Abstract
In Korea, antivenoms for the treatment of patients bitten by venomous snakes have been imported from Japan or China. Although there is cross-reactivity between these antibodies and venoms from snakes indigenous to Korea (e.g. Agkistrodon genus), protection is not optimal. Antivenoms specifically prepared to neutralize Korean snake venoms could be more effective, with fewer side effects. To this end, we established an infrastructure to develop national standards and created a standardized method to evaluate the efficacy of two horse-derived antivenoms using mouse lethal toxin test. Additionally, we determined the antivenoms neutralizing activity against lethal doses (LD50) of Agkistrodon halys (from Japan) and Jiangzhe Agkistrodon halys (from China) venoms. We also performed cross-neutralization tests using probit analysis on each pairing of venom and antivenom in order to check the possibility of using Jiangzhe A. halys venom as a substitute for A. halys venom, the current standard. Slope of A. halys venom with A. halys antivenom was 10.2 and that of A. halys venom with Jiangzhe A. halys antivenom was 9.6. However, Slope of Jiangzhe A. halys venom with A. halys antivenom was 4.7 while that of Jiangzhe A. halys venom with Jiangzhe A. halys antivenom was 11.5. Therefore, the significant difference in slope patterns suggests that Jiangzhe A. halys venom cannot be used as a substitute for the standard venom to test the anti-lethal toxin activity of antivenoms (p<0.05).
anti-lethal toxin potency; Jiangzhe Angkistrodon halys; lethal activity; probit analysis; slope
ORIGINAL PAPER
Standardization of anti-lethal toxin potency test of antivenoms prepared from two different Agkistrodon halys venoms
Lee K. H.I; Won H. J.I; Kim S. N.I; Yoo S. H.I; Shin I. S.I; Shin K. H.I; Hong S. H.I; Lee S. H.II; Min H. K.I; Park S. N.I; Hur S. J.I
IDepartment of Biologics Evaluation, Korea Food and Drug Administration, Seoul, Korea
IINational Institute of Toxicological Research, Korea Food and Drug Administration, Seoul, Korea
Correspondence to Correspondence to: Sook Jin Hur Biologics Evaluation Department Korea Food and Drug Administration, 231 Jinheungno, Eunpyeong-Gu, Seoul 122-704, Korea Phone: 82 2 380 1321. Fax: 82 2 383 8322 Email: hursj@kfda.go.kr
ABSTRACT
In Korea, antivenoms for the treatment of patients bitten by venomous snakes have been imported from Japan or China. Although there is cross-reactivity between these antibodies and venoms from snakes indigenous to Korea (e.g. Agkistrodon genus), protection is not optimal. Antivenoms specifically prepared to neutralize Korean snake venoms could be more effective, with fewer side effects. To this end, we established an infrastructure to develop national standards and created a standardized method to evaluate the efficacy of two horse-derived antivenoms using mouse lethal toxin test. Additionally, we determined the antivenoms neutralizing activity against lethal doses (LD50) of Agkistrodon halys (from Japan) and Jiangzhe Agkistrodon halys (from China) venoms. We also performed cross-neutralization tests using probit analysis on each pairing of venom and antivenom in order to check the possibility of using Jiangzhe A. halys venom as a substitute for A. halys venom, the current standard. Slope of A. halys venom with A. halys antivenom was 10.2 and that of A. halys venom with Jiangzhe A. halys antivenom was 9.6. However, Slope of Jiangzhe A. halys venom with A. halys antivenom was 4.7 while that of Jiangzhe A. halys venom with Jiangzhe A. halys antivenom was 11.5. Therefore, the significant difference in slope patterns suggests that Jiangzhe A. halys venom cannot be used as a substitute for the standard venom to test the anti-lethal toxin activity of antivenoms (p<0.05).
Key words: anti-lethal toxin potency, Jiangzhe Angkistrodon halys, lethal activity, probit analysis, slope
INTRODUCTION
Three members of the Agkistrodon genus: A. brevicaudus, A. saxatilis, and A. caliginosus, have been vastly investigated (4). Agkistrodon snakes are widely distributed throughout Korea, Japan, Siberia, China, Central Asia, and Eastern Europe. Some scientists (8) insist that metalloproteinases and disintegrins (nonenzymatic platelet aggregation inhibitors) are important components of most viperine and crotalic venoms, but their immunogenicity and toxicity are still not well understood.
