Abstract
To establish Korea National Standards for venoms and antivenoms, it is necessary to have standardized assay methods. In this study, we standardized a method to evaluate the antihemorrhagic potency of two horse-derived antivenoms using rabbit intracutaneous injection. We expressed the capability of these antivenoms to neutralize the hemorrhagic activities triggered by the venoms of Agkistrodon halys from Japan and Jiangzhe Agkistrodon halys from China as Minimum Hemorrhagic Dose (MHD). We also performed cross-neutralization tests employing the parallel line assay on different pairings of venoms and antivenoms to check the possibility of using Jiangzhe Agkistrodon halys venom as a substitute for the standard Agkistrodon halys venom in measurements of the antihemorrhagic activity, since A. halys venom is not easily available. Slope function ratio (S.R.) was 0.957 for Agkistrodon halys venom either with Agkistrodon halys antivenom or with Jiangzhe Agkistrodon halys antivenom (p>0.05). Similarly, S.R. was 0.348 for Jiangzhe Agkistrodon halys venom either with Agkistrodon halys antivenom or with Jiangzhe Agkistrodon halys antivenom (p>0.05). Thus, in this study we established antihemorrhagic potency test methods for both Agkistrodon halys and Jiangzhe Agkistrodon halys antivenoms and we could also show it is possible to use Jiangzhe Agkistrodon halys venom as a standard.
antihemorrhagic potency; minimum hemorrhagic dose; Jiangzhe Agkistrodon halys; parallel line assay
ORIGINAL PAPER
Standardization of an antihemorrhagic potency test of antivenoms prepared from two different Agkistrodon halys venoms
Lee K. H.I; Hur S. J.I; Kim S. N.I; Yoo S. H.I; Shin I. S.I; Won H. J.I; Shin K. H.I; Hong S. H.I; Lee S. H.II; Min H. K.I; Park S. N.I, II
IDepartment of Biologics Evaluation, Korea Food and Drug Administration, Seoul, Korea
IINational Institute of Toxicological Research, Korea Food and Drug Administration, Seoul, Korea
Correspondence to Correspondence to: Sue Nie Park Blood Products Division, Department of Biologics Evaluation Korea Food and Drug Administration, 231, Jinheoungno Eunpyeong-Gu, Seoul 122-704, Korea Phone: 82 2 380 1711. Fax: 82 2 383 8322 or 82 2 380 1349 Email: suenie@kfda.go.kr
ABSTRACT
To establish Korea National Standards for venoms and antivenoms, it is necessary to have standardized assay methods. In this study, we standardized a method to evaluate the antihemorrhagic potency of two horse-derived antivenoms using rabbit intracutaneous injection. We expressed the capability of these antivenoms to neutralize the hemorrhagic activities triggered by the venoms of Agkistrodon halys from Japan and Jiangzhe Agkistrodon halys from China as Minimum Hemorrhagic Dose (MHD). We also performed cross-neutralization tests employing the parallel line assay on different pairings of venoms and antivenoms to check the possibility of using Jiangzhe Agkistrodon halys venom as a substitute for the standard Agkistrodon halys venom in measurements of the antihemorrhagic activity, since A. halys venom is not easily available. Slope function ratio (S.R.) was 0.957 for Agkistrodon halys venom either with Agkistrodon halys antivenom or with Jiangzhe Agkistrodon halys antivenom (p>0.05). Similarly, S.R. was 0.348 for Jiangzhe Agkistrodon halys venom either with Agkistrodon halys antivenom or with Jiangzhe Agkistrodon halys antivenom (p>0.05). Thus, in this study we established antihemorrhagic potency test methods for both Agkistrodon halys and Jiangzhe Agkistrodon halys antivenoms and we could also show it is possible to use Jiangzhe Agkistrodon halys venom as a standard.
Key words: antihemorrhagic potency, minimum hemorrhagic dose, Jiangzhe Agkistrodon halys, parallel line assay.
