Abstract
The present study aimed at investigating the susceptibility of the microorganisms Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli, Staphylococcus aureus, and Bacillus subtilis to ethanolic extracts of propolis (EEP) from three regions of Kenya (Taita, Tana and Samburu). Propolis was extracted using four different concentrations of ethanol: pure, 70%, 50%, and 30%. Ethanol (70%) and Streptomycin were used as controls. The agar diffusion method using filter paper disks was employed. Antibacterial activity was determined as an equivalent of the inhibition zones diameters (in millimeters) after incubation at 37°C for 24h. Significant differences in the antibacterial activities of propolis were observed among the three regions, depending on the test microorganisms and on the procedure used for the preparation of propolis extract. Bacillus subtilis and Staphylococcus aureus were the most susceptible bacteria and 70% EEP had the best antibacterial effect.
Apis mellifera; propolis; antibacterial agents; disk diffusion antimicrobial tests
ORIGINAL PAPER
Antibacterial activity of Apis mellifera L. propolis collected in three regions of Kenya
Muli E. M.I; Maingi J. M.II
IInternational Center of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya
IIDepartment of Biological Sciences, Kenyatta University, Nairobi, Kenya
Correspondence to Correspondence to: Elliud M. Muli International Center of Insect Physiology and Ecology (ICIPE), P. O. Box 30772, Nairobi, Kenya Phone: +254 20 8632053. Fax: +254 20 8632001/2 E-mail: emuli@icipe.org
ABSTRACT
The present study aimed at investigating the susceptibility of the microorganisms Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli, Staphylococcus aureus, and Bacillus subtilis to ethanolic extracts of propolis (EEP) from three regions of Kenya (Taita, Tana and Samburu). Propolis was extracted using four different concentrations of ethanol: pure, 70%, 50%, and 30%. Ethanol (70%) and Streptomycin were used as controls. The agar diffusion method using filter paper disks was employed. Antibacterial activity was determined as an equivalent of the inhibition zones diameters (in millimeters) after incubation at 37°C for 24h. Significant differences in the antibacterial activities of propolis were observed among the three regions, depending on the test microorganisms and on the procedure used for the preparation of propolis extract. Bacillus subtilis and Staphylococcus aureus were the most susceptible bacteria and 70% EEP had the best antibacterial effect.
Key words: Apis mellifera, propolis, antibacterial agents, disk diffusion antimicrobial tests.
INTRODUCTION
Propolis is a complex resinous mixture collected by honeybees from plant exudates and mixed with hypopharyngeal secretions, beeswax and pollen. It is used for honeycomb construction and polishing, for maintenance of the aseptic conditions in hive environment, and for protection and adaptation of nests (7, 8, 13, 14, 28). Propolis has a complex chemical composition, depending on the diversity of plants and geographical locations from which bees collect it (1, 3). Similarly, the biological properties of propolis may vary according to different plant sources (6, 14, 29). These biological activities such as antibacterial, antiviral, antifungal, among others, continue to attract the researchers interest (1, 4, 9, 11, 12, 14, 23, 36). Most of the antibacterial, antifungal and anti-inflammatory activity of propolis is due to the presence of polar compounds, mainly phenols (flavonoids, phenolic acids and their esters) and aromatic acids (caffeic acid and p-coumaric acid) (15, 24). Propolis has also been reported to be protective against radiation-induced damage (10) and to have antimutagenic effect (38) and anti-inflammatory activity (22, 33).
Though the antibacterial activity of propolis has been extensively reported, literature on the antimicrobial activity of Kenyan propolis is scarce. The present work aimed at investigating (i) the antibacterial activity of EEP collected from three regions of Kenya and (ii) the effect of extraction procedures on the antibacterial activity of EEP. Five bacteria strains were used for the study.
