Abstract
A comparative study on the sensitivity of erythrocytes from different vertebrate species (avian, mammalian and reptilian) to the hemolytic action caused by cardiotoxin isolated from Naja naja atra venom was carried out. Cardiotoxin was able to induce direct hemolysis in washed erythrocytes from several animals, except for llama. The EC50 values from hemolysis of the most sensitive (cat) and the most resistant (snake) animal varied approximately tenfold. According to the cell behavior, it was possible to characterize four types of behavior: The first was observed in cat, horse and human cells; the second in rat, rabbit and dog erythrocytes; and the third only in llama erythrocytes, which were resistant to cardiotoxin concentrations up to 300 µg/ml. Finally, avian and reptilian erythrocytes were more resistant to cardiotoxin III-induced hemolysis than those of the mammalian species.
hemolysis; cobra; venom; erythrocytes; animals
ORIGINAL PAPER
Hemolitic action of Naja naja atra cardiotoxin on erythrocytes from different animals
Troiano J. C.II; Gould E. G.I; Gould I.I
IFundación de Estudios Biológicos, Gral. Urquiza, Buenos Aires, Argentina
IIUniversidad del Salvador, Buenos Aires, Argentina
Correspondence to Correspondence to: Juan Carlos Troiano Pringles, 760 3ro 17 Buenos Aires, (1083) Argentina Email: juancarlostroiano@hotmail.com
ABSTRACT
A comparative study on the sensitivity of erythrocytes from different vertebrate species (avian, mammalian and reptilian) to the hemolytic action caused by cardiotoxin isolated from Naja naja atra venom was carried out. Cardiotoxin was able to induce direct hemolysis in washed erythrocytes from several animals, except for llama. The EC50 values from hemolysis of the most sensitive (cat) and the most resistant (snake) animal varied approximately tenfold. According to the cell behavior, it was possible to characterize four types of behavior: The first was observed in cat, horse and human cells; the second in rat, rabbit and dog erythrocytes; and the third only in llama erythrocytes, which were resistant to cardiotoxin concentrations up to 300 µg/ml. Finally, avian and reptilian erythrocytes were more resistant to cardiotoxin III-induced hemolysis than those of the mammalian species.
KEY WORDS: hemolysis, cobra, venom, erythrocytes, animals.
INTRODUCTION
Cardiotoxins (CD) constitute a large family of small (60-62 amino acid residues) basic peptides present in the venoms of cobras (genera Naja and Hemachatus). Most of these venoms possess up to five homologous cardiotoxin polypeptides. Near 50 CD analogs have been purified and sequenced, and the tridimensional structure of some of them has been determined (2, 15). The gross structures of these CDs are considered to be similar, consisting of three extended loops radiating from a central core containing a cluster of four disulfide bonds ("three-fingered fold"). These loops are characterized by a two-stranded b-sheet configuration near the N-terminal end (loop 1) and a three-stranded b-sheet configuration next toit (loop 2 and loop 3). Cardiotoxins are known to cause multiple biological effects, such as hemolysis, local inflammation, depolarization, and contracture of smooth, skeletal and cardiac muscles (2, 5, 6, 10, 18, 22). In vitro, CDs can bind strongly to zwitterionic or acidic phospholipids, resulting in aggregation/fusion of phospholipid vesicles (3), and are able to cause lysis of different types of cells, within a distinct range of concentrations, presumably by disarranging the structure of membrane lipids. However, it is not clear how these interactions are related to their different biological activities.
Sensitivity to CD-induced hemolysis can be used to investigate some properties of erythrocytes from different species. However, two important facts must be considered. The first one is that although all CDs are able to produce almost all the above-mentioned effects, some of them are more efficient to produce fusion of phospholipid vesicles and depolarization of muscular cells, while others present a higher hemolytic activity (2, 3, 18). Therefore, the source and purity of the CD to be employed must be specified. The second fact is the contamination with phospholipase A2, which constitutes a major problem in assaying the hemolytic activity of cardiotoxins (12), since this enzyme is known to have a synergistic effect on CD-induced hemolytic activity (5, 20).