Hospital reports of about 410 snakebite accidents per year are most frequent from the last 10 days of April to the middle of October in Korea. These incidents often occur in farm villages and mountains but their exact reason is unknown. As Agkistrodon saxatilis and Agkistrodon usuriensis inhabit relatively high altitudes, the incidence of bites by these two species is small.
To ensure the efficacy of any antivenom, the interaction between venom and antivenom should always be evaluated (9). The first preparations of Japanese A. halys antivenom were commercially available in 1990; Chinese Jiangzhe A. halys antivenom came out to the market in 1997. Since then, their effectiveness in treating envenomation has been widely accepted.
According to bioassay principles (7), the potency of any antitoxin should be determined relative to a stable standard antitoxin, similarly to the titration of diphtheria or tetanus antitoxin. It has been reported that A. halys venom contains at least two lethal fractions and two hemorrhagic fractions, as demonstrated by DEAE-cellulose chromatography (12), and that there is a close association between the major part of its toxic activity and its main hemorrhagic fraction.
In the present paper, we indicate a new method of establishing an infrastructure to develop national standards and standardized a method to evaluate the anti-lethal toxin potency of two horse-derived antivenoms using an intravenous (mouse tail vein) test. This study was carried out during 1999 in our laboratory to confirm the hypothesis that Jiangzhe A. halys and A. halys venoms have similar lethal effects and to compare the efficacy of both A. halys and Jiangzhe A. halys antivenoms.
MATERIALS AND METHODS
Snake lethal venoms
The venoms used were Mamushi venom (Lethal toxin titer: 530 test doses/ampoule, Lot 3-2, 50 mg protein/ampoule), supplied by the National Institute of Infectious Diseases (NIID), Japan, and Jiangzhe A. halys venom, supplied by the Shanghai Institute of Biological Products (SIBP), China. Jiangzhe A. halys venom contains 10 mg protein/ampoule. For the lethal test, both stock venoms were diluted to a desired potency immediately before use.
Antivenoms
Test antivenoms were: national standard equine Mamushi antivenom (Anti-lethal toxin titer: 14,200 U/ampoule, Lot C), produced by NIID, Japan, and equine Jiangzhe Agkistrodon halys antivenom, produced by SIBP, China.
Experimental animals
White inbred ICR mice of both sexes, aged 4-6 weeks (14-16 g), were used to determine the venoms lethal toxicity and the antivenoms anti-lethal toxin potency. The animals were fed on Purina Rodent Chow and received water ad libitum for 10 days prior to the study.
Venoms lethal toxicity
The venoms lethal toxicity was assayed by using intravenous (1) and intraperitoneal (3) injections into mice. Venom was subjected to 4-5 serial dilutions (1.4-fold increments) in 17 mM phosphate buffered sodium chloride solution (pH 7.0) containing 0.2% gelatin. Then, an aliquot of 0.1 ml of each dilution was either intravenously or intraperitoneally injected into 4-8 mice. The dilutions range was selected to cover the entire mortality range (from 0%-100%). Deaths up to 48 h after injections were ascribed to venom toxicity, although most deaths occurred within 24 h. The LD50 was calculated using both the Reed-Muench method (15) and probit analysis (2, 10, 16). The lethal toxicity of venoms was determined by the interpolation of their dose-response curves using Statistical Analysis Program (STA77), which was established by National Institute of Infectious Diseases (NIID), Japan, on March 2002.
Antivenoms effective dose 50% (ED50)
Aliquots of diluted A. halys and Jiangzhe A. halys venoms were mixed with the same volume of each serial dilution of antivenoms (4-5 serial dilutions, graded with 1.25-fold intervals from 5 U to 19.2 U). Each mixture was kept at room temperature for 1 h; then, 0.2 ml was intravenously or intraperitoneally injected into 8 mice. Animals were observed for 48 h, the number of dead animals was recorded and the antivenom ED50 was calculated using probit analysis (2). The antivenom protective potency was estimated according to the method of anti-lethal toxin potency of Jiangzhe A. halys antivenom described in the Results.
