Abstract
Background
Tityus serrulatus scorpion venom (TsV) contains toxins that act on K + and Na + channels and account for the venom’s toxic effects. TsV can activate murine peritoneal macrophages, but its effects on human lymphocytes have been poorly investigated. Considering that lymphocytes may play an important role in envenomation, we assessed whether TsV affects the expression of phenotypic (CD3, CD4, and CD8) and activation (CD69, CD25, and HLA-DR) markers, cell proliferation, and cytokine production in peripheral blood mononuclear cells.
Methods
Cytotoxicity of TsV was evaluated via the MTT assay. Cell proliferation, expression of phenotypic and activation markers, and release of cytokines were assessed using flow cytometry, after treatment with non-cytotoxic concentrations of TsV. The combined use of carboxyfluorescein diacetate succinimidyl ester and monoclonal antibodies against phenotypic and activation markers enabled us to simultaneously assess cell proliferation extent and cell activation status, and to discriminate among cell subpopulations.
Results
TsV at concentrations of 25 to 100 μg/mL were not cytotoxic towards peripheral blood mononuclear cells. TsV did not induce significant changes in lymphocyte subpopulations or in the expression of activation markers on CD4 + and CD8 + T cells. TsV inhibited the phytohemagglutinin-stimulated lymphocyte proliferation, particularly in the CD8 + CD25 + T lymphocyte subset. TsV alone, at 50 and 100 μg/mL, did not induce peripheral blood mononuclear cell proliferation, but elicited the production and release of IL-6, a proinflammatory cytokine that plays an important role in innate and adaptive immune responses.
Conclusions
TsV is a potential source of molecules with immunomodulatory action on human T lymphocytes.
Immunomodulation; Immunophenotyping; T lymphocyte; Cell proliferation; Cytokine; Tityus serrulatus venom
Background
Scorpions from the family Buthidae are responsible for the majority of worldwide envenomations, especially in South Asia, the Middle East, Central and South America, and North Africa [11. Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal . Acta Trop. 2008;107(2) :71-9.], [22. Chippaux JP. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: from obvious facts to contingencies . J Venom Anim Toxins incl Trop Dis.2015; 21 :13.].Tityus is one genus of this family, of which Tityus serrulatus is the most dangerous species – it causes serious envenomation that may provoke fatalities, the majority of the victims are children[33. Cupo P, Jurca M, Azevedo-Marques MM, Oliveira JSM, Hering SE.Severe scorpion envenomation in Brazil: clinical, laboratory and anatomopathological aspects . Rev Inst Med Trop São Paulo. 1994; 36(1) :67-76.]–[77. Bahloul M, Chabchoub I, Chaari A, Chtara K, Kallel H, Dammak H et al.. Scorpion envenomation among children: clinical manifestations and outcome (analysis of 685 cases) . Am J Trop Med Hyg. 2010; 83(5) :1084-92.].
Scorpion venom is composed of numerous toxins, mostly of peptides and neurotoxins, which act by deregulating cell membrane ion channels. Such toxins may cause pain, deregulation of cardiovascular and autonomic nervous systems, vomiting, abdominal pain, stimulation of peripheral nervous system, neuromuscular excitation, among other deleterious effects in humans[88. Isbister KG, Bawaskar HS. Scorpion envenomation .N Engl J Med. 2014;371(5) :457-63.].
Massive activation of the immune system seems also to participate in the pathogenesis of T. serrulatus envenomation. The plasma levels of NO and cytokines – such as interleukin-1 (IL-1), IL-6, IL-8 and interferon-γ (IFN-γ) produced by immune cells – are increased in patients presenting moderate or severeT. serrulatus envenomation, and correlate with the severity of envenomation[99. Fukuhara YD, Reis ML, Dellalibera-Joviliano R, Cunha FQ, Donadi EA. Increased plasma levels of IL-1beta, IL-6, IL-8, IL-10 and TNF-alpha in patients moderately or severely envenomed by Tityus serrulatus scorpion sting . Toxicon. 2003;41(1) :49-55.]. The participation of proinflammatory cytokines in the moderate and severe envenomation pathophysiology indicates that these patients may benefit from treatment with glucocorticoids.
Recent studies reported that the venom of T. serrulatus and its toxins activate murine macrophages, which are critical cells for the immune response because they participate in humoral and cellular responses [1010. Fialho EM, Maciel MC, Silva AC, Reis AS, Assunção AK, Fortes TS et al.. Immune cells recruitment and activation by Tityus serrulatus scorpion venom . Toxicon. 2011;58(6–7) :480-5.]–[1515. Zoccal KF, Paula-Silva FW, Bitencourt CS, Sorgi CA, Bordon KC, Arantes EC et al.. PPAR-γ activation by Tityus serrulatus venom regulates lipid body formation and lipid mediator production .Toxicon. 2015; 93 :90-7.]. However, the effect of Tityus serrulatus scorpion venom (TsV) on human lymphocytes remains to be investigated. Considering that TsV contains several toxins that act on membrane K + channels, and that these channels are involved in the regulation of cellular functions, in the present study we examined whether TsV interferes in peripheral blood mononuclear cell (PBMC) cytokine production, as well as in T lymphocyte phenotype, proliferation, and expression of activation markers.
Methods
Source and preparation of venom
TsV was obtained from the serpentarium of the Ribeirão Preto Medical School, University of São Paulo, Brazil, by using the electrical stimulation method with the minimal voltage required (− 12 V)[1616. Lowe RM, Farrell PM. A portable device for the electrical extraction of scorpion venom .Toxicon. 2011;57(2) :244-7.].
