Abstract
The purpose of the present study was to investigate the biodistribution profile of the venom of Hemiscorpius lepturus, the most dangerous scorpion in Iran. Blood and tissue samples were taken at various predetermined intervals during a 400-minute period for the venom and a 360-minute period for the antivenom in rats. The radio-iodination was carried out using the chloramine-T method. The results showed that the descending order of venom uptake was skin, kidneys and intestine, respectively. The descending order of polyclonal antivenom uptake was kidneys, intestine, heart and lungs. The calculated pharmacokinetic parameters of the venom were Telimination half-life = 521.5 ± 12.6 minutes; Vd/F (apparent volume of distribution) = 14.9 ± 3.3 mL; clearance (CL/F, apparent total clearance of the drug from plasma) 0.02 ± 0.005 mL/minute and for the antivenom Telimination half-life = 113.7 ± 7.4 minutes; Vd/F = 13 ± 1.2 mL and CL/F 0.08 ± 0.01 mL/minute. The pharmacokinetics profile comparison of the venom with that of the antivenom shows that serotherapy may be more effective if administered within 2-4 hours following envenomation by H. lepturus.
Hemiscorpius lepturus scorpion venom; polyvalent antivenom; pharmacokinetic parameters; tissue distribution
ORIGINAL PAPER
A biodistribution study of Hemiscorpius lepturus scorpion venom and available polyclonal antivenom in rats
Seyedian RI; Jalali AII; Babaee MHII; Pipelzadeh MHIII; Rezaee SIV
IDepartment of Pharmacology and Toxicology, Bushehr University of Medical Sciences, Bushehr, Iran
IIDepartment of Pharmacology and Toxicology, School of Pharmacy, and Toxicology Research Centre, Jundishapur University of Medical Sciences, Ahvaz, Iran
IIIDepartment of Pharmacology, School of Medicine, and Toxicology Research Centre, Jundishapur University of Medical Sciences, Ahvaz, Iran
IVDepartment of Pharmaceutics, School of Pharmacy, Jundishapur University of Medical Sciences, Ahvaz, Iran
Correspondence to Correspondence to: Amir Jalali Department of Pharmacology and Toxicology, School of Pharmacy, and Toxicology Research Centre of Jundishapur University of Medical Sciences Ahvaz, Iran Phone: + 0098 611 3738380. Fax: 0098 611 3738381 Email: amjalali@hotmail.com
ABSTRACT
The purpose of the present study was to investigate the biodistribution profile of the venom of Hemiscorpius lepturus, the most dangerous scorpion in Iran. Blood and tissue samples were taken at various predetermined intervals during a 400-minute period for the venom and a 360-minute period for the antivenom in rats. The radio-iodination was carried out using the chloramine-T method. The results showed that the descending order of venom uptake was skin, kidneys and intestine, respectively. The descending order of polyclonal antivenom uptake was kidneys, intestine, heart and lungs. The calculated pharmacokinetic parameters of the venom were Telimination half-life = 521.5 ± 12.6 minutes; Vd/F (apparent volume of distribution) = 14.9 ± 3.3 mL; clearance (CL/F, apparent total clearance of the drug from plasma) 0.02 ± 0.005 mL/minute and for the antivenom Telimination half-life = 113.7 ± 7.4 minutes; Vd/F = 13 ± 1.2 mL and CL/F 0.08 ± 0.01 mL/minute. The pharmacokinetics profile comparison of the venom with that of the antivenom shows that serotherapy may be more effective if administered within 2-4 hours following envenomation by H. lepturus.
Key words: Hemiscorpius lepturus scorpion venom, polyvalent antivenom, pharmacokinetic parameters, tissue distribution.
INTRODUCTION
Envenomation by various species of scorpions is a serious public health issue in many regions of the world. In Iran, so far 24 species of scorpions have been identified, 12 of which are found in the Khuzestan province in the southwest region of this country (1). The medically relevant species found in this region are Androctonus crassicauda, Mesobuthus eupeus and Hemiscorpius lepturus. Unlike the first two, which belong to Buthidae family and produce sympathomimetic and parasympathetic overstimulation, the latter is a member of Scorpionidae family (subfamily Hemiscorpiidae), the most venomous of all types of scorpions, contributing to 95% of scorpion-associated mortalities (2-4). Dermonecrotic reactions, anemia, hemolysis, renal failure, cardiovascular and central nervous system disorders are usual clinical manifestations of envenomation by this scorpion (2, 3, 5).