The venom of Naja naja atra (Taiwanese cobra) contains a high amount of cardiotoxins, which account for about 45% of the dried venom mass. Four or five homologs (I to V) have been purified and sequenced. Cardiotoxins I-IV are 60 amino acid residues peptides with different amino acid sequences (3, 15), while CD V is a 62 amino acid residues peptide, previously called "cardiotoxin-like basic peptide" (CLBP 3). The isoform with higher hemolytic activity is the 60 amino acid residues peptide CD III, the only protein characterized by the presence of proline (Pro) at the position 30 (P-type, 3) and the sequence VATPK (27-31) at the tip of loop 2.The isoform III was employed in this study in order to compare the sensitivity of erythrocytes from different species to CD-induced hemolysis.
MATERIALS AND METHODS
Venom
Venom of Taiwanese cobra Naja naja atra was purchased from Miami Serpentarium (Punta Gorda, FL).
Purification of cardiotoxin isoform III
One gram of venom was dissolved in 10.0 ml of 50 mM sodium phosphate buffer, pH 7.5, and centrifuged for 10 min at 5000 r.p.m. In step 1, the supernatant was carefully aspired and applied to a 35x2.6 cm column of SP-Sepharose FF, pre-equilibrated with 50 mM sodium phosphate buffer, pH 7.5, at 23ºC. Elution was started with the same buffer until the absorbance A280 of the eluate returned to the baseline. The column was then eluted stepwise with 0.1 M NaCl, 50 mM sodium phosphate buffer, pH 7.5; the "cardiotoxin" fraction was eluted with 0.5 M NaCl, 50 mM sodium phosphate buffer, pH 7.5. The "cardiotoxin" fraction (approximately 380 mg) was concentrated by ultrafiltration using a YM-3 Amicon membrane, desalted by chromatography on a Sephadex G-25 column, and lyophilized. In step 2, the lyophilized material from step 1 was dissolved in 10.0 ml of 50 mM sodium phosphate buffer, pH 7.5, and applied to a 40x1.6 cm column of SP-Sepharose FF, pre-equilibrated with the same buffer. Elution was performed using a FPLC (Pharmacia) equipment with 50 mM sodium phosphate buffer, pH 7.5, and 5.0-ml fractions were collected at a flow rate of 0.5 ml/min. Cationic proteins were eluted by means of 400 ml of a linear gradient (0-0.5 M) of NaCl in 50 mM phosphate buffer, pH 7.5. Cardiotoxin isoform III (approximately 120 mg) was eluted at about 0.27 M NaCl; fractions under the peak were combined, concentrated by ultrafiltration, desalted, and lyophilized as described above.
In step 3, the material recovered from step 2 was rechromatographed on the same system. The final yield of solid material was 110 mg.
SDS-PAGE of the purified cardiotoxin isoform III (CD III) yielded a single band with mobility corresponding to a molecular weight of 6500 ± 300. On the other hand, SDS-PAGE analysis of the native protein in a reverse polarity system (b alanine-acetate, pH 3.8) yielded a single band with a characteristic mobility, showing less than 2% contamination with cardiotoxin isoform II. Regarding contaminants, it was important to rule out contamination with phospholipase A2. Samples of 0.5 ml containing dihexanoyllecithin (2-15 mM) in 0.2 M NaCl, 2.0 mM CaCl2 and 20.0 mM HEPES buffer, pH 7.5, were incubated at 25°C for 60 to 180 min with 1-5 mg of CD III from step 3 of the purification procedure or 1.0 mg crude venom. Reaction was stopped by the addition of 5.0 mM EDTA, and after the addition of 5.0 ml of absolute ethanol, the samples were evaporated to dryness. Residues were redissolved in chloroform/methanol 20:1 (by vol.) and applied to silica gel TLC plates (Merck). Control samples containing dihexanoyllecithin and 1-hexanoyl lysolecithin were applied to the same plate. Plates were developed in chloroform/methanol/water (65: 25: 4 by vol.) and revealed with a sulfuric acid-formaldehyde spray. Differently from the sample of crude venom, no lysoderivative spot was observed in the sample treated with the purified preparation of CD III, therefore it appears to be devoid of phospholipase A2 activity.