Lyophilized TsV (4 mg) was diluted with 10 mL of sterile distilled water and fractionated by centrifugation (10,015 × g, 4 °C, 10 min), yielding an insoluble and non-toxic fraction composed of mucoproteins and membrane debris. The soluble part was rich in basic neurotoxic proteins, but also contained various enzymes and organic (lipids, carbohydrates, nucleosides, free amino acids) and inorganic (ions) compounds. Protein concentrations in crude soluble venom were determined by absorbance readings at 280 nm using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and the extinction coefficient of the soluble venom [1717. Pucca MB, Amorim FG, Cerni FA, Bordon KC, Cardoso IA, Anjolette FA et al.. Influence of post-starvation extraction time and prey-specific diet in Tityus serrulatus scorpion venom composition and hyaluronidase activity . Toxicon. 2014;90 :326-36.]:
Peripheral blood mononuclear cell (PBMC) isolation and culture
PBMC were isolated from venous blood of 20 healthy adult volunteers (7 males and 13 females, 20–40 years old) via the Ficoll-Hypaque (Hystopaque®-1077; Sigma-Aldrich, USA) discontinuous density centrifugation method. PBMC were washed twice, suspended in RPMI 1640 medium (GIBCO®, USA) to a concentration of 5 × 10 6 cells/mL. Cells were cultured in RPMI with phytohemagglutinin (PHA; 2 μg/mL; Sigma-Aldrich, USA) and/or TsV (25, 50, and 100 μg/mL) at 37 °C and under 5 % CO 2 , during the proliferation assay (96 h) and before the immunophenotyping assay (24 h). The Human Research Ethics Committee at FCFRP-USP approved the research protocol, registered under number CEP/FCFRP 166.
Cytotoxicity evaluation
The cytotoxic activity of TsV was evaluated using the MTT method described by Mosmann [1818. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays .J Immunol Methods. 1983;65(1–2) :55-63.]. PBMC (5 × 10 5cells/well) were cultured in 96-well plates in RPMI 1640 complete culture medium containing 10 % fetal calf serum (FCS) in the presence of TsV (12.5; 25.0; 50.0; 62.5; 100.0; 125.0; 250.0; 500.0; 1000.0; 2000.0 μg/mL) or cyclophosphamide (Cyclo; 25 mg/mL; positive control; Sigma-Aldrich, USA) previously diluted in culture medium, for 24 h at 37 °C, and under 5 % CO 2 . Next, 20 μL of MTT (Sigma-Aldrich, USA) solution at 5 mg/mL was added to each well, and the plates were incubated for 4 h, at 37 °C, and under 5 % CO 2 . After incubation, the precipitated formazan crystals were dissolved with 200 μL of 20 % SDS (sodium dodecylsulfate), and absorbance was recorded at 570 nm. Absorbance values recorded for untreated cells (negative control) represent 100 % of PBMC viability, and were used as reference to calculate the percentage of cell viability in the presence of each sample concentration.
Immunophenotyping of lymphocyte subsets and activation markers
Flow cytometry analysis was performed to identify and quantify lymphocyte subpopulations (CD3-FITC, CD4-PE, and CD8-PE monoclonal antibodies), and to measure the levels of cellular activation markers expression (CD69, CD25, and HLA-DR monoclonal antibodies conjugated with APC) (Becton-Dickinson, USA).
PBMC (2 × 10 5 cells per well) were cultured with TsV (25; 50; 100 μg/mL) for 24 h at 37 °C, and under 5 % CO 2 . Cells were collected, suspended to a concentration of 1 × 10 6 cells in 100 μL of FACS buffer (phosphate buffered saline – PBS, with 10 % FCS and 1 % sodium azide), and incubated with 1 μL of monoclonal antibodies at the following concentrations (mg/mL): 0.0031(CD4); 0.0125 (CD8); 0.012 (CD25); 0.2 (CD69); 0.05 (HLA-DR); and 0.1 (CD3), for 30 min at 4 °C, in the dark. Then, the cells were washed with PBS and suspended in 100 μL of PBS-FCS.
A total of 30,000 events per tube was acquired on the flow cytometer (FACSCanto, Becton-Dickinson, USA), the lymphocyte gate was selected according to its forward and side scatter distribution, and further analyzed with the aid of the software FACSDiva (Becton-Dickinson, USA). The results were expressed as percentages of stained cells. Additional file 1 depicts representative forward/side scatter dot-plots of human PBMC treated with TsV.
Cell proliferation assay
The use of carboxyfluorescein diacetate succinimidyl ester (CFSE) in combination with monoclonal antibodies against phenotypic and activation markers enabled the concomitant determination of cell proliferation and activation status and discrimination of cell subpopulations.
PBMC were centrifuged, suspended to the concentration of 5 × 10 6cells/mL in PBS containing 0.1 % human albumin, and labeled with CFSE (2.5 μM; Molecular Probes, USA) for ten minutes at 37 °C. The labeling process was stopped by addition of five volumes of ice-cold RPMI 1640 containing 10 % FCS (RPMI-FCS) followed by incubation for five minutes in an ice bath, in the dark. The cells were washed three times with 20 mL of RPMI-FCS, and further suspended in the same medium.
PBMC were plated in 96-well plates (5 × 10 5 cells/well) and cultured with PHA (2 μg/mL) and/or TsV(25, 50, or 100 μg/mL) for 96 h at 37 °C, and under 5 % CO 2 . The cells (1 × 10 6 ) were transferred to flow cytometry tubes and labeled with 2 μL of the following monoclonal antibodies: anti-CD3/PerCP (Peridinin-chlorophyll-protein complex); anti-CD8, or anti-CD4 PE; anti-CD25, anti-HLA-DR, or anti-CD69 APC (Becton-Dickinson, USA). A total of 30,000 events per tube was acquired on the flow cytometer (FACSCanto, Becton-Dickinson, USA) and analyzed with the aid of the software FACSDiva (Becton-Dickinson, USA). The lymphocyte gate was set on light-scatter properties (forward scatter vs. side scatter). The results were expressed as percentages of stained cells.
Cytokine quantification
The levels of tumor necrosis factor-α (TNF-α), IL-2, IL-4, IL-6, IL-10, IL-17, and IFN-γ in culture supernatants of PBMC (1.0 × 10 5 cells) treated with PHA (2 μg/mL) and/or TsV (25, 50, and 100 μg/mL) for 96 h were quantified by flow cytometry, using the assay kit Cytometric Bead Array (CBA) Human Th1/Th2/Th17 Cytokine (BD Biosciences, USA) according to the manufacturer’s instructions. The samples were analyzed on a FACSCanto cytometer with the aid of the CBA analysis software FCAP Array (version 1.01; Becton-Dickinson, USA). The cytokine concentration was expressed as pg/mL.
Statistical analysis
The results were expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by the Tukey’s test, using the software Graph Pad Prism 5. Significance was defined as p< 0.05.