A specific polyvalent antivenom is produced by the Razi Vaccine and Serum Research Institute through the immunization of horses against the six medically relevant scorpion species in Iran: Odontobuthus doriae, Mesobuthus eupeus, Androctonus crassicauda, Buthotus saulcyi, Buthotus sach and H. lepturus (6). Immunotherapy by polyvalent antivenom produced by the Razi Institute of Iran is the conventional treatment for envenomation caused by H. lepturus, though the efficacy of such treatment by intravenous route is controversial. Envenomed patients by this scorpion are usually treated by intravenous administration of antivenom. Nevertheless, there is disagreement among physicians on the best way to neutralize the toxic effects of envenomation and the correct time for antivenom administration (7). It has been generally accepted that the ideal antivenom must reach the different tissues in which the venom produces its toxic effects and once bonded the complex must be rapidly eliminated (8). As a whole, there is no general consensus about the dose, route of administration and its effectiveness in treating human envenomation by this scorpion.
International pharmaceutical regulations recommend pharmacokinetic studies concerning the drugs to be used by humans in order to evaluate their safety and the appropriate dosage. In summary, pharmacokinetics contributes to the understanding of the relationship between dose and response in the design of new therapies and/or modifications of the current medicinal products.
Labeled antivenoms have been used in biodistribution and pharmacokinetics studies for determining the efficacy of the antivenom. These studies were done with antivenom labeled with 125I (9, 10). Previous experimental pharmacokinetic studies employed radiolabeling techniques to show the pharmacokinetics of Androctonus amoreuxi, Leiurus quinquestriatus, Buthotus judaicus and Androctonus crassicauda venoms (11-13).
Despite well known severe toxic manifestations, no pharmacokinetic study has been previously carried out on the venom of H. lepturus. Intravenous (IV) administration of the available antivenom is normally prescribed by physicians for the treatment of H. lepturus envenomation. This toxicokinetic study, therefore, was designed to evaluate the pharmacokinetic parameters of the venom and the available polyvalent antivenom. For this purpose, a protocol was selected to closely simulate human accidental envenomation and its distribution in various body tissues was determined in Sprague Dawley rats. Trichloroacetic acid (TCA), precipitated radiolabeled venom and antivenom were employed in the study and pharmacokinetic parameters of the raw venom of H. lepturus (after subcutaneous administration) and Razi Institute polyvalent antivenom (via intravenous route) were also evaluated.
MATERIALS AND METHODS
Animals
Sprague Dawley rats (100 to 150 g body weight) were used in this experiment. The rats were purchased from Shaheed Beheshti Breeding Laboratories and housed in groups of three in PVC cages with free access to tap water and hard food pellets. They were kept at 23 ± 2ºC, and maintained at a 12-hour light/dark cycle, starting at 7 am.
Materials
The lyophilized venom was prepared from electrically stimulated telson of H. lepturus collected during summer in the Khuzestan province. The polyvalent scorpion antivenom (ampoules of 5 mL stored at 2 to 8ºC) was provided by the the Razi Institute and the Hesarak Karaj facilty in Iran. This product is a pepsin-digested, refined and concentrated preparation obtained from equine hyperimmune serum (6) ). The final antivenom is adjusted to contain the desired neutralizing antibody titer in about 10.3 mg of protein/mL. The pH was adjusted to 6.4.
Sodium iodine Na125I (specific activity = 0.2 mCi/µL of iodide), chloramine-T and sodium metabisulfite were purchased from Sigma-Aldrich Co (USA) and Sephadex G-50 column was provided by Pharmacia (Sweden).
Labeling of H. lepturus Venom and Polyvalent Antivenon
H. lepturus venom was radioiodinated using the chloramine-T method as previously described (14) with some modifications. The reaction was initiated by addition of 10 µL (5mg/mL) chloramine-T solution - prepared in a 0.05 M sodium phosphate buffer (pH 7.6) - to 10 µL of aqueous solution of lyophilized venom in distilled water (1 mg/mL) mixed with 10 µL of 125I-Na solution (2 mCi). After one minute, the reaction was stopped using 10 µL of a 5 mg/mL sodium metabisulfite. All the unbound [125I] iodide was displaced by addition of 100 µL of potassium iodide (100 mg/mL). 125I-venom was separated from free iodine by gel filtration through a Sephadex G50 column equilibrated with PBS, pH 7.4, 30 mL/hour. Radiolabeled fragment was collected sequentially (1 mL) by loading with PBS and its radioactivity assessed by gamma counter. Six samples with greatest radioactivity were separated and used in the experiment.