The purified CD III from step 3 had an amino acid composition similar to what was previously reported (15). Protein concentration determined by the method of Lowry et al. (20) allowed to calculate molar absorptivity e280 = 3950 M-1.cm-1.
Animals
Beagle dogs, New Zealand rabbits, common European cats, Wistar rats, Leghorn hens, and llamas (Lama glama) were kept at the Animal Facility of the Fundación de Estudios Biológicos (Buenos Aires, Argentina). Horses (mixed bred) and specimens of two crotalids from Argentina, Bothrops alternatus and Crotalus durissus terrificus, were kept at the serpentarium of Fundación de Estudios Biológicos (Buenos Aires, Argentina). Except for Wistar rats, whose blood was obtained by cardiac puncture, in all the cases, 2-3 ml blood was obtained by venipuncture using sterile and disposable syringes and needles. Human blood samples (3.0 ml) were obtained by venipuncture from healthy, voluntary donors.
Hemolysis analysis
Blood samples were collected in glass tubes containing heparin (lithium salt) as anticoagulant and transported to the laboratory in polyestyrene boxes on crushed ice for conservation. Samples were centrifuged for 10 min at 800 rpm; the plasma and the buffy coat were carefully removed by means of sterile Pasteur pipettes. The pelleted red blood cells were resuspended in 0.15 M NaCl, 10 mM phosphate buffer, pH 7.0 (PBS), washed three times by centrifugation, suspended in PBS at a density of 1.2 x 109 cells/ml, and monitored by counting in a Neubauer chamber.
For hemolysis experiments, triplicate samples containing 250 µl of washed red blood cell suspensions were incubated with different concentrations of CD III (1-100 µg/ml) in 1.0 ml (final volume) of PBS. The samples were incubated at 25°C with gentle stirring. After 60, 90, 120, 150, and 180 min incubation, they were centrifuged for 20 min at 8000 rpm. Aliquots (100 µl) of the supernatants were mixed with 900 µl of either distilled water or Drabkin solution, and the absorbance at 540 nm was determined. A negative control (0% hemolysis) was prepared by mixing 250 µl of RBC suspension with 750 µl PBS, pH 7.0, without cardiotoxin, and a positive control (100% hemolysis) was prepared using 250 µl of erythrocyte suspension and 750 µl of PBS plus 0.1% Triton X-100.
Statistics and data analysis
The mean absorbance for each cardiotoxin concentration (mean ± SD) was employed to produceplots of percentage of hemolysis as a function of cardiotoxin concentration for each species. Curve fitting and nonlinear regression analysis were performed using the combined Prisma-Stat Mate software (GraphPad Software, San Diego, CA). For the different species studied, experimental data were fitted to a standard equation for a sigmoidal dose-response curve (Equation 1):
Hemolysis (%) = PlateauL + [(PlateauH PlateauL)(1 + 10 (log EC50 log CD))-1)]
Or to a four-parameter logistic equation, representing a sigmoidal dose-response curve with a variable slope (Equation 2):
Hemolysis (%) = PlateauL + [(PlateauH PlateauL)(1 + 10(log EC50 log CD).nH)-1)]
Where PlateauL stands for the percentage of hemolysis at the bottom plateau; PlateauH represents the percentage of hemolysis at the top plateau; log EC50 is the logarithm (base 10) of the CD concentration, which results in a percentage of hemolysis halfway between the top and the bottom plateausL; and nH is the Hill coefficient. The fit to these equations was compared by means of the F-test and the associated p value.