Results
TsV at lower concentrations is not cytotoxic towards PBMC
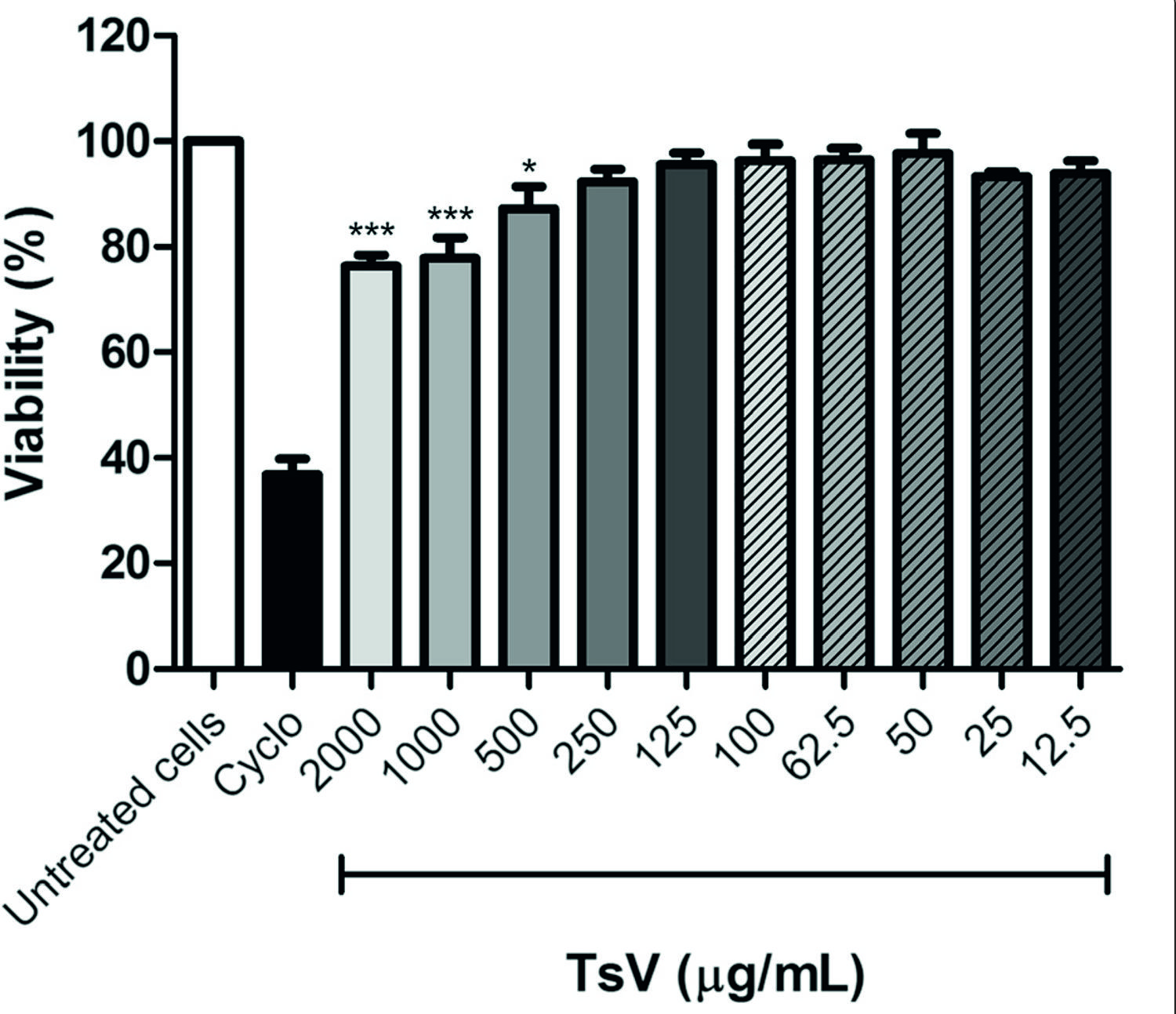
We used the MTT assay to examine whether TsV is cytotoxic towards PBMC. Compared with untreated cells, TsV at concentrations of 500, 1000, and 2000 μg/mL reduced cell viability by 12.7, 22.2, and 23.7 %, respectively. TsV was not cytotoxic towards PBMC at concentrations lower than 250 μg/mL (Fig. 1). Based on these results, we selected the TsV concentrations of 25, 50, and 100 μg/mL for further experiments.
Effect of Tityus serrulatus scorpion venom (TsV) on peripheral blood mononuclear cell (PBMC) viability. PBMC (5 × 10 5 cells/well) were treated with different concentrations of TsV for 24 h, and cell viability was assessed by the MTT method. Cyclophosphamide (Cyclo; 25 mg/mL) was used as positive control. Results are expressed as mean (± SEM) percentage of cell viability calculated in relation to untreated cells (n= 6 experiments). *p< 0.05 and ***p< 0.001 vs. untreated cells
TsV does not induce changes in T lymphocyte subpopulations and in the expression of PBMC activation markers
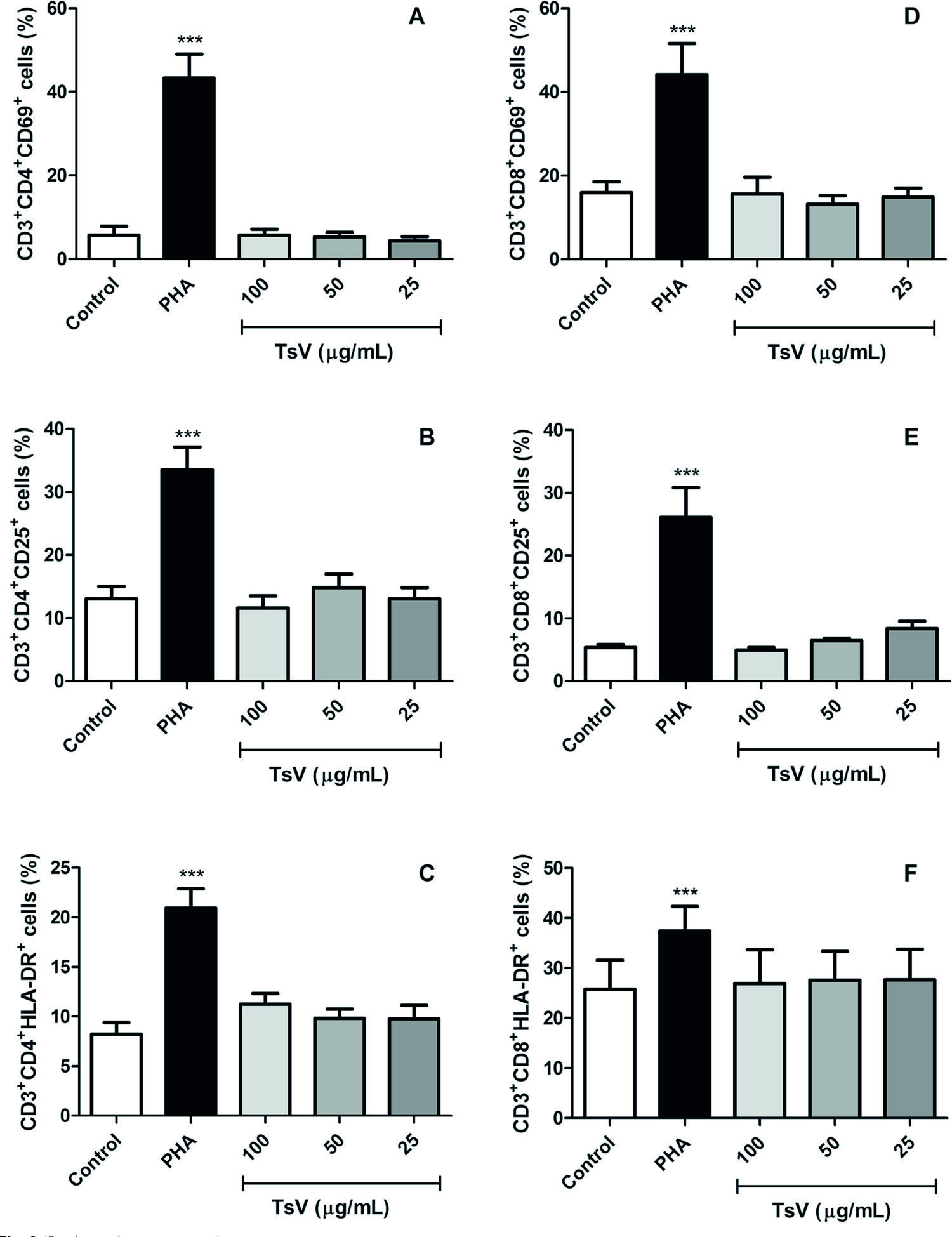
After 24 h of culture, TsV at concentrations of 25, 50, and 100 μg/mL did not induce significant changes in TCD3 + CD4 + and CD3+ CD8 + lymphocyte subpopulations, and did not modulate the expression of three activation markers – CD69, CD25 and HLA-DR – in PBMC (Fig. 2).