Bicinchoninic acid [BCA] protein assay (6)
Enzyme-linked immunosorbent assay (ELISA) was carried out to detect the amount of proteins in A. halys antivenom and Jiangzhe A. halys antivenoms. We used bovine serum albumin diluted to various concentrations (25, 125, 250, 500, 750, 1000, 1500 and 2000 µg) to generate a standard concentration curve. Two different venom proteins were adsorbed to a solid surface. Total protein concentrations of antivenoms against A. halys and Jiangzhe A. halys venoms were determined by using an ELISA reader (Spectra-max 340 PC Molecular Device, U.S.A.). Absorbance of each dilution concentration was recorded at 562 nm. Graphical representation of the relationship between the standard dose of bovine serum albumin and the solution protein concentration was used to plot the results. Linear function was determined using Microsoft Excel 97 for Windows. The linear function of antivenom protein concentrations was computed.
Electrophoresis and immunoglobulin concentration
Electrophoresis was performed in order to analyze the mobility of immunoglobulin. It was carried out on cellulose acetate strip, pH 8.6, using veronal buffer. Protein was stained with Ponceau S solution and destained with a mixture of 7.5% acetic acid and 5% methanol. Protein purities were determined using a densitometer (Helena Lab. U.S.A.). Antivenoms nitrogen concentration was estimated according to the micro-Kjeldahl method, and equine whole IgG was evaluated using a densitometer. Standard protein markers (horse serum) were used.
Ouchterlony techniques (14)
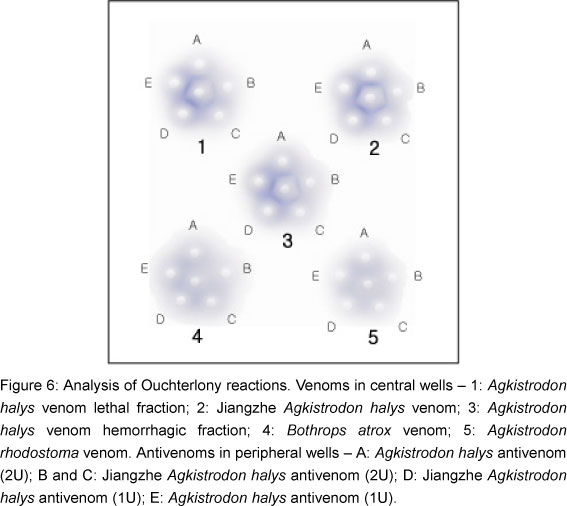
Ouchterlony gel diffusion was carried out on 1-mm thin plates of 1.2% agar. First, venoms (A. halys venom lethal fraction, Jiangzhe A. halys venom, A. halys venom hemorrhagic fraction, Bothrops atrox venom, and Agkistrodon rhodostoma venom; 10 µg/ µl each) were placed in central wells. Antivenoms (2 U / 10 µl or 1 U / 10 µl) against their respective venoms were added to peripheral wells; plate was kept at room temperature for 24 h. The precipitin line was stained with Coomassie brilliant blue reagents.
RESULTS
Venoms lethal toxicity
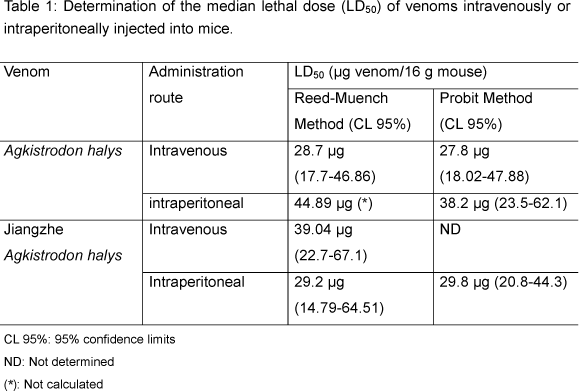
According to the Reed-Muench method, the LD50 of A. halys and Jiangzhe A. halys venoms, when intravenously injected, was 28.7 µg / 16g and 39.04 µg / 16 g mouse, respectively. When intraperitoneally injected, the LD50 of A. halys and Jiangzhe A. halys venom was 44.89 µg/16 g and 29.2 µg/16 g mouse, respectively (Table 1).