To determine the pharmacokinetics of radiolabeled Razi Institute polyvalent antivenom in the envenomed rats, 300 µL were added to the same volume of phosphate buffer solution. Subsequently, 300 µL of the mixture were removed and the radiolabeling method was performed similar to that in the previous experiment. After labeling, radiolabeled venom and antivenom were kept at 4ºC until the time of use. The antivenom contained a dilution of the F (ab´) 2 fraction of equine immunoglobulins obtained after double saline precipitation and pepsin digestion. The mean protein content of the antivenom from the used batches was 3.6 mg/mL, with a neutralizing ability of 26 LD50/mL. The specific radioactivity of the substrates (venom and antivenom) was approximately 220000 cpm/mg protein (cpm: count per minute).
Animal Experiments
On the day of the experiment, 0.2 mL of the labeled H. lepturus venom and polyvalent antivenom solution were injected subcutaneously and intravenously, respectively, in separate experiments to seven groups of rats (n = 3). Subcutaneous injections of the venom were administered to the gluteal region and all antivenom injections were performed from tail vein. Animals (three for each time point) were euthanized with carbon dioxide 10, 40, 60, 120, 240, 360 and 400 minutes (for venom) and 5, 10, 40, 60, 120, 240 and 360 minutes (for polyvalent antivenom) post injection, to determine the distribution of radiolabeled compounds in blood and internal organs.
The blood was obtained from the heart after euthanasia. The organs were deposited in separate pre-weighed disposable tubes. Selected organs plus the skin at the site of venom injection were weighed, and their radioactivity was measured by the nucleus automatic well-type γ-counter (Model 600 B, Gammatec II, INC, Oakridge). The skin from the site of injection was used to determine the radioactivity percent. A 2-centimeter incision (diameter) was made to the skin at the inoculated site in order to determine radioactivity/gram. At least two standards of the injected material were set aside and counted at the same time to correct for physical decay of [125I]. The percent of the injected dose (based on the radioactivity count) per organ weight (%ID/g) was determined. Rat blood density (1.05) was used for calculation of percent of initial count per milliliter of blood to determine pharmacokinetic parameters of the venom and antivenom (15). The Ethic Committee of the Jundishapur University, Ahvaz approved of the design of the experiment, and the protocol conforms to the guidelines of the National Institutes of Health (NIH).
Determination of Pharmacokinetic Parameters
The sparse radioactive counts per milliliter of blood data relative to the initial count versus time were fitted to a one-compartment open model after an IV bolus dose for antivenom and to a one-compartment open model with first-order absorption for venom using a non-linear mixed effects modeling approach. Exponential and additive error models were used to describe inter- and intra- animal variability, respectively. The modeling was done using Monolix 3.1 software (16).
Statistical Analysis
The biodistribution of venom and antivenom were presented as mean ± standard deviation. The means were calculated from at least three separate experiments. Pharmacokinetic parameters were expressed as mean ± SEM. The significance of the data between two groups was analyzed by the Paired Students' t-test. Statistical comparison between groups was made by one-way ANOVA. The level of significance was set at p < 0.05.
RESULTS
Radiolabeled Venom Distribution
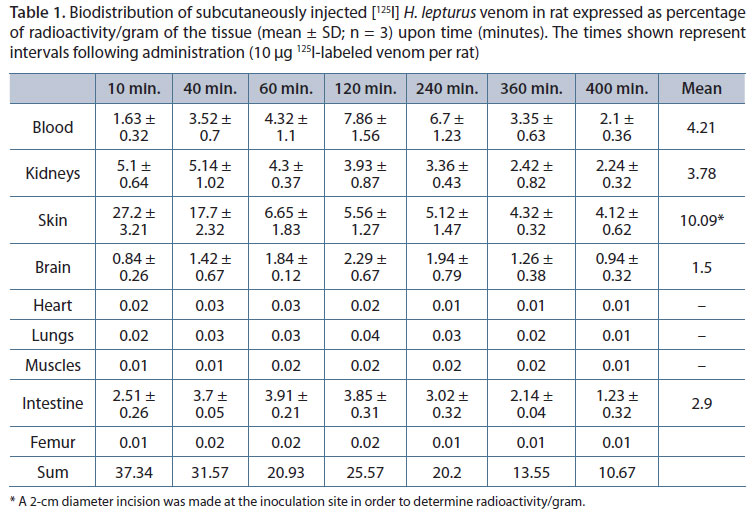
The distribution profile of the radiolabeled venom in nine different organs during the experimental period of 400 minutes is shown in Table 1. In addition, the trend of change in concentration of the venom in four main organs where the venom was mostly distributed (blood, kidneys, intestine and brain) and the absorption from the site of injection is illustrated in Figure 1 and 2, respectively. Ten minutes after subcutaneous administration, the highest radioactivity was measured in the skin followed by the kidneys and intestine. Although the initial concentration of the venom in the intestine was lower than that measured in the kidneys, after 60 minutes their levels of distribution were very similar. The highest measured radioactivity in the small intestine at 60 minutes was 3.91%ID/g and at 400 minutes its concentration reached 1.23.