RESULTS
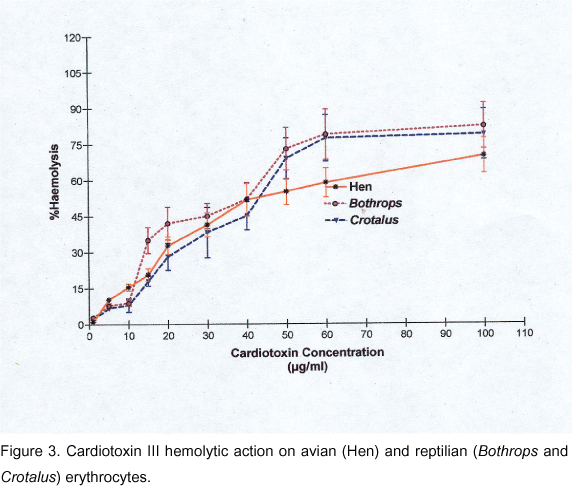
With the types of erythrocytes tested, the amount of hemoglobin released after 60 min incubation with different concentrations of CD III did not increase with time, thus interaction with CD and the consequent membrane damage was complete in 60 min and did not progress, suggesting further kinetic effects. Curves of the degree of hemolysis as a function of the CD III concentration for each species tested are shown in Figures 1, 2 and 3. Except for the Lama glama erythrocytes, CD III was able to induce direct hemolysis in washed erythrocytes from several mammalian, avian and reptilian species.
As shown in Table 1, the EC50 values from hemolysis of the most sensitive (cat) and the most resistant (B. alternatus) erythrocytes varied approximately tenfold. However, most of the changes were observed among mammalian erythrocytes. The EC50 values for avian and reptilian erythrocytes varied only from 4 to 5 x 10-6µg.
In addition, the dose-effect curves for mammalian erythrocytes were significantly different and allowed to characterize three types of behavior. The first type includes cat, horse and human erythrocytes (Figure 1) and is characterized by dose-effect curves which fit Equation 1, with EC50 values of about 5.1 (± 2.4) x 10-7 M. The second one includes rat, rabbit and dog erythrocytes (Figure 2) and is characterized by dose-effect curves fitting to Equation 2. The dose-effect curves for rat and rabbit erythrocytes were similar in both, the positive Hill coefficients (nH = 2.7 to 3.7) and the EC50 values (1.58 [± 0.53] x 10-6 µg). Dog erythrocytes seem to be less sensitive to the hemolytic action, since addition of CD III up to 20 µg/ml (2.95 x 10-6 M and higher than the EC50 for any of the sensitive mammalian erythrocytes studied) results in only 5% hemolysis. A small increase in CD III concentration (up to 30 µg/ml = 4.4 x 10-6 µg) results in a high degree (>95%) of hemolysis. Thus, the dose-effect curve exhibits a high positive Hill coefficient (nH = 8.5 ± 3.5) and an EC50 value of 26.5 ± 1.7 µg/ml = 3.9 (± 0.25) x 10-6 µg.
The third group is composed by Lama glama (an American camelid) erythrocytes (Figure 2), which is completely resistant to CD III concentrations up to 300 µg/ml (4.4 x 10-5 µg).
Avian and reptilian erythrocytes (Figure 3) showed to be more resistant to CD III-induced hemolysis than sensitive mammalian erythrocytes, since concentrations of CD III equal to 1.5 x 10-5 µg, which produced 100% hemolysis of sensitive mammalian erythrocytes, resulted in 58%-65% hemolysis of avian or reptilian erythrocytes. Dose-effect curves for hen, C. d. terrificus and B. alternatus erythrocytes presented similar features.
These curves are shallow, and the best fit was obtained with Equation 2, although Hill coefficients were close to unity, i.e., 1.34 ± 0.07 (hen); 1.49 ± 0.14 (B. alternatus); and 1.7 ± 11.4 (C. d. terrificus).
The EC50 values were 4.3 (± 0.03) x 10-6 µg (hen); 4.53 (± 0.1) x 10-6 µg (C. d. terrificus); and 5.2 (± 0.1) x 10-6 µg (B. alternatus).
