 1964, 119, 269-75.
-
14HJORTRUP A., NORDKILD P., KJERGAARD J., STONTOFT E., OLESEN HP. Fibrin adhesive versus sutured anastomosis: a comparative intra-individual study in the small intestine pigs. Br. J. Surg., 1986, 73, 760-1.
-
15IUAN FC., THOMAZINI IA., GIANNINI MJSM., VITERBO S., TOSCANO E., MORAES RA., BARRAVIERA B. Preparation of peripheral nerves with fibrin glue prepared from snakes venous. Preliminary results. Rev. Paul. Med, 1995, 113, 1000-2.
-
16JIBORN H., AHONEN J., ZEDERFELDT B. Healing of experimental colonic anastomoses. II Breaking strength of the colon after left colon resection and anastomosis. Am. J. Surg, 1978, 136, 595-9.
-
17KJAERGAARD J., NORDKILD P., SJONTOFT E., HJORTRUP A. Non-sutured fibrin adhesive versus sutured anastomosis. A comparative intra-individual study in dog colon. Acta Chir. Scand., 1987, 153, 599-601.
-
18KOEHNLEIN HE., LEMPERLE G. Experimental studies with a new gelatin - resorcin - formaldehyde glue. Surgery, 1969, 66, 377-82.
-
19KRAM HB., REUBEN BI., FLEMING AW., SHOEMAKER WG. Use of fibrin glue in hepatic trauma. J. Trauma, 1988, 28, 1195-201.
-
21LEITE CVS., NARESSE LE., KOBAYASI S., MINOSSI JG., BURINI RC., CURI PR., HOSSNE WS. Efeito da desnutrição protéica na anastomose do cólon distal no rato. Efeito da força ruptura e do colágeno tecidual. Acta Cir. Bras, 1993, 8, 145-50.
-
22MALAMENT M. Experimental bladder closure with a tissue adhesive. Investig. Urol., 1966, 3, 429-38.
-
23MARTINOVITZ V., SCHULMAN S. Fibrin sealant in surgery of patient with hemorrhage diathesis. Thromb. Haemost., 1995, 74, 486-92.
-
24MATHES GL., TERRY JJW. Non-suture closure of nephrotomy. J. Urol, 1963, 89,122-4.
-
25MEDEIROS AC., RAMOS CCF. FREIRE TMGL., PINTO JR FEL., MEDEIROS PJ., MELLO LEB., AZEVEDO FC. Uso de adesivo cirúrgico em anastomose do cólon. Estudo experimental em ratos. Acta Cir. Bras, 1990, 5, 136-4.
-
26NARESSE LE, LEITE CVS., RODRIGUES MAM., ANGELELI AYO., MINOSSI JG., KOBAYASI S. Efeito da peritonite fecal na cicatrização do cólon distal no rato. Acta Cir. Bras., 1993, 8, 48-53.
-
27SILBERSTEIN LE., WILLIAMS JJ., HUGHLETT MA., MAGEE DA., WEISMAN RA. An autologous fibrinogen: based adhesive for use in othologic surgery. Transfusion, 1988, 28, 319-21.
-
28SPOTNITZ WB. Fibrin sealant in the United States: clinical use at the University of Virginia. Thromb. Haemost, 1995, 74, 482-5.
-
29YOHO AV., DRACH G., KOLETSKY S., PERSKY L. Experimental evaluation of tissue adhesives in urogenital surgery. J. Urol, 1964, 92, 56-9.
 CORRESPONDENCE TO:
CORRESPONDENCE TO:Publication Dates
-
Publication in this collection
22 Sept 2000 -
Date of issue
2000
History
-
Accepted
23 Aug 1999 -
Received
27 Aug 1998