The level of radioactivity reached its peak in the blood (7.86% ID/g) after 120 minutes and then decreased slowly. However, after 400 minutes the radioactivity of the venom in the blood was still higher than in other organs except the skin and equaled that of the kidneys. A much lower concentration was found to be distributed in other organs such as bones, lungs, muscles, and heart. Among these organs, the main site was the brain. This site reached its peak at 120 minutes. Such time interval, 120 minutes, is correlated with the maximum plasma radioactivity/gram and dropped to 0.94ID/g after 400 minutes.
The radioactivity of the blood was significantly elevated and subjected to definitive changes within 10-40 minutes compared with that of the beginning of the experiment (p < 0.001). This amount remained without a significant change for 120 minutes (120-240 minutes following venom administration (Table 1).
Absorption of the venom from the injection site was relatively quick after subcutaneous injection, with a rapid initial phase that lasted 60 minutes, followed by a slight decline. After 60 minutes until the end of the experimental period (400 minutes) the venom concentration at the injection site remained almost constant (Figure 2). The means of measured radioactivity reflect the presence of the toxins in the tissues during the period of the study. The highest measured mean radioactivity was in the skin (10.09 radioactivity/gram of the tissue), followed by the blood (4.21), kidneys (3.78) and intestine (2.9), respectively.
Analysis of the pharmacokinetic data showed that the elimination-half life T1/2 of the venom was 521 minutes. The value of Vd/F for the venom and its apparent total body clearance (CL/F) rate were 14.9 mL and 0.02 mL/minute, respectively (Table 2).
Distribution of Radiolabeled Antivenom in Rats
The mean ± SD values of distribution in various organs after intravenous administration of 0.2 mL of radiolabeled polyclonal antivenom are shown in Table 3. High amounts of radioactivity were detected in the blood (21.42%ID/g) and kidneys (19.56%ID/g) at five minutes post intravenous injection. Radioactivity measured in other organs during the study period of 360 minutes was more significant than the venom in the intestine (6.74%ID/g), brain (3.21%ID/g),heart (10.52%ID/g) and lungs (11.65% ID/g) at five minutes (p < 0.05) (Table 3). Antivenom reached its maximum level in the heart and brain within five minutes following administration. Peak level in heart declined significantly (p < 0.001) and rapidly within five minutes during 5 to 10 minutes following administration. After ten minutes, the antivenom level in the heart declined gradually until the end of the period of the study. Antivenom reached its highest concentration only five minutes following administration. Peak concentration of antivenom in the blood declined significantly (p < 0.001) within five minutes following administration. Antivenom level in the intestine was higher than the blood concentration during 40 to 240 minutes following antivenom administration.
The level of radioactivity reached its peak in the brain (3.21%ID/g) after five minutes and then declined gradually during 360 minutes. The rate of decline of the radioactivity in the kidneys was well correlated with that in the blood. The rate of decline of the radioactivity in the brain was almost similar to that in the blood. The maximum radioactivity in muscles and intestine were achieved at delayed times 40 and 60 minutes, respectively when the blood concentration had already declined. After 40 minutes, the antivenom amount in the intestine was higher than in the kidneys. The mean measure of radioactivity reflects the presence of the antivenom in the tissues during the whole period of the study. The highest measured mean of radioactivity was in the blood (7.61 radioactivity/gram of the tissue), followed by the kidneys (6.92), intestine (6.5) and heart (3.64), respectively (Table 3).