Agkistrodon halys venom showed higher LD50 when intrapeitoneally injected than when intravenously injected. Jiangzhe A. halys venom had higher LD50 when intravenously injected. There was no difference between Reed-Muench and probit method.
One lethal dose determination
To determine one lethal dose, 0.1 ml of venom solution was intravenously or intraperitoneally injected into mice. For the intraperitoneal injection tests, five serial dilutions containing 51; 71.4; 100; 140; 196 µg A. halys and 85.7; 120; 168; 235.2; 329.28 µg Jiangzhe A. halys venom were used.
Ten units (U) of Jiangzhe A. halys or A. halys antivenom were tested against 0.1 ml of either Jiangzhe A. halys or A. halys venom solution. The relative potency of the two parenteral administration methods was estimated by measuring the animals' response to various concentrations of each antivenom preparation. Control mice were given 0.2 ml PBS alone, without antivenom. When given intraperitoneally, the venoms lethal test doses were significantly higher than when administered intravenously (Table 2).
Cross-neutralization test
The neutralizing capacity of both antivenoms was designated as ED50, which was defined as the unit of antivenom per test dose of venom (µg) capable of reducing the venom effects by 50%.
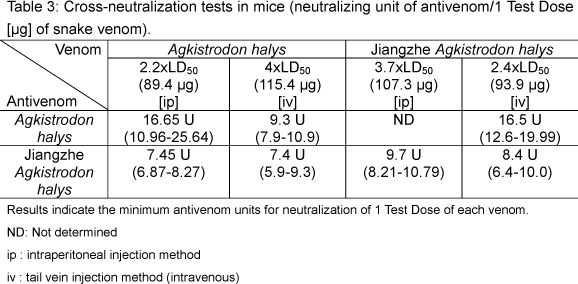
The ED50 of A. halys and Jiangzhe A. halys antivenoms intravenously administered against 115.4 µg A. halys venom [4 x LD50] was 9.3 U and 7.4 U, respectively (p<0.05) The ED50 of A. halys and Jiangzhe A. halys antivenoms intravenously administered against 93.9 µg Jiangzhe A. halys venom [2.4 x LD50] was 16.5 U and 8.4 U, respectively. There was a statistically significant difference between the neutralizing activity of A. halys and Jiangzhe A. halys antivenoms (p<0.05; Table 3).
When intraperitoneally injected into mice, the ED50 of A. halys and Jiangzhe A. halys antivenom against 89.4 µg [2.2 x LD50] was 15.3 U and 7.3 U, respectively. In this case, the potency of the anti-lethal toxin effect of Jiangzhe A. halys antivenom was two-fold higher than that of A. halys antivenom (p>0.05; Table 3). The ED50 of Jiangzhe A. halys antivenom intraperitoneally injected against 107.3 µg Jiangzhe A. halys venom [3.7 x LD50] was 9.7 U. For the A. halys antivenom intraperitoneal injection against 107.3 µg Jiangzhe A. halys venom [3.7 x LD50], the regression response on the logarithmic scale was nonlinear (Table 3).
Antivenoms effective dose 50%
According to probit analysis of intravenous administration of antivenoms against 115.4 µg A. halys venom [4 x LD50], the ED50 of A. halys antivenom was 10.12, and that of Jiangzhe A. halys antivenom was 7.52. Jiangzhe A. halys antivenom potency was 1.34-fold higher than that of A. halys antivenom. When administered intravenously, A. halys and Jiangzhe A. halys antivenoms were capable of completely neutralizing one test dose of 115.4 µg A. halys venom [4 x LD50] (Table 4.1 and Figure 1); 19.2 U Jiangzhe A. halys antivenom was sufficient to completely neutralize 93.9 µg Jiangzhe A. halys venom [2.4 x LD50], but the same amount of A. halys antivenom did not completely neutralize it (Table 4.2 and Figure 2).