Lymphocyte subsets and their expression levels of activation markers after TsV treatment. Peripheral blood mononuclear cells (2 × 105 cells) were cultured with Tityus serrulatusscorpion venom (TsV; 25, 50, and 100 μg/mL) or phytohemagglutinin (PHA; 2 μg/mL; positive control) for 24 h, at 37 °C, under 5 % CO 2 , and further stained with monoclonal antibodies to simultaneously detect CD3 + CD4 + (a, b, c) and CD3 + CD8+ (d, e, f) T lymphocyte subsets, and the expression of three activation markers: CD69 (a, d), CD25 (b, e), and HLA-DR (c, f). Control: untreated cells. Data are expressed as mean ± SEM of six experiments. ***p <0.001 vs. control
TsV inhibits the proliferation of CD8 + CD25 + T lymphocytes
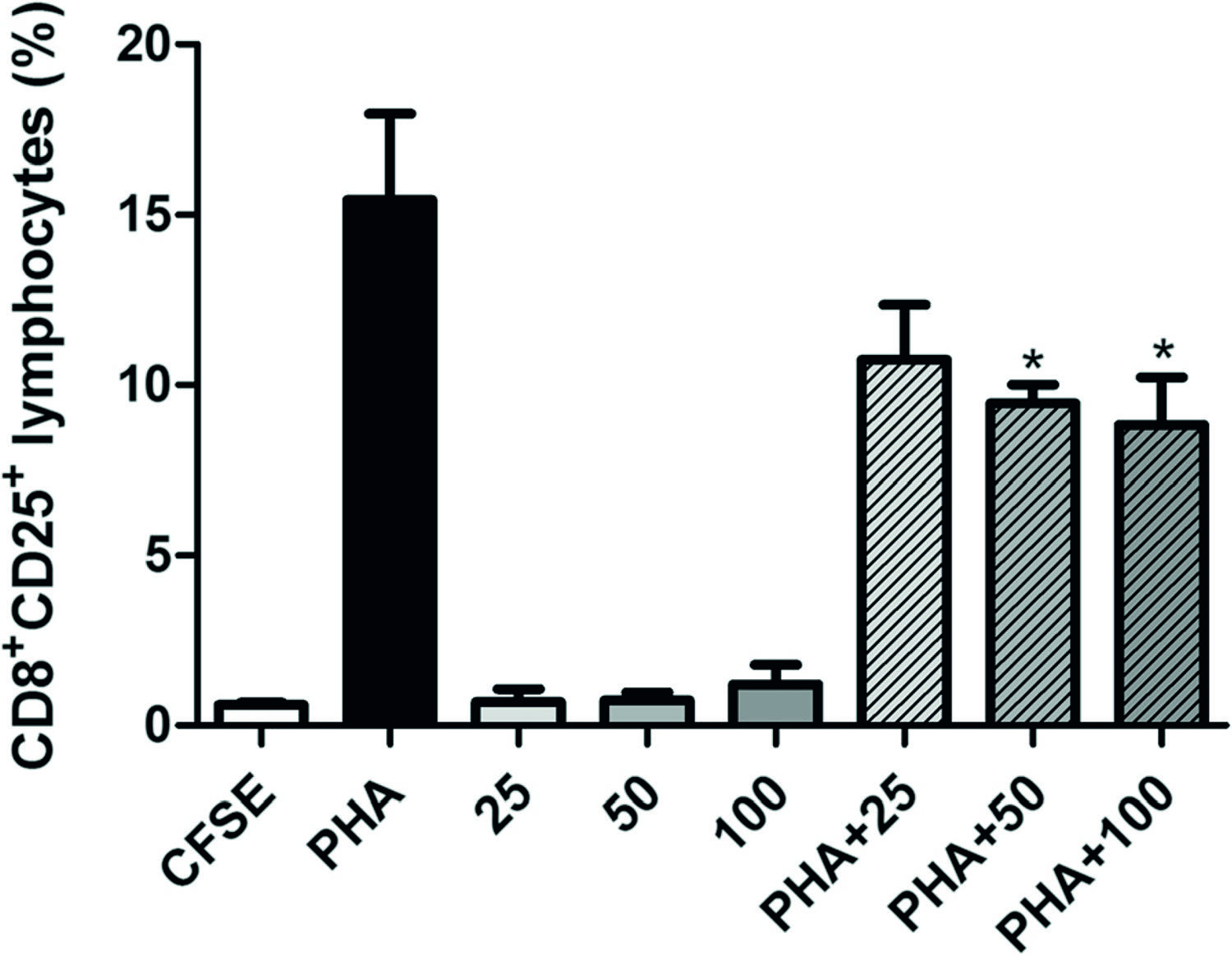
In PBMC previously stimulated with PHA, the 96-h treatment with TsV at 25, 50 or 100 μg/mL reduced the percentage of CD8 + CD25 +lymphocytes by 35.7, 45.5, and 53.5 %, respectively, i.e. in a concentration-dependent manner (Fig. 3). These values were statistically different from the respective control (PHA) at TsV concentrations of 50 and 100 μg/mL (Fig. 3). In contrast, TsV did not alter either T lymphocyte proliferation extent or the percentage of CD3 + CD4 + and CD3+ CD4+ HLA-DR + lymphocyte subsets in non-stimulated PBMC, as compared with PHA- and PHA + TsV-stimulated PBMC (data not shown).