The measured pharmacokinetic parameters showed that half-life of the antivenom was 113.7 minutes, with Vd/F and apparent clearance of 13 mL and 0.08 mL/minute, respectively (Table 2). Statistical analyses of biodistribution values of venom and antivenom showed a significant difference in all investigated organs at different time intervals. These differences are distinctive especially for heart, lungs and muscles (Table 1 and 3).
DISCUSSION
Scorpion sting mortality in Iran is mostly due to H. lepturus, thus it can be stated that this creature is the most dangerous scorpion of this country (4). Development of severe forms of intoxication and regular reports of treatment failure following accidental H. lepturus scorpion envenomation, especially among children and in late referral in adults, is a common occurrence. One underlying reason is the lack of knowledge of the pharmacokinetic parameters of the venom for this scorpion. Therefore, the aim of this study was to clarify the pharmacokinetic parameters of this venom and that of its available antivenom allowing for the most appropriate form of treatment to be given to envenomed patients.
In this study, we report, for the first time, the biodistribution profile of the venom of H. lepturus and its available antivenom, using chloramine-T method according to previous experiments (17). As to drug administration, 10 µg of H. lepturus venom and 0.2 of mL antivenom were given via SC and IV route, respectively. The selected dose of venom was the average amount secreted by a scorpion sting. The amount of antivenom administered to the rats was calculated taking into account their body weight in relation to the standard dose of 10 mL given to human subjects. Due to limitations of earlier sampling, i.e. less than five minutes, any attempt to fit two or three compartment models were not successful.
As shown, the volume of distribution of antivenom was 14.9 mL which is not different compared to the Vd/F of venom (13 mL). Considering the venom concentration in the blood, the venom was absorbed and reached its maximum at 120 minutes. We found a rapid increase in the measured concentration of the venom during the 60 to 120 minutes (Table 1), especially in the blood. The volume of distribution of this venom was 15 mL, which was different from previous studies. The apparent volume of distribution of other studied venoms is different (11, 13, 18-21).
After tracer administration (labeled venom with iodine) the pattern of plasma radioactivity is different under the same conditions to yield a maximum peak. The route of administration had a major influence on the time for maximum plasma radioactivity. The Tmax was longer than those reported for venoms such as Andercotonus australis hector (21), Tityus serrulatus (18), Centruroides limpidus limpidus (19), Androctonus mauretanicus mauretanicus (20) and shorter lower than those reported by Ismail and Abd-Elsalam in 1988 (12). The underlying reason for such discrepancies in the results may be due to the route of administration and pharmacokinetics of scorpion venom and animal model used, or due to differences in the quantifying methods used (radioactivity versus ELISA).
The clearance of H. lepturus venom (0.02 mL/minute) was lower than Odonthubuthus doriae (2.12 mL/minute). The gradual absorption and distribution of the H. lepturus venom when compared with that of O. doriae indicate that H. lepturus venom has a slower biodistribution. This finding may be related to the delayed clinical manifestations of the envenomation by this scorpion (3, 22).
The elimination of venom is quite slow, as could be observed from the small value of its clearance and long elimination half life. These facts could clearly explain the prolonged adverse effects of the venom. It is important to note that intravenous administration results in a fast neutralizing efficacy compared to the intramuscular route (13). Based on the data (Table 1), it is reasonable to suggest that if a neutralizing dose of antivenom is infused over a period of 120 to 240 minutes following sting, more neutralization of toxicity is likely to occur. This is advisable in order to compensate for the slow rate of distribution of venom in tissue compartments.
Comparison of the measured biodistribution values of venom and antivenom showed distinguishing differences in all organs at different time intervals especially for the heart, lungs and muscles.
Based on the results, the amount of radiolabeled venom at the site of injection (skin) was very high in comparison to other organs. Radioactivity of all tissues together at ten minutes was around 37.34% and skin contained around 27.2% of injected counts, but after 40 minutes its percentage was 17.7%. The reason for consistent presence of the venom at the injection site of skin may be due to two possible mechanisms: firstly, the venom is bound to underlying skin layers or is taken up by cells within this layer. This suggestion has clinical implications since patients stung by this scorpion develop necrotic skin reactions that gradually become more severe, and require surgical debridement. If this assumption is true, then early removal of the skin at the sting site may be helpful. Secondly, the measured radioactivity is not due to the toxin, but its degraded products are indistinguishably counted by our radioactivity method of measurement. About 7% of the total initial activity could be detected at the site of injection one hour after the administration. Thus it can be inferred that the skin is not a major depot site of the venom and hence the late toxic effects of the venom could be related to its slow elimination from the body.