MATERIALS AND METHODS
Propolis Extracts Preparation
Propolis samples were collected from five colonies in each of the following three regions of Kenya: Taita District (between 37°35E, 4°8.2S and 39°13E, 2°40.8S), Tana River District (between 38°26E, 3°4.4S and 40°43.8E, 0°0.9S) and Samburu District (between 36°17E, 0°33.9N and 38° 4.9E, 2°30.9N). Propolis samples from each place were ground to powder and subjected to extraction with varying ethanol concentrations. Thirty grams (30g) of ground crude propolis from each place were added to 100ml of each of the following solvents: pure absolute ethanol (Sigma-Aldrich, Germany); mixtures of absolute ethanol and distilled water containing 70%, 50% and 30% ethanol (v/v). The solutions were kept at room temperature in the absence of light for 20 days and shaken once daily. After 20 days, filtration was done and resultant solvents were totally evaporated using a water bath at temperatures not exceeding 50°C. The dry extracts were then re-dissolved in 70% ethanol in order to obtain solutions containing 10% (w/v) propolis extracts (2).
Bacterial Cultures
Five bacteria strains obtained from Inoclaine International, Nairobi, and Pseudomonas aeruginosa (American Type Culture Collection [ATCC] 27853), Salmonella typhi (ATCC 2202), Escherichia coli (Standard Culture 25922), Staphylococcus aureus (ATCC 20591), and Bacillus subtilis (ATCC 6633) strains were used.
Antibacterial Activity Tests
The agar disk diffusion method (5) was employed to test the antimicrobial activity of EEP. Inoculum was prepared using fresh cultures of bacteria strains cultured on nutrient agar. A loop full of bacteria culture was inoculated into a nutrient broth medium and incubated for 24h at 37°C. The size was adjusted to 0.5 McFarland standard turbidity, approximately 108 colony-forming units (CFU/ml). Cell suspensions (100m l of target strain) were introduced into the nutrient agar plates and spread thinly on the plates using a glass spreader. Disks of 6mm diameter were impregnated with 25µl of each EEP and with ethanol 70% (control). The disks were then placed on inoculated agar plates, which were incubated at 37°C for 24h under aerobic conditions. The diameter of the inhibition zones (in millimeters) around the disks was measured after 24h. Tests were performed in duplicate.
Statistical Analysis
Results were analyzed using Analysis of Variance (ANOVA) and the probability p=0.05 was considered the critical value for all tests. Tukeys post-hoc test was used for separation of statistically significant means.
RESULTS AND DISCUSSION
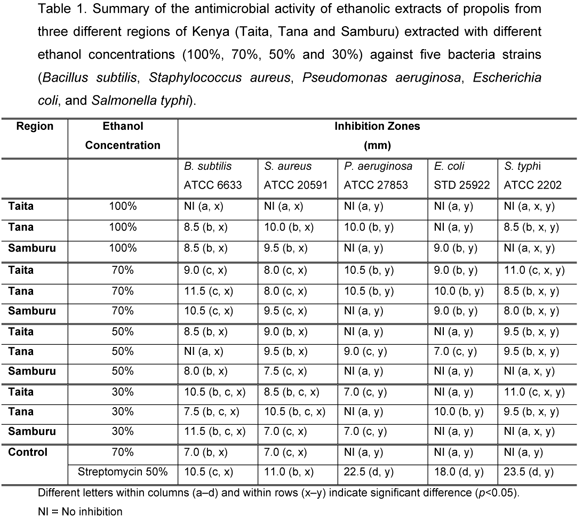
Larger inhibition zones were verified for the Gram-positive bacteria B. subtilis and S. aureus, compared with the Gram-negative P. aeruginosa and E. coli. However, the inhibitory effects of EEP were similar between the Gram-negative S. typhi and the two Gram-positive bacteria strains.
Among the Gram-positive bacteria, B. subtilis had larger inhibition zones than S. aureus but such difference was not statistically significant (p=0.05). The antibacterial effect of Kenyan EEP on S. aureus and B. subtilis agreed with those reported by other authors. Detoma and Ozino (8), Krol et al. (25), Kujumgiev et al. (26), Park et al. (34), Ivan et al. (20) and Gonsales et al. (15), among others, have reported that S. aureus is susceptible to propolis effects. Varied inhibition zones by EEP have also been reported: 1012mm by Massuda (30), 13mm by Brumfitt et al. (5), 10mm by Nieva et al. for Argentine propolis (31), and 011mm by Sato (35), suggesting there is variability in the biological activity of EEP, depending on its botanical origin and thus on its chemical composition. The antimicrobial activity of EEP was demonstrated by Ivan et al. (19, 20) against B. subtilis and by Orsi et al. (32) against Salmonella sp.