DISCUSSION AND CONCLUSIONS
Cardiotoxin (CD) III was able to induce direct hemolysis on suspensions of washed erythrocytes from several vertebrate species. Among biconcave mammalian erythrocytes, the lowest EC50 (the most sensitive to the hemolytic action of CD III) were observed in cat, human and horse erythrocytes, followed by rabbit, rat, and finally dog erythrocytes. While these results partially agree with previous reports (7, 8, 11, 14, 16, 22), they differ in regard to relative sensitivities. Condrea et al. (9) found that guinea pig and dog erythrocytes were more sensitive to the hemolityc action of Hemachatus haemachatus venom, while human and rabbit erythrocytes have a moderate susceptibility. Conversely, Klibansky et al. (16) reported that, at 200 µg/ml, the "Direct Lytic Factor" (DLF) isolated from H. haemachatus venom was able to cause no more than 10% hemolysis in human red blood cells, while dog erythrocytes were more resistant to the cardiotoxin action. Possible differences from the results presented here may be due to the use of a different cardiotoxin.
It must be pointed out that the curves presented above express the degree of membrane perturbation resulting in hemoglobin release as a function of the total CD concentration added. While hemolysis requires previous binding of CD to the erythrocyte membrane, the measurement of the hemoglobin released as a function of CD concentration does not represent the CD binding curve, so that Hill's coefficients, as employed here, allow the characterization of the dose-effect curves and do not imply cooperativity in CD binding. The hemolysis curves as a function of CD III concentration, as well as the CD III EC50 of erythrocytes from different species, may reflect the specificity of binding and/or the CD-membrane interaction, which depends on the architectural properties of the different cell membranes.
The interaction of CDs with biological membranes is complex, and possible protein targets for the CDs have been postulated (4, 5, 6, 12, 17, 21). However, no such protein targets have been identified so far. On the other hand, CDs are also able to interact with the lipid component of the membranes. Since the binding of CDs to zwitterionic phospholipids seems to be high and since these are the most common phospholipid components in the outer leaflet of biological membranes, there is no reason why they would not serve as the most likely targets for CDs (2).
The molecular conformation of CDs consists of three extended loops radiating from a central core containing a cluster of 4 disulfide bonds. The configuration of these loops is characterized by a two-stranded b-sheets near the N-terminal (loop 1) and a three-stranded b-sheet next to it (loops 2 and 3). Their molecular shape is that of a flat oblate ellipsoid with two faces defined by the plane formed by the b-sheets of the loops. This kind of structure has been called "three-fingered" fold. Amino acid residues at positions 6 to 13 (loop 1) form a continuous hydrophobic patch. In addition, CD III has a Pro residue at position 31 (a P-type cardiotoxin) and if the side chain of Lys is long, flexible and hydrophobic near the backbone of the polypeptide chain (2), the amino acid residues near the tip of loop 2 (positions 24 to 37) form another continuous hydrophobic patch able to act as a lipid anchoring site.
Besides conformational considerations, this may be the reason why CDs of the P-type (like CD III) possess hemolytic activity higher than those containing Ser at position 31 (S-type, like CD I, II and IV). The amino acid residues located at both ends of these positively charged hydrophobic patches (Lys-6 and Lys-13 for loop 1, and Lys-24 and Lys-37 for loop 2) have been proposed to interact electrostatically with the negatively charged phosphate groups of phospholipids (2). On the other hand, the sequence Cys-Pro-Ala-Gly-Lys-Asn-Leu-Cys at positions 15-22 may constitute an interaction site with zwitterionic head groups (3). Evidences of fluorescence binding in the presence of lipids and chemical modifications indicate that loop 1 of CD penetrates the hydrophobic core of the membrane, while the lipid binding site in loop 2 is anchored (2). According to this interpretation, the rigid CD molecule inserts the edge of loop 1 into the membrane, which is consistent with an apparent molecular area of 420 A2 during the CD insertioninto phospholipid monolayers at surface pressures higher than 25 mM/m (1). In fact, this surface area per molecule agrees with that of a molecule in an "edgewise" configuration as might occur at a surface pressure of about 30 mN/m existing at an erythrocyte membrane. Further CD binding requires space, which becomes incompatible with the membrane structure due to the formation of CD dimmers or trimmers and/or because the CD molecule reorients itself into a "flat" configuration in relation to the plane of the membrane (1), resulting in lysis.