In order to confirm either of these suggestions, ELISA method needs to be carried out with the specific H. lepturus toxin. In order to make more accurate estimation of our findings, radiolabeled albumin method will need to be carried out in future studies (23). The radioactivity uptake in the small intestine increased until 60 minutes (3.91%ID/g) and then decreased slowly. The radioactivity uptake in the small intestine showed a smaller fraction than the kidneys. These data may indicate that hepatobilliary secretion of this venom plays a less important role than the kidneys in elimination from the body. At ten minutes, the highest radioactivity was measured in the kidneys followed by intestine suggesting that the venom is primarily and rapidly eliminated by the renal and hepatic (as reflected by its detection in the intestine) systems.
The overall radioactivity level rate of decrease of the venom was slow during the 10 to 60 minutes following administration. So we can conclude that the kidneys showed high radioactivity at times where the blood radioactivity was lower (12). The identified radioactivity in the kidneys can be associated with partial elimination or secretion of H. lepturus venom to renal tubules. The consistent presence of the venom in the renal system during the period of study suggest that the renal system is the target organ in which the venom causes direct toxicity, as has been commonly reported in patients.
The gradual increase of venom in plasma and its peaking at 120 minutes with more gradual decline suggest that venom has a relatively prolonged contact with blood cells. Previous clinical and experimental results have demonstrated hemolytic characteristics of this venom. The renal toxicity seems to be compounded by RBC hemolysis as well as the persistent presence of the venom. Accumulation of venom in other organs like heart and brain was very low compared with other organs, so it seems that venom concentration is not directly responsible for all manifestations of envenomation like seizure, tachycardia and arrhythmia (3).
The complex mathematical formulas used in explaining the pharmacokinetics of a given agent, despite being useful and essential for planning treatment protocols, are merely reflecting approximations of the trends and normally used to express the changes by computational and exponential terms. The obtained data cannot fully explain the underlying reasons for the delayed and devastating toxic ministrations seen in patients stung by this scorpion, who do not feel pain and do not show immediate symptomatic manifestations. In order to explain these discrepancies, we need to look more closely to the general distribution profiles of the venom in these tissues, especially the skin, intestine and kidneys.
ACKNOWLEDGMENTS
We would like to thank Professor Simin Dadashzadeh (School of Pharmacy, Shaheed Beheshti University of Medical Sciences, Tehran), Dr Ali Hassan Rahmani (School of Medicine), Dr Alireza Droudi (School of Pharmacy, Jundishapur University), Dr Saghir Shakil (Battelle Pacific Northwest National Laboratory, Richland, Washington, USA) and Nazanin Shobeiry for their suggestions and guidance in performing this study. We also would like to thankfully acknowledge the financial support of Deputy of Research Affairs of Ahvaz Jundishapur University of Medical Sciences and the Ministry of Health and Medical Education of Iran.
Received: March 19, 2012.
Accepted: June 15, 2012.
Abstract published online: June 29, 2012.
Full paper published online: November 30, 2012.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
FINANCIAL SOURCE: The Deputy of Research Affairs of Ahvaz Jundishapur University of Medical Sciences and the Ministry of Health and Medical Education of Iran provided the financial grants.
ETHICS COMMITTEE APPROVAL: The present study was approved by the Ethics Committee of the Jundishapur University, Ahvaz (Ref. n. IA/P/2100). Moreover, the protocols were conducted according to the guidelines of the National Institutes of Health (NIH).
- 1. Farzanpay R. A catalogue of the scorpions occurring in Iran, up to January 1986. Rev Arachn. 1998;8(2):33-44.
- 2. Pipelzadeh MH, Dezfulian AR, Jalali MT, Mansouri AK. In vitro and in vivo studies on some toxic effects of the venom from Hemiscorpius lepturus scorpion. Toxicon. 2006;48(1):93-103.
- 3. Pipelzadeh MH, Jalali A, Taraz M, Pourabbas R, Zaremirakabadi A. An epidemiological and a clinical study on scorpionism by the Iranian scorpion Hemiscorpius lepturus Toxicon. 2007;50(7):984-92.
- 4. Jalali A, Pipelzadeh MH, Sayedian R, Rowan EG. A review of epidemiological, clinical and in vitro physiological studies of envenomation by the scorpion Hemiscorpius lepturus (Hemiscorpiidae) in Iran. Toxicon. 2010;55(2-3):173-9.