In Gram-negative bacteria, the highest antibacterial activity was recorded for S. typhi compared with E. coli and P. aeruginosa. However, the differences in their inhibition zones diameters were not statistically significant (p=0.05). There are conflicting data on the susceptibility of E. coli to EEP. Shub et al. (37), Ivan et al. (20) and Gonsales et al. (15) reported that EEP were ineffective against E. coli. On the other hand, Grange and Davey (16), Fernandes et al. (11), Hegazi and Abd el Haddy 2002 (18) and Sato (35) reported complete or minimal susceptibility. Grange and Davey (16) and Ivan et al. (20) showed P. aeruginosa inhibition by EEP.
The antibacterial activity of EEP varied among the three regions studied; EEP from Tana and Samburu had better antibacterial activity than those from Taita (Table 1). Taita EEP extracted using pure ethanol showed no inhibition on any of the five bacteria strains; Samburu EEP extracted using pure and 70% ethanol had no inhibitory effect on P. aeruginosa. Taita and Samburu EEP extracted with 50% ethanol and Tana EEP extracted using 30% ethanol had no inhibitory effect on P. aeruginosa. Similarly, Tana and Samburu EEP extracted using pure ethanol had no inhibitory effect on E. coli and S. typhi, respectively. Taita and Samburu EEP extracted using 30% and 50% ethanol had no inhibitory effect on E. coli. Taita EEP extracted using pure ethanol as well as Samburu EEP extracted using pure, 30% and 50% ethanol had no inhibitory effect on S. typhi. Control (70% ethanol) had no inhibitory effect on P. aeruginosa, E. coli and S. Typhi. Streptomycin presented inhibitory effects on all five bacteria strains.
A high content of polyphenols and flavonoids in alcoholic extracts of propolis is associated with significant microbial activity (16, 27). In a recent study, Volpi and Bergonzini (39) demonstrated that EEP collected in Kenya had no identified flavonoids and was less rich in polyphenols; however, the specific place(s) from where the Kenyan propolis was collected is not mentioned. This probably explains the low inhibition zones or lack of inhibition recorded in the current study, compared with the EEP inhibitory effects reported by other researchers like Massuda (30), Brumfitt et al. (5), Nieva et al. (31) and Sato (35).
The present results allow the conclusion that Gram-positive bacteria (B. subtilis and S. aureus) are more susceptible than Gram-negative bacteria (E. coli and P. aeruginosa) to Kenyan propolis. These findings agree with earlier reports by Grange and Davey (16), Grecianu and Enciu (17), Ivan et al. (20), and Keskin et al. (21). However, susceptibility of Gram-negative bacteria to EEP is still an important subject for further investigations. It can also be concluded that the extraction procedures determine the EEP antibacterial activity. Probably, different extraction procedures lead to extraction of different compounds, which ultimately contribute to differences in the antibacterial activity.
Received: October 23, 2006
Accepted: January 30, 2007
Abstract published online: February 10, 2007
Full paperpublished online: August 31, 2007
Conflicts of interest:There is no conflict.
- 1 ALEXANDRA CHS., KIRLLIAN SS., MARIA CM., ILDENIZE BSW., MARIO TS. Analysis of the composition of Brazilian propolis extracts by chromatography and evaluation of their in vitro activity against gram-positive bacteria. Braz. J. Microbiol, 2004, 35, 1-2.
- 2 BANKOVA V., BOUDOUROVA-KRASTEVA G., SFORCIN JM., FRETE X., KUJUMGIEV A., MAIMONI-RODELLA R., POPOV S. Phytochemical evidence for the plant origin of Brazilian propolis from São Paulo State. Z. Naturfosch., 1999, 54, 401-5.
- 3 BANSKOTA AH., TEZUKA Y., KADOTA S. Recent progress in pharmacological research of propolis. Phytotherapy Res., 2001, 15, 561-71.