To test the efficacy of intraperitoneal administration of antivenoms versus the currently used method (mouse tail vein), we carried out cross-neutralization studies for each pairing of venom and antivenom. Both A. halys and Jiangzhe A. halys antivenoms were diluted to five different concentrations (6.4; 8.0; 10.0; 12.5; 16.0 U) in a total volume of 0.1 ml and tested against 89.4 µg A. halys venom [2.2 x LD50].
Intraperitoneal administration of 10.0 U Jiangzhe A. halys antivenom completely neutralized 89.4 µg A. halys venom, but a similar response was not seen when A. halys antivenom was used, even up to the dose of 16.0 U (Table 5.1 and Figure 3).
The ED50 of A. halys and Jiangzhe A. halys antivenoms intraperitoneally administered against 107.3 µg Jiangzhe A. halys venom [3.7 x LD50] was assessed (Table 5.2 and Figure 4). Although 16.0 U of A. halys antivenom was not capable of completely neutralizing 107.3 µg Jiangzhe A. halys venom, a dose as little as 12.5 U of Jiangzhe A. halys antivenom could lead to complete neutralization.
Slopes homogeneity
We compared the slopes of regression lines of the relationship between death rate and dose of both Chinese and Japanese antivenoms.
After substituting Jiangzhe A. halys venom for A. halys venom in the current method, we performed slope assays and comparisons between intravenous and intraperitoneal administration for each antivenom against each venom (Table 6).
Slope ratio can be used to test the relative potency of two preparations and is estimated by the relation between the two regression coefficients.
Agkistrodon halys and Jiangzhe A. halys antivenom intravenously injected against 115.4 µg A. halys venom [4 x LD50] showed slopes of 10.2 and 9.6, respectively.
We then investigated the linearity and parallelism of log-dosage response curves for both antivenoms intravenously injected against 115.4 µg A. halys venom [4 x LD50]. Results reinforced the above-stated conclusion that the log-dosage response curves were linear and parallel with each other. Properties of slopes ratio indicate relative potency of control (Japan antivenom) and tested materials (China antivenom). In terms of efficacy, this means that Jiangzhe A. halys antivenom is an adequate treatment for Japanese snakebite patients.
Slopes of A. halys and Jiangzhe A. halys antivenoms intravenously administered against 93.9 µg Jiangzhe A. halys venom [2.4 x LD50] were 4.7 and 11.5, respectively. So, in this case, results were linear but non-parallel with each other, demonstrating that Jiangzhe A. halys venom cannot be used as a standard toxin to test the anti-lethal toxin potency of both antivenoms using the mouse tail vein injection method. This difference in the neutralizing capacity is probably related to antigenicity.
The intraperitoneal injection method showed that the slopes of A. halys and Jiangzhe A. halys antivenoms against 89.4 µg A. halys venom [2.2 x LD50] were 2.5 and 15.5, respectively. Slopes of A. halys and Jiangzhe A. halys antivenoms against 107.3 µg Jiangzhe A. halys venom [3.7 x LD50] were 1.4 and 8.8, respectively. Such results were linear but non-parallel with each other.
Anti-lethal toxin potency of Jiangzhe A. halys antivenom
One test dose of 10 L+ leveled A. halys toxin (the minimum amount of toxin which when combined with 10 I.U. of antitoxin is capable of killing a 16-g mice in two days) was determined by the multiple level beta-procedure. The anti-lethal toxin titer of A. halys antivenom (control) was 300 units potency, whereas that of Jiangzhe A. halys antivenom (tested material) was 323 units for the same mouse samples (Table 7), and the potency of A. halys and Jiangzhe A. halys antivenoms against A. halys venom was almost the same.
Therefore, the main lethal fraction of A. halys venom should be used as a test toxin. The ED50 of each test and standard antivenom was determined using one lethal dose of test toxin. The parallelism and linearity of the neutralization curves were assessed by statistical analysis.
Anti-lethal toxin potency of test antivenom was determined by comparing their relative potency with that of the standard antivenom using probit analysis.