INTRODUCTION
Hemorrhagic proteinases, or hemorrhagic factors [HRs] (7), are largely present in the venoms of snakes from the genus Agkistrodon (Crotalidae family) and those from the Viperidae family. They have been purified from several snake venoms and some of their properties have been investigated (4). It has been reported that HRs are metalloproteinases but their enzymatic properties are apparently different from one another.
Hemorrhagic activities seem to play a leading role in the lethality of Agkistrodon halys venom (8). Some pharmacists insist that purified hemorrhagic components of A. halys venom are immunologically related (2) but their mechanism of hemorrhagic activity is still unclear.
The antihemorrhagic potency of A. halys antivenom can be determined by multiple level beta-procedure in which the amount of antivenom varies with a fixed dose of venom (6). The hemorrhagic fractions of A. halys venom should be used as test toxins. The effective dose (ED) of both the test and the standard antivenoms should be determined with no less than three doses of the test toxin according to the method described by Kondo et al. (5). Parallelism and linearity of the neutralization curves should be ascertained by statistical analyses. Antihemorrhagic potency of the test antivenom should be determined by measuring the relationship between its potency and the standard antivenom potency according to the parallel line assay (3) using neutralization curves.
In our laboratory, we established an antihemorrhagic potency test using A. halys antivenoms against A. halys venom. We confirmed that the antivenoms are effective in neutralizing the most relevant hemorrhagic effects of A. halys venom.
In this study, we first tested and compared A. halys and Jiangzhe A. halys antivenoms against A. halys venoms. We also attempted to determine whether cross-neutralization of hemorrhage occurs experimentally and whether antivenoms produced from Jiangzhe A. halys and A. halys venoms are capable of neutralizing not only their own specific venom but also venoms from other species by using rabbit intracutaneous injection. This way we tried to check the possibility of using Jiangzhe A. halys venom as a substitute for the standard A. halys venom in antihemorrhagic activities, since A. halys venom is not readily available in Korea.
MATERIALS AND METHODS
Antivenom
Test antivenoms were: national standard equine Mamushi antivenom (Lot C, antihemorrhagic titer: 23,430 U/ampoule), produced by the National Institute of Infectious Diseases (NIID) in Japan and equine Jiangzhe Agkistrodon halys antivenom, produced by the Shanghai Institute of Biological Products (SIBP) in China and commercially available in Korea.
Snake hemorrhagic venom
The venoms used were Mamushi venom (Hemorrhagic hemorrhagic titer: 1,100 test doses/ampoules, Lot 3-2, 50 mg protein/ampoule) supplied by the National Institute of Infectious Diseases (NIID) in Japan and Jiangzhe A. halys venom by the Shanghai Institute of Biological Products (SIBP), in China.
Immediately before use, venoms were dissolved in 17 mM phosphate buffered sodium chloride solution (PBS, pH 7.0) containing 0.2 w/v % gelatin to a desired concentration.
Experimental animals
Male and female adult white rabbits of approximately 2.5 kg were utilized to determine the hemorrhagic activity of venoms and the antihemorrhagic activity of antivenoms.
Hemorrhagic activity
The hemorrhagic activity of venoms was assayed by the method developed by Kondo et al. (5). From four to five different doses of each venom diluted in 0.1 ml PBS at increasing concentrations as needed were intracutaneously injected into the depilated skin on the back of 3 rabbits. Rabbits were sacrificed by chloroform inhalation 24 h after injection and the tested skin areas were immediately removed and placed on a glass plate so as not to distort their original shape. The cross-diameter of each hemorrhagic spot was measured from the visceral side of the skin through the glass plate, mean value was calculated and the results were statistically analyzed. The Minimum Hemorrhagic Dose (MHD) of each venom was defined as the lowest dose of venom causing hemorrhagic reaction over an area of approximately 9 mm in diameter 24 h after the intracutaneous injection. The MHD of a test sample is determined by interpolation of its dose-response curve using the Statistical Analysis Program STA77.