- 4 BOSIO K., AVANZINI C., DAVOLIO A., OZINO O., SAVOIA D. In vitro activity of propolis against Streptococcus pyogenes. Lett. Appl. Microbiol., 2000, 31, 174-177.
- 5 BRUMFITT W., HAMILTON-MILLER JMT., FRANKLIN I. Antibiotic activity of natural products. 1 Propolis. Microbios, 1990, 63, 19-22.
- 6 BURDOCK GA. Review of the biological properties and toxicity of bee propolis (propolis), Food Chem. Toxicol., 1998, 36, 346-63.
- 7 CHENG PC., WONG G. Honeybee propolis: Prospects in Medicine. Bee World, 1996, 77, 8-15.
- 8 DETOMA P., OZINO OI. Azione della propoli su microorganismi dellambiente ospedaliero. Ann. Microbiol. Enzimol., 1991, 41, 231-2.
- 9 EL-GHAZALY MA., KHAYYAL MT. The use of aqueous propolis extract against radiation-induced damage. Drugs Exp. Clin. Res, 1995, 21, 229-36.
- 10 FERNANDES JR. A., LEOMIL L., FERNANDES AAH., SFORCIN JM. The antibacterial activity of propolis produced by Apis mellifera L. and Brazilian stingless bees. J. Venom. Anim. Toxins, 2001, 7, 73-182.
- 11 FERNANDES JR A., LOPES CAM., SFORCIN JM., NAQVI SA., DDIYA PC., FUNARI SRC. Population analysis of susceptibility of propolis in reference strains of Staphylococcus aureus and Escherichia coli. J. Venom. Anim. Toxins, 1997, 3, 287-94.
- 12 GARCIA-VIGUERA C., GREENWAY W., WHATLEY FR. Composition of propolis from two different Spanish regions. Z. Naturforsch., 1992, 47c, 634-7.
- 13 GHISALBERTI EL. Propolis: a review. Bee World, 1960, 60, 59-64.
- 14 GHILSALBERTI EL. Propolis: a review. Bee World, 1979, 60, 59-84.
- 15 GONSALES GZ., ORSI RO., FERNANDES JR A., RODRIGUES P., FUNARI SRC. Antibacterial activity of propolis collected in different regions of Brazil. J. Venom. Anim. Toxins incl. Trop. Dis., 2006, 12, 276-84.
- 16 GRANGE JM., DAVEY RW. Antibacterial properties of propolis (bee glue). J. R. Soc. Med., 1990, 83, 159-60.
- 17 GRECIANU A., ENCIU V. Activity in vitro of propolis against bacterial strains of animal origin. Institutal Agronomic Ion Ionescu de la Brad (Zootehnie. Medicina Veterinara), 1976, p.90-2.
- 18 HEGAZI A., ABD EL HADY FK. Egyptian Propolis: 3. Antioxidant, antimicrobial activities and chemical composition of propolis from reclaimed lands. Z. Naturforsch., 2002, 57c, 395-402.
- 19 IVAN KM., BAKMAZ S., PEPELJNJAK S. Analysis of propolis from the continental and Adriatic regions of Croatia. Acta Pharm., 2003, 53, 275-85.
- 20 IVAN KM., PEPELJNJAK S., BAKMAZ, M., VLADMIR-KNEZEVIC S. Flavonoid analysis and antimicrobial activity of commercially available propolis products. Acta Pharm., 2005, 55, 423-30.
- 21 KESKIN N., HAZIR S., BASER KH., KURKCUOGLU M. Antibacterial activity and chemical composition of Turkish propolis. Z. Naturforsch., 2001, 56, 1112-15.
- 22 KHAYYAL MT., EL-GHAZALLY MA., EL-KHATIB AS. Mechanisms involved in the anti-inflammatory effect of propolis extract. Drugs Exp. Clin. Res, 1993, 19, 197-203.
- 23 KOO H., GOMES BPFA., ROSALEN PL., AMBROSANO GMB., PARK IK., CURY JA. In vitro antimicrobial acitivity of propolis and Arnica montana against oral pathogens. Arch. Oral. Biol., 2000, 45, 141-8.