Percentage of CD8 + T lymphocytes expressing CD25. Peripheral blood mononuclear cells (PBMC; 5 × 10 6 cells/mL) were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; 2.5 μM) and cultured with Tityus serrulatus scorpion venom (TsV; 25, 50, and 100 μg/mL) alone or in combination with phytohemagglutinin (PHA; 2 μg/mL) for 96 h at 37 °C, and under 5 % CO 2. Then, PBMC were collected, labeled with anti-CD3/PerCP, anti-CD8/PE, and anti-CD25/APC monoclonal antibodies, and further analyzed by flow cytometry. CFSE: CFSE-stained cells cultured in RPMI; PHA: PHA-stimulated cells (positive control); 25, 50, 100: cells incubated with 25, 50, and 100 μg/mL TsV, respectively; PHA + 25, PHA + 50, PHA + 100: cells incubated with PHA plus 25, 50 and 100 μg/mL TsV, respectively. Each column represents the mean ± SEM of five experiments.*p< 0.05 vs. PHA
TsV induces IL-6 release by PBMC
TsV induced IL-6 release by PBMC in a concentration-dependent manner. The IL-6 levels in culture supernatants of non-stimulated PBMC were significantly increased at TsV concentrations of 50 and 100 μg/mL (p< 0.05 and p< 0.01, respectively), compared with the respective control (CFSE) (Fig. 4). TsV did not alter IL-6 release in PHA-stimulated cells, or the release of other cytokines (IL-2, IL-4, IL-10, IL-17, IFN-γ, and TNF-α) by non-stimulated and PHA-stimulated PBMC (data not shown).
Interleukin-6 (IL-6) release by PBMC treated with Tityus serrulatusscorpion venom (TsV) for 96 h. Peripheral blood mononuclear cells (PBMC; 5.0 × 10 5 cells) were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; 2.5 μM) and cultured with TsV (25, 50, and 100 μg/mL) alone or in combination with phytohemagglutinin (PHA; 2 μg/mL) for 96 h. The levels of IL-6 in culture supernatants were determined by flow cytometry. CFSE: CFSE-stained cells cultured in RPMI 1640; PHA: PHA-stimulated cells (positive control); 25, 50, 100: cells incubated with 25, 50, and 100 μg/mL TsV, respectively; PHA + 25, PHA + 50, PHA + 100: cells incubated with PHA plus 25, 50, and 100 μg/mL TsV, respectively. Each column represents the mean ± SEM of five experiments. *p< 0.05, **p< 0.01, ***p< 0.001 vs. CFSE
Discussion
In Brazil, the yellow scorpion T. serrulatus is the most dangerous Buthidae species and the main cause of serious accidents [44. Lourenço WR. What do we know about some of the most conspicuous scorpion species of the genus Tityus? A historical approach . J Venom Anim Toxins incl Trop Dis. 2015;21 :20.]. Its venom (TsV) contains toxins that act on K +and Na + channels, a property that accounts for great portion of the venom’s toxic effects. Several studies have reported that scorpion venoms and toxins, including TsV, elicit macrophage activation [1111. Ortiz E, Gurrola GB, Schwartz EF, Possani LD. Scorpion venom components as potential candidates for drug development .Toxicon. 2015; 93 :125-35.]–[1313. Zoccal KF, Bitencourt CS, Sorgi CA, Bordon KC, Sampaio SV, Arantes EC et al.. Ts6 and Ts2 from Tityus serrulatus venom induce inflammation by mechanisms dependent on lipid mediators and cytokine production .Toxicon. 2013; 61 :1-10.],[1515. Zoccal KF, Paula-Silva FW, Bitencourt CS, Sorgi CA, Bordon KC, Arantes EC et al.. PPAR-γ activation by Tityus serrulatus venom regulates lipid body formation and lipid mediator production .Toxicon. 2015; 93 :90-7.], [1919. Petricevich VL. Scorpion venom and the inflammatory response. Mediators Inflamm. 2010; Article ID 903295, 16 pages, 2010. doi:10.1155/2010/903295.]. TsV and Ts1 bind Toll-like receptors to mediate cytokine and lipid mediator production[1414. Zoccal KF, Bitencourt CS, Paula-Silva FW, Sorgi CA, Bordon KCF, Arantes EC et al.. TLR2, TLR4 and CD14 recognize venom-associated molecular patterns from Tityus serrulatus to induce macrophage-derived inflammatory mediators . PLoS ONE. 2014;9(2) :e88174.]. Such reports have helped to elucidate how inflammatory mediators are produced after envenomation.