Except for those of Lama glama, all the mammalian erythrocytes studied are non-nucleated and biconcave. If the interaction of CD III with the erythrocyte membranes was similar to that proposed for phospholipids, since the weight percentage of total zwitterionic phospholipids (phosphatidylcholine + sphingomyelin) accounts for 53% to 60% in all mammalian erythrocytes (23), the EC50 values should be related to the cell surface area.
While no data on the surface area of cat and horse erythrocytes are available, reported data on human, rabbit and camel erythrocytes (13, 19) suggest no correlation between the surface areas of mammalian erythrocytes and either the EC50 values or the hemolysis versus CD III concentration curves. On the other hand, except for human erythrocytes, the EC50 for CD III-induced hemolysis increases with the surface area/volume ratio as well as with erythrocyte deformability in low shear field (24). Small red blood cells with low surface/volume ratio exhibited low deformability and low CD III EC50. As the erythrocyte size increases, the surface area/volume ratios increase: 1.6-1.75 µm-1 (rabbit), 1.68-1.8 (rat), and 2.05-2.65 (dog); erythrocyte deformability and CD III EC50 values also increase with the erythrocyte size. Finally, the flat, thin erythrocytes from Lama glama which do not deform under shear stress (24) are resistant to CD-induced hemolysis.
Avian and reptilian erythrocytes are large, oval and nucleated cells. The volumes and surface areas of C. d. terrificus and B. alternatus cells have been reported previously (25). Since they present a format similar to that of reptilian cells, their surface area calculated as previously described results in 240 ± 40 µm2. The plot of the CD III EC50 as a function of the surface area for avian and reptilian erythrocytes is linear: slope 118.1 (± 3.25), intercept at -770.3 (± 30.62), r2 = 0.9992, statistically significant for p=0.0175.
Since EC50 was measured using an equal number of erythrocytes, the number of CD III molecules per cell at the EC50 could be calculated as 3.46 x 106 for rabbit; 3.93 x 106 for rat, 7.82 x 106 for dog, 8.53 x 106 for hen, 9.09 x 106 for C. d. terrificus, and 1.05 x 107 for B. alternatus erythrocytes. Assuming that all these molecules were bound to the erythrocyte's surface, the number of molecules per µm2 may be in the range of 2.5-3.5 x 105, regardless the species and an area per molecule from 250 to 400 A2.
Received: February 11, 2005
Accepted: May 30, 2005
Published online: February 24, 2006
- 1. BOUGIS P., ROCHAT H., PIERONI G., VERGER R. Penetration of phospholipid monolayers by cardiotoxins. Biochemistry, 1981, 20, 4915-20.
- 2. CHIEN KY., CHIANG CM., HSEU YC., VYAS AA., RULE GS., WU W. Two distinct types of cardiotoxin as revealed by the structure and activity relationship of their interaction with zwitterionic phospholipid dispersions. J. Biol. Chem. 1994, 269, 14473-83.
- 3. CHIEN KY., HUANG WN., JEAN JH., WU WG. Fusion of sphingomyelin vesicles induced by proteins from Taiwan cobra (Naja naja atra) venom. J. Biol. Chem. 1991, 266, 3252-9.
- 4. CONDREA E. Hemolytic effects of snake venoms. In: LEE C.Y. Ed. Snake Venoms Berlin, Springer. 1979: 448-79.
- 5. CONDREA E. Membrane-active polypeptides from snake venom: cardiotoxins and haemocytoxins. Experientia, 1974, 30, 121-9.
- 6. CONDREA E., BARZILAY M., MAGER J. Role of cobra venom direct lytic factor and Ca2+ in promoting the activity of snake venom phospholipase A. Biochim. Biophys. Acta, 1970, 210, 65-73.
- 7. CONDREA E., DE VRIES A, MAGER J. Hemolysis and splitting of human erythrocyte phospholipids by snake venoms. Biochim. Biophys. Acta, 1964, 84, 60-73.
- 8. CONDREA E., KENDZERSKY I., DE VRIES A. Binding of ringhals venom direct hemolytic factor to erythrocytes and osmotic ghosts of various animal species. Experientia, 1965, 21, 461-2.