Antivenom effective dose by the skin test
An effective dose (ED) is defined as the quantity of antivenom that, when mixed with a given dose of a test toxin, causes a hemorrhagic spot of approximately 9 mm in diameter. We prepared several dilutions of a test toxin containing 47 MHD of A. halys venom in a 0.1-ml volume. To determine dose-response curves and measure cross-reactivity, venoms were mixed with equal volumes of antivenom diluted in solutions of 1.25-fold increments in concentration. All mixtures were incubated for 1 h at room temperature. Then, 0.2 ml of each mixture was injected into the depilated skin on the back of three rabbits and hemorrhagic response after 24 h was observed from the visceral side of the removed skin using the method developed by Kondo et al. (5).
Unit of antihemorrhagic activity
One unit of antihemorrhagic activity was defined as the amount of specific antibody that neutralized 47 MHD of A. halys venom and 43 MHD of Jiangzhe A. halys venom. The antihemorrhagic potency is expressed as units per ml of antivenom. The effective dose (ED10mm) of an antivenom with a test toxin at 47 MHD of A. halys venom resulting in a 10mm-diameter hemorrhagic spot corresponds to one unity of antihemorrhagic activity.
RESULTS
Hemorrhagic activities of venoms
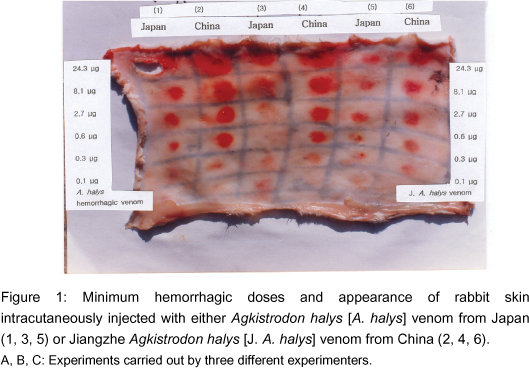
Each venom (4-5 different doses) was intracutaneously injected into the depilated skin on the back of 3 rabbits. Agkistrodon halys venom was diluted to six concentrations: 0.1 µg, 0.3 µg, 0.9 µg, 2.7 µg, 8.1 µg, and 24.3 µg in 0.1-ml solutions; Jiangzhe A. halys venom was also diluted to: 0.1 µg, 0.3 µg, 0.9 µg, 2.7 µg, 8.1 µg, and 24.3 µg in 0.1-ml solutions (Table 1).
Expression of the hemorrhagic activities of A. halys toxins usually does not directly correlate with their weight in µg. It is necessary to express their weight and biological activities (MHD, etc) to represent their characteristics because the purities of toxins from A. halys and Jiangzhe A. halys are different.
The MHD of each venom was determined by the emergence of a hemorrhagic spot of approximately 9 mm in diameter on the visceral side of the removed skin of rabbits (Figure 1). The MHD of A. halys venom was 1.78 µg / 0.1 ml and that of Jiangzhe A. halys venom was 0.42 µg / 0.1 ml (Table 2).
When the hemorrhagic activity of A. halys venom was considered as 1 unit of virulence, the relative virulence of Jiangzhe A. halys venom was approximately 4.2 (Table 2).
Response to the MHD showed a linear correlation between A. halys venom and Jiangzhe A. halys venom (data not presented). The MHDs of A. halys and Jiangzhe A. halys venoms were estimated by the Reed and Muench method (9). When the mean diameter of the red spot exceeded 9 mm, it was considered as a positive reaction.
Determination of test doses of venoms
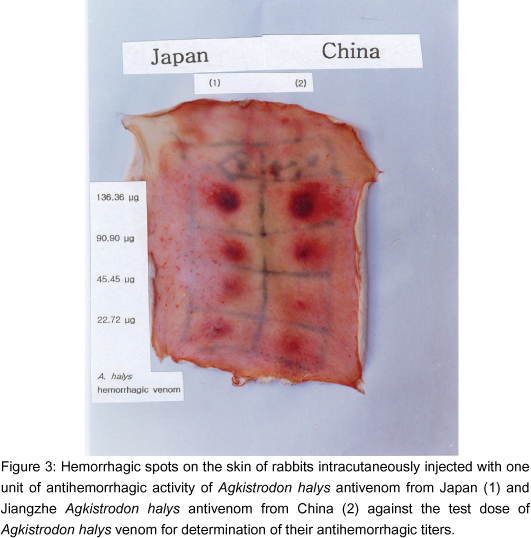
The hemorrhagic activities of A. halys and Jiangzhe A. halys venoms were determined using fixed amounts of A. halys and Jiangzhe A. halys antivenoms. Results of the tests carried out to determine one test dose of A. halys venom against 1 unit of antihemorrhagic activity of A. halys and Jiangzhe A. halys antivenoms are shown in Table 3 and Figures 2 and 3.