- 24 KROL W., SCHELLER S., CZUBA Z., MATSUNO T., ZYDOWICZ G., SHANI J., MOS M. Inhibition of neutrophils chemiluminescence by ethanol extract of propolis (EEP) and its phenolic components. J. Ethnopharmacol., 1996, 55, 19-25.
- 25 KROL W., SCHELLER S., SHANI J., PETSZ G., CZUBA Z. Synergistic effect of ethanolic extract of propolis and antibiotics on the growth of Staphylococcus aureus. Arzneimittelforschung, 1993, 43, 607-9.
- 26 KUJUMGIEV A., TSVETKOVA I., SERKEDJIEVA Y., BANKOVA V., CHRISTOV R., POPOV S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol., 1999, 64, 235-40.
- 27 MALIMON GL., SHUB TA., KAGRAMANOVA KA., KIVMAN GYA. Comparative study of alcoholic extracts of propolis from different geographic zones by spectrophotometric and antimicrobial analysis. Khimiko-farmatsevficheskii Zhural, 1980, 14, 114-117.
- 28 MARCUCCI MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie, 1995, 26, 83-99.
- 29 MARKHAM KE., MITCHEL KA., WILKINS AL., DALDY JA., LU Y. HPLC and GC-MS identification of the major organic constituents in New Zealand propolis. Phytochemistry, 1996, 42, 205-11.
- 30 MASSUDA KF. Parâmetros físico-químicos e atividade biológica da própolis submetida a diferentes tipos de extração. Rio Claro: Universidade Estadual Paulista, Instituto de Biociências, 2003. 27f. [End of Course Paper].
- 31 NIEVA MMI., ISLA MI., CUDMANI NG., VATTUONE MA., SAMPIETRO AR. Screening of antibacterial activity of Amaicha del Valle (Tucuman, Argentina) propolis. J. Ethnopharmacol, 1999, 68, 97-102.
- 32 ORSI RO., SFORCIN JM., RALL VLM., FUNARI SRC., BARBOSA L., FERNANDES JR A. Susceptibility profile of Salmonella against the antibacterial activity of propolis produced in two regions of Brazil. J. Venom. Anim. Toxins incl. Trop. Dis., 2005, 11, 109-16.
- 33 ORZTURK F., KURT E., CERCI M., EMIROGLU L., INAN U., TURKER M., ILKER S. The effect of propolis extract in experimental chemical corneal injury. Opthalmic Res., 2000, 32, 13-8.
- 34 PARK YK., IKEGAKI M., ALENCAR SM. Classificação das própolis brasileiras a partir de suas características físico-químicas e propriedades biológicas. Mensagem doce, 2000, 58, 2-7.
- 35 SATO PM. Inter-relações das características físicas, químicas e biológicas de própolis das regiões sul e sudeste do Brasil. Rio Claro: Universidade Estadual Paulista, Instituto de Biociências, 2002. 33f. [End of Course Paper].
- 36 SFORCIN JM., FERNANDES JR A., LOPES CAM., BANKOVA V., FUNARI SRC. Seasonal effect on Brazillian propolis antibacterial activity. J. Ethnopharmacol, 2000, 73, 243-9.
- 37 SHUB TA., KAGRAMANOVA KA., VOROPAEVA, SD., KIVMAN GYA. Effect of propolis on strains of Staphylococcus aureus resistant to antibiotics. Antibiotiki, 1981, 26, 268-71.
- 38 VARANDA EA., MONTI R., TAVARES DC. Inhibitory effect of propolis and bee venom on the mutagenicity of some direct- and indirect-acting mutagens. Teratog. Carcinogen Mutagen, 2000, 19, 403-13.
- 39 VOLPI N., BERGONZINI G. Analysis of flavonoids from propolis by on-line HPLC-electrospray mass spectrometry. J. Pharm. Biomedical Anal., 2006, 6, 1676-2525.
Publication Dates
-
Publication in this collection
14 Sept 2007 -
Date of issue
2007
History
-
Accepted
30 Jan 2007 -
Received
23 Oct 2006