Antivenom immunological analysis
The electrophoresis profile of each antivenom is shown in Figure 5. Protein composition of Jiangzhe A. halys antivenom (Figure 5, Lane 3) showed the mobility of a pure immunoglobulin preparation. In contrast, only 84.9% of A. halys antivenom demonstrated immunoglobulins mobility (Figure 5, Lane 4). So, Jiangzhe A. halys antivenom preparation is more purified than that of A. halys antivenom. Comparison of the antivenoms activity is shown in Table 8.
Total protein concentration of Jiangzhe A. halys antivenom is 12.60 mg/ml versus 13.44 mg/ml of A. halys antivenom, and the specific activity of A. halys antivenom is 1.1-fold higher than that of the Jiangzhe A. halys antivenom (Table 8).
Ouchterlony test demonstrated that A. halys and Jiangzhe A. halys antivenoms cross-reacted with both venoms, which indicates that both A. halys and Jiangzhe A. halys venoms share identical antigenic structures. But, when exposed to either B. atrox or A. rhodostoma venoms, these antivenoms did not show a precipitated line using the precipitin test (Figure 6).
DISCUSSION
There are approximately 16 snake species in Korea. Among them, only three belong to the Agkistrodon genus. Snake venom biological specificity has been determined through intra and inter-species analysis. To obtain a precise classification of snakes, it is important to know their geographical distribution, age, the season when accidents occurred, and whether their venoms are homologous or heterologous. Effective treatment for snake envenomation is difficult. It is recommended that, in each country, antivenoms be produced from venoms obtained from its indigenous snakes (13). However, if an antivenom has significant immunological cross-reactivity with a venom from a different species of a different region or country, it can be used as an effective treatment for snakebites (9). In order to treat envenomation by snakes of the Agkistrodon genus, which is indigenous to Korea, the commercial antivenoms Jiangzhe A. halys (from China) and A. halys (from Japan) have been imported, and the quality control test for these products have been based on A. halys antivenom and venom standards.
Grasset (5) proposed that the method for antivenom potency determination should be unified using a multiple-level or single-level procedure (choosing the suitable level), a highly active and stable standard venom, and a standard antivenom. He also suggested that expression of the antivenom potency should be standardized to a suitable international unit. This proposal will only hold true if the neutralization curves of both standard and test antivenoms against the standard venom are all linear and parallel with each other. So, to comply with this suggestion, our first step was to select both standard antivenom and venom. Although A. halys venom has been used as standard, the present study used Jiangzhe A. halys venom as its substitute and demonstrated anti-lethal toxin potency methods for Jiangzhe A. halys and A. halys antivenoms.
Jiangzhe A. halys snakes (18) were captured from Chiangsu and Chechiang, provinces of China. Agkistrodon halys snakes were captured from the mainland of Japan. Japanese partially-purified test toxins (11) were prepared using zone electrophoresis of crude venom in a column packed with starch. Each antivenom was prepared by obtaining venom-specific neutralizing globulins present in the serum of healthy horses hyperimmunized against crude Agkistrodon venoms. To standardize anti-lethal toxin potency assays (17) for the characterization of Jiangzhe Agkistrodon halys venoms, we verified the relationship between injection route and lethality (LD50) using two groups of mice: the first group was injected with A. halys venom and the second received Jiangzhe A. halys venom. The test dose, intraperitoneally administered, was significantly higher than that intravenously injected.
The capacity of two horse-derived antivenoms to neutralize lethal toxin activities induced by A. halys and Jiangzhe A. halys venoms was tested.
To substitute the standard method of intraperitioneal injection by the method of mouse tail vein injection, we used cross-neutralization test of A. halys and Jiangzhe A. halys venoms and antivenoms. In the intraperitioneal test, A. halys antivenom against A. halys venom showed linearity (p>0.05). So, the intraperitoneal test can be used as the anti-lethal toxin activity test.
To substitute Jiangzhe A. halys venom for A. halys venom in the anti-lethal activity test, we also performed cross-neutralization test. Slope of A. halys venom with A. halys antivenom was 10.2 and that of A. halys venom with Jiangzhe A. halys antivenom was 9.6. However, slope of Jiangzhe A. halys venom with A. halys antivenom was 4.7 and that of Jiangzhe A. halys venom with Jiangzhe A. halys antivenom was 11.5. Therefore, it is considered that Jiangzhe A. halys venom cannot be used as a standard to test anti-lethal activity (p<0.05).