To date, very little is known about the direct effect of scorpion venoms on human lymphocyte functions. As lymphocytes have membrane K + channels and TsV contains toxins that interact with these channels, we hypothesized that TsV and its toxins could affect lymphocyte functions mediated by the activity of K +channels, such as PHA-induced cell proliferation [1111. Ortiz E, Gurrola GB, Schwartz EF, Possani LD. Scorpion venom components as potential candidates for drug development .Toxicon. 2015; 93 :125-35.], [2020. Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes .Immunol Rev. 2009;1(1) :59-87.]–[2222. DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K + channels in human T lymphocytes: a role in mitogenesis?Nature. 1984; 307(5950) :465-8.].
Cell stimulation by pathogens in infectious and inflammatory processes and by mitogens induces the expression of activation markers in T, B, and NK cells. PHA, a commonly used mitogen, is a plant lectin that binds to TCR and activates T lymphocytes [2323. Licastro F, Davis LJ, Morini MC. Lectins and superantigens: membrane interactions of these compounds with T-lymphocytes affect immune responses . Int J Biochem. 1993;25(6) :845-52.]. In the present study, we examined the expression of very early (CD69), middle-to-late (CD25), and late (HLA-DR) T cell surface activation markers. CD69 expression can be detected within 1 to 2 h after T cell stimulation [2424. Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69 . Stem Cells. 1994;12(5) :456-65.]. It can initiate tyrosine kinase and calcium flux activity, and transcription of IL-2 and TNF-α [2525. Rea IM, McNerlan SE, Alexander HD. CD69, CD25, and HLA-DR activation antigen expression on CD3+ lymphocytes and relationship to serum TNF-alpha, IFN-gamma, and sIL-2R levels in aging . Exp Gerontol. 1999; 34(1) :79-93.]. The expression of CD25 (the α-chain of the IL-2 receptor) and HLA-DR (a human class II major histocompatibility complex antigen) increases progressively after 24 h of stimulation with PHA [2626. Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U.Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function .J Immunol Methods. 2004;293(1–2) :127-42.].
After establishing the TsV concentrations and incubation time with PBMC, we examined whether TsV alters the percentage of T lymphocytes, and the extent of CD4+ and CD8 + T cell proliferation. In the presence of TsV, the percentage of T lymphocytes (data not shown), as well as the percentage of CD3+ CD4 + and CD3 + CD8 + lymphocyte subpopulations expressing CD69, CD25, and HLA-DR were similar to the untreated controls. However, maximum expression of some activation markers, especially CD25 and HLA-DR, may occur later. It suggests that the PBMC culture periods used (24 h and 96 h) were not sufficient to detect significant alterations in the expression level of these markers. To confirm this hypothesis, it is necessary to perform additional experiments using longer culture times. Another possibility is to use higher TsV concentrations to better evaluate its action on the expression of lymphocyte activation markers.
To assess lymphocyte proliferation, we selected CFSE due to its compatibility with several other fluorochromes, which enabled us to simultaneously evaluate several parameters including the percentage of activated cells and proliferation of distinct populations of activated cells, using multi-color flow cytometry [2727. Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester . Nat Protoc. 2007; 2(9) :2049-56.]. With the aid of this technique, we found that TsV at 50 and 100 μg/mL significantly decreased the percentage of CD8+ CD25 + T cells in PHA-stimulated PBMC (Fig. 3). Considering that ion channels participate in an early stage of cell activation by PHA, it is possible that toxins that exist in TsV act on ions channels in CD8 + CD25 + T cells and thereby impair the action of PHA on these cells [2222. DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K + channels in human T lymphocytes: a role in mitogenesis?Nature. 1984; 307(5950) :465-8.]. It is well documented that ion channels play important roles in the activation and proliferation of lymphocytes, as well as in the production of cytokines [2020. Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes .Immunol Rev. 2009;1(1) :59-87.], [2828. Cahalan MD, Wulff H, Chandy KG. Molecular properties and physiological roles of ion channels in the immune system . J Clin Immunol. 2001; 21(4) :235-52. PubMed Abstract|],[2929. Urrego D, Tomczak AP, Zahed F, Stühmer W, Pardo LA.Potassium channels in cell cycle and cell proliferation .Philos Trans R Soc Lond B Biol Sci. 2014;369(1638) :20130094.]. Blockage of the K+ channel Kv1.3 inhibits mitogen-induced T cell proliferation, protein synthesis and IL-2 production [2020. Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes .Immunol Rev. 2009;1(1) :59-87.].