- 9. CONDREA E., MAMMON Z., ALOOF S., DE VRIES A. Susceptibility of erythrocytes of various animals species to the hemolytic and phospholipid splitting action of snake venom. Biochim. Biophys. Acta, 1964, 84, 365-75.
- 10. FAJNHOLC N., CONDREA E., DE VRIES A. Activation of enzymes in red blood cell membranes by a basic protein isolated from cobra venom. Biochim. Biophys. Acta, 1972, 255, 850-7.
- 11. FLETCHER JE., RAPUANO BE., CONDREA E., YANG CC., ROSENBERG P. Relationship between catalysis and toxicological properties of three phospholipases A2 from elapid snake venoms. Toxicol. Appl. Pharmacol, 1981, 59, 375-88.
- 12. GHARAIBEH NS., AL TALAFEEH K., GHARAIBEH M., BATCHOUN R. A comparative analysis of osmotic fragility of mammalian blood (human, camel and rabbit). Indian J. of Anim. Sci., 1993, 63, 942-5.
- 13. GUL S., SMITH AD. Haemolysis of washed human red cells by the combined action of Naja naja phospholipase A2 and albumin. Biochim. Biophys. Acta, 1972, 288, 237-40.
- 14. HAYASHI K., TAKECHI M., SASAKI T. Amino acid sequence of cytotoxin I from the venom of the Indian cobra (Naja naja). Biochem. Biophys. Res. Commun., 1971, 45, 1357-72.
- 15. IBRAHIM SA., THOMPSON RHS. Action of phospholipase on human red cell ghosts and intact erythrocytes. Biochim. Biophys. Acta, 1965, 99, 331-41.
- 16. KLIBANSKY C., LONDON Y., FRENKEL A., DE VRIES A. Enhancing action of synthetic and natural basic polypeptides on erythrocyte ghost phospholipid hydrolysis by phospholipase A. Biochim. Biophys. Acta, 1968, 150, 15-23.
- 17. LEE CY., CHANG CC., CHIU TH., CHIU PJS., TSENG TC., LEE SY. Pharmacological properties of cardiotoxin isolated from Formosan cobra venom. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol., 1968, 259, 360-74.
- 18. LIVNE A., KUIPER PJC. Unique properties of the camel erythrocyte membrane. Biochim. Biophys. Acta, 1973, 318, 41-9.
- 19. LOUW VR. Snake venom toxins. The purification and properties of five neurotoxic polypeptides from Naja mossambica mossambica venom. Biochem. Biophy. Acta 1978, 336, 470-80.
- 20. LOWRY OH., ROSEBROUGH NJ., FARR AL., RANDALL RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem, 1951, 93, 265-75.
- 21. MOHAMED AH., KAMEL A. Direct hemolysis induced by some Egyptian snake venoms and other allied venoms. Indian J. Med. Res. 1972, 60, 1756-63.
- 22. NELSON GJ. Lipid composition of erythrocytes in various mammalian species. Biochim. Biophys. Acta, 1967, 144, 221-32.
- 23. SMITH JE., MOHANDAS N., SHOHET SB. Variability in erythrocytes deformability among various mammals. Am. J. Physiol., 1979, 236, 725-30.
- 24. TROIANO JC., VIDAL JC., GOULD EF., GOULD J. Hematological reference intervals of the South American rattlesnake (Crotalus durissus terrificus, Laurenti, 1768) in captivity. Comp. Haem. Int. 1997, 1, 109-12.
- 25. TROIANO JC., VIDAL JC., GOULD EF., HEKER J., GOULD J., VOGT AU., SIMONCINI C., AMANTINI E., DE ROODT AR. Hematological values of some Bothrops species (Ophidia Crotalidae) in captivity. J. Venom. Anim. Toxins, 1999, 6, 194-204.
Publication Dates
-
Publication in this collection
22 Mar 2006 -
Date of issue
2006
History
-
Received
11 Feb 2005 -
Accepted
30 May 2005