The weight range of A. halys venom used was between 22.72 µg and 136.36 µg.
When the antihemorrhagic potency of A. halys antivenom was considered as 1 unit/ml that of Jiangzhe A. halys antivenom was 0.837 unit/ml. Agkistrodon halys antivenom appears to be slightly more effective than Jiangzhe A. halys antivenom when their weights are compared.
We also determined one test dose of Jiangzhe A. halys venom against 1 unit of antihemorrhagic activity of either A. halys or Jiangzhe A. halys antivenom (Table 4 and Figure 4). The weight range of Jiangzhe A. halys venom used was between 6.0 µg and 486 µg.
In this case, when the antihemorrhagic potency of A. halys antivenom was considered as 1 unit/ml that of Jiangzhe A. halys antivenom was 1.287 units/ml.
Determination of hemorrhagic test doses of the toxins
The hemorrhagic test doses of A. halys and Jiangzhe A. halys venoms were calculated and are shown in Table 5.
Neutralization reactions of A. halys venom correlated well with the antivenoms used and demonstrated a first-order relationship (Figure 4). The test dose of A. halys venom against 1 unit of A. halys antivenom was 60.78 µg, and for 1 unit of Jiangzhe A. halys antivenom it was about 76.43 µg (Table 5). The test dose of Jiangzhe A. halys venom against either 1 unit of A. halys or Jiangzhe A. halys antivenom was approximately 10.4 µg.
Antihemorrhagic Potency of the antivenoms
Three different amounts of A. halys antivenom and Jiangzhe A. halys antivenom against A. halys venom were used to determine antihemorrhagic potency test doses (Table 6). The test dose of A. halys venom used was 83.0 µg (47 MHD). Antihemorrhagic potency of an antivenom was estimated by analyzing the relationship between its potency and the standard antivenom potency in the parallel line assay. Relative potency of the two antivenom preparations against A. halys venom was estimated by the ratio between their regression coefficients. Slope ratios of both A. halys and Jiangzhe A. halys antivenoms against A. halys venom were 0.957 (Figures 5 and 6). Results of the log-dosage response curves were linear and parallel (p>0.05). When the antihemorrhagic potency of A. halys antivenom was considered as 1 unit/ml that of Jiangzhe A. halys antivenom was 0.991 unit/ml (Table 6).
Five different amounts of A. halys antivenom and Jiangzhe A. halys antivenom against Jiangzhe A. halys venom were used to determine antihemorrhagic potency test doses (Table 7). The test dose of Jiangzhe A. halys venom was 18 µg (43 MHD). Relative potency of the two antivenom preparations against Jiangzhe A. halys venom was estimated by the ratio between their regression coefficients. Slope ratios of both A. halys and Jiangzhe A. halys antivenoms against Jiangzhe A. halys venom were 0.348 (Figure 7). Results of the log-dosage response curves were linear and parallel (p>0.05). When the antihemorrhagic potency of A. halys antivenom was considered as 1 unit/ml that of Jiangzhe A. halys antivenom was 1.156 units/ml (Table 7).