Cytokines are produced during the effector phases of innate and acquired immune responses and regulate immune and inflammatory responses [3030. Van der Meide PH, Schellekens H. Cytokines and the immune response . Biotherapy. 1996;8(3–4) :243-9.], [3131. Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways .Blood. 2011; 118(1) :9-18.]. Besides the knowledge that TsV and its toxins are potent inducers of cytokine and lipid mediator production in vitro and in vivo [1111. Ortiz E, Gurrola GB, Schwartz EF, Possani LD. Scorpion venom components as potential candidates for drug development .Toxicon. 2015; 93 :125-35.]–[1515. Zoccal KF, Paula-Silva FW, Bitencourt CS, Sorgi CA, Bordon KC, Arantes EC et al.. PPAR-γ activation by Tityus serrulatus venom regulates lipid body formation and lipid mediator production .Toxicon. 2015; 93 :90-7.],[1919. Petricevich VL. Scorpion venom and the inflammatory response. Mediators Inflamm. 2010; Article ID 903295, 16 pages, 2010. doi:10.1155/2010/903295.], little is known about the effect of TsV on the production of cytokines by human PBMC. We measured the levels of various cytokines – IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ and TNF-α – in the supernatant of PBMC cultures after 96 h of treatment with PHA and/or different concentrations of crude TsV. Compared with untreated cells, TsV at 50 and 100 μg/mL significantly increased IL-6 release in non-stimulated cells (p< 0.05 and p< 0.01, respectively) (Fig. 4). In contrast, TsV did not alter the PHA-induced IL-6 production by PBMC. Our results partially agree with those reported by several in vitro and in vivo studies [1111. Ortiz E, Gurrola GB, Schwartz EF, Possani LD. Scorpion venom components as potential candidates for drug development .Toxicon. 2015; 93 :125-35.]–[1515. Zoccal KF, Paula-Silva FW, Bitencourt CS, Sorgi CA, Bordon KC, Arantes EC et al.. PPAR-γ activation by Tityus serrulatus venom regulates lipid body formation and lipid mediator production .Toxicon. 2015; 93 :90-7.], [1919. Petricevich VL. Scorpion venom and the inflammatory response. Mediators Inflamm. 2010; Article ID 903295, 16 pages, 2010. doi:10.1155/2010/903295.]. TsV fractions stimulate the macrophage production of proinflammatory cytokines such as TNF-α, IL-1, and IL-6 [3232. Petricevich VL, Lebrun I. Immunomodulatory effects of the Tityus serrulatus venom on murine macrophage functions in vitro .Mediators Inflamm. 2005;2005(1) :39-49.]. TsV and the toxins Ts1 and Ts6 stimulate the production of NO, IL-6, and TNF-α in J774.1 cells [1212. Zoccal KF, Bitencourt CS, Secatto A, Sorgi CA, Bordon KC, Sampaio SV et al.. Tityus serrulatus venom and toxins Ts1, Ts2 and Ts6 induce macrophage activation and production of immune mediators .Toxicon. 2011;57(7–8) :1101-8.]. When inoculated in mice, Ts2 and Ts6 induce the production of the proinflammatory cytokines IL-6, TNF-α, IL-1β, and IFN-γ, and the lipid mediators prostaglandin E2 and leukotriene B4 [1313. Zoccal KF, Bitencourt CS, Sorgi CA, Bordon KC, Sampaio SV, Arantes EC et al.. Ts6 and Ts2 from Tityus serrulatus venom induce inflammation by mechanisms dependent on lipid mediators and cytokine production .Toxicon. 2013; 61 :1-10.]. Several authors have reported that IL-6 levels are increased after envenomation in humans [99. Fukuhara YD, Reis ML, Dellalibera-Joviliano R, Cunha FQ, Donadi EA. Increased plasma levels of IL-1beta, IL-6, IL-8, IL-10 and TNF-alpha in patients moderately or severely envenomed by Tityus serrulatus scorpion sting . Toxicon. 2003;41(1) :49-55.], [3333. Sofer S, Gueron M, White RM, Lifshitz M, Apte RN.Interleukin-6 release following scorpion sting in children .Toxicon. 1996; 34(3) :389-92.]–[3535. Voronov E, Apte RN, Sofer S. The systemic inflammatory response syndrome related to the release of cytokines following severe envenomation. J Venom Anim Toxins. 1999;5(1):5–33. Available at: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-79301999000100002. Accessed 10 Nov 2015.
http://www.scielo.br/scielo.php?script=s...
].
To the best of our knowledge, this is the first study that shows immunomodulatory actions of TsV on human T lymphocytes. Taken together, our results suggest that TsV is a potential source of molecules with immunomodulatory effects on these cells, and should stimulate further research to elucidate the action mechanisms involved, including the participation of T lymphocyte ion channels in the phenomena observed.
Conclusions
TsV is a potential immunomodulator of human T lymphocyte functions. The results reported herein stimulate further investigations to identify the venom components responsible for the observed effects and to elucidate the molecular mechanisms involved in TsV immunomodulatory action on lymphocytes. These investigations may facilitate the development of new tools to study the pathophysiological mechanisms of envenomation and to discover new treatment alternatives for diseases mediated by the immune system.
Additional file
Additional file 1:. Representative picture showing forward/side scatter dot-plot of human PBMC treated with TsV. Peripheral blood mononuclear cells (PBMC; 2 × 106 cells/mL) were cultured with Tityus serrulatus scorpion venom (TsV; 25, 50, and 100 μg/mL), phytohemagglutinin (PHA; 2 μg/mL; positive control) for 24 h, at 37 °C, and under 5 % CO 2. Untreated PBMC represents the negative control. PBMC were labeled with anti-CD3/FITC and anti-CD8/PE monoclonal antibodies and further analyzed by flow cytometry. The lymphocyte gate was selected and analyzed to calculate the percentage of stained cells. The figure depicts a representative analysis from six independent experiments. The percentages in parentheses refer to CD3+ CD8+ cells (P2 region). (A) Negative control (5.5 %). (B) PHA-stimulated cells (26.1 %). (C) 100 μg/mL of TsV (4.9 %). (D) 50 μg/mL of TsV (6.4 %). (E) 25 μg/mL of TsV (8.4 %). (DOCX 204 kb) http://www.jvat.org/content/supplementary/s40409-015-0046-3-s1.docx
Acknowledgments
The authors would like to thank the Nucleus for Research on Animal Toxins (NAP-TOXAN-USP, grant n. 12–125432.1.3), the State of São Paulo Research Foundation (FAPESP, grant n. 2011/23236-4), the Coordination for the Improvement of Higher Education Personnel (CAPES, ACM, JCP and SMB grants) and the National Council for Scientific and Technological Development (CNPq, SMB grant) for their funding of this research. We also thanks to T. M. Casare-Ogasawara for the technical support. Thanks are also due to the Center for the Study of Venoms and Venomous Animals (CEVAP) of UNESP for enabling the publication of this special collection (CNPq process 469660/2014-7).
References
-
1Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal . Acta Trop 2008;107(2) :71-9.
-
2Chippaux JP. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: from obvious facts to contingencies . J Venom Anim Toxins incl Trop Dis.2015; 21 :13.
-
3Cupo P, Jurca M, Azevedo-Marques MM, Oliveira JSM, Hering SE.Severe scorpion envenomation in Brazil: clinical, laboratory and anatomopathological aspects . Rev Inst Med Trop São Paulo 1994; 36(1) :67-76.
-
4Lourenço WR. What do we know about some of the most conspicuous scorpion species of the genus Tityus? A historical approach . J Venom Anim Toxins incl Trop Dis 2015;21 :20.
-
5Ministério da Saúde, Secretaria de Vigilância em Saúde. Manual de Controle de Escorpiões. Brasília: MS; 2009. http://bvsms.saude.gov.br/bvs/publicacoes/manual_controle_escorpioes.pdf. (Accessed 10 Nov 2015).
» http://bvsms.saude.gov.br/bvs/publicacoes/manual_controle_escorpioes.pdf -
6Reckziegel GC, Pinto VL. Scorpionism in Brazil in the years 2000 to 2012. J Venom Anim Toxins incl Trop Dis. 2014;20:46. http://www.jvat.org/content/20/1/46. (Accessed 10 Nov 2015).