ACKNOWLEDGEMENTS
We thank Dr. Motohide Takahashi (National Institute of Infectious Diseases, Japan), Dr. Setsuji Ishida (Tokyo Women's Medical College, Japan), Dr. Wei Zhu (Shanghai Institute of Biological Products, China) for their valuable collaboration, and Korea Food and Drug Administration for financial support.
Received: August 8, 2005
Accepted: December 8, 2005
Abstract published online: January 20, 2006
Full paper published online: November 30, 2006
- 1 ASSOCIATION OF BIOLOGICAL MANUFACTURERS OF JAPAN. Freeze-dried mamushi antivenom, equine. Minimum Requirements for Biological Products, 1986, 106-110.
- 2 FINNEY DJ. Statistical method in biological assay. Charles Griffin & Company Ltd, London, 3.ed., 1978, 69-104.
- 3 GINGRICH W., HOHENADEL J. Standardization of polyvalent antivenin. In: BUCKLEY E., PORGES N. Eds. Venoms Washington: American Association for the Advancement of Science, 1956: 381-385.
- 4 GLOYD HK. The Korean snakes of the genus Agkistrodon (Crotalidae). Proc. Biol. Soc. Wash, 1972, 85, 557-78.
- 5 GRASSET E. Survey of assay methods of antivenins; immunological factors influencing antivenin standardization. Bull. World Health Organ, 1957, 16, 79-122.
- 6 HILL GD., STRAKA JG. Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal. Biochem, 1988, 170, 203-8.
- 7 JERNE NK., WOOD EC. The validity and meaning of the results of biological assays. Biometrics, 1949, 5, 272-99.
- 8 KAMIGUTI AS., ZUZEL M., THEAKSTON RDG. Snake venom metalloproteinases and disintegrins: interactions with cells. Braz. J. Med. Biol. Res, 1998, 31, 853-62.
- 9 KAWAMURA Y. Study of the immunological relationships between venom of six Asiatic Agkistrodons. The Snake, 1974, 6, 19-26.
- 10 KUROGAWA M. Are vaccines safe? Tokyo: Au Tsuki-Publishing Company, 1993. 128p.
- 11 OHSAKA A., OMORI-SATOH T., KONDO S., MURATA R. Biochemical and pathological aspects of hemorrhagic principles in snake venoms with special reference to Habu (Trimeresurus flavoviridis) venoms. Mem. Inst. Butantan, 1966, 33, 193-205.
- 12 OMORI T., IWANAGA S., SUZUKI T. The relationship between the hemorrhagic and lethal activities of Japanese mamushi (Agkistrodon halys blomhoffii) venom. Toxicon, 1964, 15, 1-4.
- 13 OTERO R., NUNEZ V., GUTIERREZ JM., ROBLES A., ESTRADA R., OSORIO RG., DEL-VALLE G., VALDERRAMA R., GIRALDO CA. Neutralizing capacity of a new monovalent anti-Bothrops atrox antivenom: comparison with two commercial antivenoms. Braz. J. Med. Biol. Res, 1997, 30, 375-79.
- 14 OUCHTERLONY O. Antigen-antibody reactions in gels. Acta Path Microbiol. Scand, 1949, 26, 516-24.
- 15 REED LJ., MUENCH H. A simple method of estimating 50% end points. Am. J. Hyg, 1938, 27, 493-97.
- 16 SNEDECOR GW., COCHRAN WG. Statistical method 7.ed. Ames: The Iowa State University Press, 1980. 17p.
- 17 THEAKSTON RDG., REID HA. Development of simple standard assay procedures for the characterization of snake venoms. Bull. World Health Organ, 1983, 61, 949-56.
- 18 ZHU W., LIU W., MA Y. Neutralizing capacity of purified Jiangzhe Agkistrodon halys antivenom to Korean snake venoms. Chin. J. Biol, 2000, 13, 211-14.
Publication Dates
-
Publication in this collection
11 Jan 2007 -
Date of issue
2006
History
-
Accepted
08 Dec 2005 -
Received
08 Aug 2005