- 5. Radmanesh M. Clinical-study of Hemiscorpion lepturus in Iran. J Trop Med Hyg. 1990;93(5):327-32.
- 6. Latifi M, Tabatabai M. Immunological studies on Iranian scorpion venom and antiserum. Toxicon. 1979;17(6):617-21.
- 7. Abroug F, ElAtrous S, Nouira S, Haguiga H, Touzi N, Bouchoucha S. Serotherapy in scorpion envenomation: A randomised controlled trial. Lancet. 1999;354(9182):906-9.
- 8. Krifi MN, El-Ayeb M, Dellagi K. The improvement and standardization of antivenom production in developing countries: Comparing antivenom quality, therapeutical efficiency and cost. J Venom Anim Toxins.1999;5(2):128-41.
- 9. Rochat C, Sampieri F, Rochat H, Miranda F. Iodination of neurotoxins I and II of the scorpion Androctonus australis Hector. Biochimie. 1972;54(4):445-9.
- 10. Bazin-Redureau M, Pepin S, Hong G, Debray M, Scherrmann JM. Interspecies Scaling of Clearance and Volume of Distribution for Horse Antivenom F(ab')2 Toxicol Appl Pharmacol. 1998;150(2):295-300.
- 11. Ismail M, Abdullah ME, Morad AM, Ageel AM. Pharmacokinetics of 131I-labelled venom from the scorpion Androctonus amoreuxi (Aud. and Sav). Toxicon. 1980;18(2):301-8.
- 12. Ismail M, Abd-Elsalam MA. Are the toxicological effects of scorpion envenoming related to tissue venom concentration? Toxicon. 1988;26(3):233-56.
- 13. Ismail M, Abd-Elsalam MA. Pharmacokinetics of 131 I-labelled of IgG. Fab2 and Fab fractions of scorpion and snake antivenins: merits and potential for therapeutic use. Toxicon. 1998;36(11):1523-8.
- 14. Hunter WM, Greenwood FC. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962;194:495-6.
- 15. Waynforth HB, Flecknell PA. Experimental and Surgical Technique in the Rat. 2nd ed. London: Academic Press. 1979.
- 16. Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal. 2005;49(4):1020-38.
- 17. Novakova K, Laznicek M, Rypacek F, Machova L. 125I-labeled PLA/PEO block copolymer: Biodistribution studies in rats. J Bioact Compat Polym. 2002;17(4):285-96.
- 18. Revelo MP, Bambirra EA, Ferreira AP, Diniz CR, Chávez-Olórtegui C. Body distribution of Tityus serrulatus scorpion venom in mice and effects of scorpion antivenom. Toxicon. 1996;34(10):1119-31.
- 19. Calderón-Aranda ES, Rivière G, Choumet V, Possani LD, Bon C. Pharmacokinetics of the toxic fraction of Centruroides limpidus limpidus venom in experimentally envenomed rabbits and effects of immunotherapy with specific F(ab')2 Toxicon. 1999;37(5):771-82.
- 20. El-Hafny B, Chgoury F, Adil N, Cohen N, Hassar M. Intraspecific variability and pharmacokinetic characteristics of Androctonus mauretanicus mauretanicus scorpion venom. Toxicon. 2002;40(11):1609-16.
- 21. Hammoudi-Triki D, Lefort J, Rougeot C, Robbe-Vincent A, Bon C, Laraba-Djebari F, et al. Toxicokinetic and toxicodynamic analyses of Androctonus australis hector venom in rats: Optimization of antivenom therapy. Tox Applied Pharm. 2007;218(3):205-14.
- 22. Jalali A, Moazen S, Babaee M, Dadashzadeh S, Droudi A. The pharmacokinetic of Iranian scorpion Odonthubuthus doriae venom and the available antivenom. J Venom Res. 2010;1:48-53.
- 23. Vasconcelos CM, Valenca RC, Araújo EA, Modesto JCA, Pontes MM, Brazil TK, et al. Distribution of 131 I-labeled Bothrops erythromelas venom in mice. Brazilian J Med Biol Res. 1998;31(3):439-43.
Correspondence to:
Publication Dates
-
Publication in this collection
07 Dec 2012 -
Date of issue
2012
History
-
Received
19 Mar 2012 -
Accepted
15 June 2012