» http://www.jvat.org/content/20/1/46 -
7Bahloul M, Chabchoub I, Chaari A, Chtara K, Kallel H, Dammak H et al.. Scorpion envenomation among children: clinical manifestations and outcome (analysis of 685 cases) . Am J Trop Med Hyg 2010; 83(5) :1084-92.
-
8Isbister KG, Bawaskar HS. Scorpion envenomation .N Engl J Med 2014;371(5) :457-63.
-
9Fukuhara YD, Reis ML, Dellalibera-Joviliano R, Cunha FQ, Donadi EA. Increased plasma levels of IL-1beta, IL-6, IL-8, IL-10 and TNF-alpha in patients moderately or severely envenomed by Tityus serrulatus scorpion sting . Toxicon 2003;41(1) :49-55.
-
10Fialho EM, Maciel MC, Silva AC, Reis AS, Assunção AK, Fortes TS et al.. Immune cells recruitment and activation by Tityus serrulatus scorpion venom . Toxicon 2011;58(6–7) :480-5.
-
11Ortiz E, Gurrola GB, Schwartz EF, Possani LD. Scorpion venom components as potential candidates for drug development .Toxicon 2015; 93 :125-35.
-
12Zoccal KF, Bitencourt CS, Secatto A, Sorgi CA, Bordon KC, Sampaio SV et al.. Tityus serrulatus venom and toxins Ts1, Ts2 and Ts6 induce macrophage activation and production of immune mediators .Toxicon 2011;57(7–8) :1101-8.
-
13Zoccal KF, Bitencourt CS, Sorgi CA, Bordon KC, Sampaio SV, Arantes EC et al.. Ts6 and Ts2 from Tityus serrulatus venom induce inflammation by mechanisms dependent on lipid mediators and cytokine production .Toxicon 2013; 61 :1-10.
-
14Zoccal KF, Bitencourt CS, Paula-Silva FW, Sorgi CA, Bordon KCF, Arantes EC et al.. TLR2, TLR4 and CD14 recognize venom-associated molecular patterns from Tityus serrulatus to induce macrophage-derived inflammatory mediators . PLoS ONE 2014;9(2) :e88174.
-
15Zoccal KF, Paula-Silva FW, Bitencourt CS, Sorgi CA, Bordon KC, Arantes EC et al.. PPAR-γ activation by Tityus serrulatus venom regulates lipid body formation and lipid mediator production .Toxicon 2015; 93 :90-7.
-
16Lowe RM, Farrell PM. A portable device for the electrical extraction of scorpion venom .Toxicon 2011;57(2) :244-7.
-
17Pucca MB, Amorim FG, Cerni FA, Bordon KC, Cardoso IA, Anjolette FA et al.. Influence of post-starvation extraction time and prey-specific diet in Tityus serrulatus scorpion venom composition and hyaluronidase activity . Toxicon 2014;90 :326-36.
-
18Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays .J Immunol Methods 1983;65(1–2) :55-63.
-
19Petricevich VL. Scorpion venom and the inflammatory response. Mediators Inflamm. 2010; Article ID 903295, 16 pages, 2010. doi:10.1155/2010/903295.
-
20Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes .Immunol Rev 2009;1(1) :59-87.
-
21Cerni FA, Pucca MB, Peigneur S, Cremonez CM, Bordon KCF, Tytgat J et al..Electrophysiological characterization of Ts6 and Ts7, K + channel toxins isolated through an improved Tityus serrulatus venom purification procedure . Toxins 2014;6(3) :892-913.
-
22DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K + channels in human T lymphocytes: a role in mitogenesis?Nature 1984; 307(5950) :465-8.
-
23Licastro F, Davis LJ, Morini MC. Lectins and superantigens: membrane interactions of these compounds with T-lymphocytes affect immune responses . Int J Biochem 1993;25(6) :845-52.
-
24Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69 . Stem Cells 1994;12(5) :456-65.
-
25Rea IM, McNerlan SE, Alexander HD. CD69, CD25, and HLA-DR activation antigen expression on CD3+ lymphocytes and relationship to serum TNF-alpha, IFN-gamma, and sIL-2R levels in aging . Exp Gerontol 1999; 34(1) :79-93.
-
26Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U.Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function .J Immunol Methods 2004;293(1–2) :127-42.
-
27Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester . Nat Protoc 2007; 2(9) :2049-56.
-
28Cahalan MD, Wulff H, Chandy KG. Molecular properties and physiological roles of ion channels in the immune system . J Clin Immunol 2001; 21(4) :235-52. PubMed Abstract|
-
29Urrego D, Tomczak AP, Zahed F, Stühmer W, Pardo LA.Potassium channels in cell cycle and cell proliferation .Philos Trans R Soc Lond B Biol Sci 2014;369(1638) :20130094.
-
30Van der Meide PH, Schellekens H. Cytokines and the immune response . Biotherapy 1996;8(3–4) :243-9.
-
31Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways .Blood 2011; 118(1) :9-18.
-
32Petricevich VL, Lebrun I. Immunomodulatory effects of the Tityus serrulatus venom on murine macrophage functions in vitro .Mediators Inflamm 2005;2005(1) :39-49.
-
33Sofer S, Gueron M, White RM, Lifshitz M, Apte RN.Interleukin-6 release following scorpion sting in children .Toxicon 1996; 34(3) :389-92.
-
34Magalhães MM, Pereira ME, Amaral CF, Rezende NA, Campolina D, Bucaretchi F et al..Serum levels of cytokines in patients envenomed by Tityus serrulatus scorpion sting . Toxicon 1999;37(8) :1155-64.
-
35Voronov E, Apte RN, Sofer S. The systemic inflammatory response syndrome related to the release of cytokines following severe envenomation. J Venom Anim Toxins. 1999;5(1):5–33. Available at: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-79301999000100002. Accessed 10 Nov 2015.
» http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-79301999000100002
-
Ethics committee approvalThe present study was approved by the Human Research Ethics Committee at FCFRP-USP registered under number CEP/FCFRP 166.
Publication Dates
-
Publication in this collection
2015
History
-
Received
19 Feb 2015 -
Reviewed
5 Nov 2015 -
Accepted
11 Nov 2015