Antihemorrhagic potency of Jiangzhe A. halys antivenoms
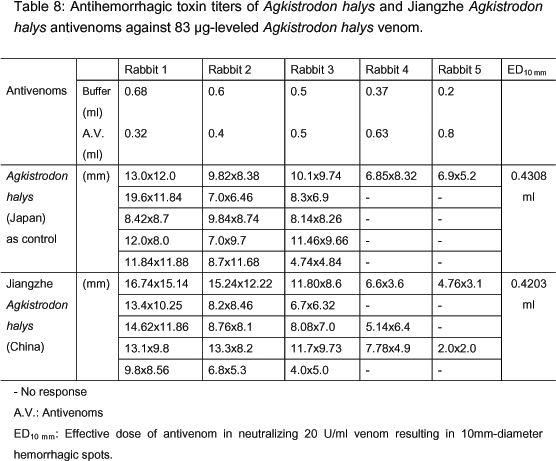
The test method established in this study was utilized in order to check the possibility of adopting it in the determination of antihemorrhagic potency of an unknown antivenom sample in the lot release test of Jiangzhe A. halys antivenom. The current lot release test in Korea requires potency higher than 300 units/ml. The antihemorrhagic activity of the unknown sample applied for lot release was tested using 20 units of A. halys antivenom (330 U/ml) as control. Detailed experiments are shown in Table 8. We used 5 different rabbits for testing 5 different dilutions of antivenoms. For each dilution, five spots were injected. ED10 mm for the control and the unknown sample was 0.4308 ml and 0.4204 ml, respectively. Antivenom potency of the unknown sample was found to be 300 units/ml not only by the current lot release test method in Korea (data not shown) but also by the newly established method in this study.
DISCUSSION
It has been demonstrated that the hemorrhagic factors present in A. halys and Jiangzhe A. halys venoms have distinct immunological specificities. Furthermore the antihemorrhagic activities of their antivenoms can be determined only when individual factors are used separately as test toxins instead of the crude venom.
The most widely used type of simultaneous trial is that in which a simple response metameter has a linear regression on its log dose (3).
For such an assay, it is required that the lines for the standard (A. halys antivenom) and the test preparation (Jiangzhe A. halys antivenom) be parallel. In view of the important role played by the hemorrhagic as well as the lethal activity of A. halys venom in snake envenoming, the potency of A. halys antivenom should be determined according to its antihemorrhagic and anti-lethal potencies. Hemorrhagic activity of venoms (7) was only suppressed by treatment with chelating agents, such as EDTA-2Na (Disodium Ethylene Diamine Tetraacetate Dihydrate), EGTA (ethyleneglycol-bis [beta-aminoethylether] N,N'-tetraacetic acid), and o-phenanthroline, suggesting that they are metalloproteinases.
During the course of our study, we found out that two hemorrhagic principles (A. halys HR and Jiangzhe A. halys HR) showed indistinguishable hemorrhagic action on rabbit skin. We also noticed that there is an extensive cross-reactivity between the hemorrhagic principles from the two venoms studied, evidenced by the capability of their antivenoms to neutralize the hemorrhagic activity of heterologous venoms. Thus, hemorrhagic metalloproteinases from the two Agkistrodon halys venoms tested may share relevant neutralizing epitopes (1).
Both antivenoms tested were effective in neutralizing the hemorrhagic activities of the two snake venoms used. So, in emergencies, Jiangzhe A. halys antivenom may be useful as an antitoxin for Japanese snakebite cases.
The MHD of A. halys venom for hemorrhagic response was 1.78 µg / 0.1 ml and that of Jiangzhe A. halys venom was 0.42 µg / 0.1 ml. We found out that 1 unit/ml of A. halys antivenom neutralized 50% of the hemorrhagic activity induced by 83.0 µg (47 MHD) of A. halys venom (as a challenge dose). When the test dose of A. halys venom was 83 µg (47 MHD), the antihemorrhagic potency of A. halys antivenom was 1 unit/ml in the rabbit samples used, whereas that of Jiangzhe A. halys was 0.991 unit/ml in the same samples. Although A. halys antivenom appears to be slightly more effective than that of Jiangzhe A. halys, they may be considered comparable. So, we can use these two antivenoms in the quality control of A. halys venoms. Agkistrodon halys and Jiangzhe A. halys antivenoms showed almost the same efficacy against Jiangzhe A. halys venom: 1 and 1.156 U/ml, respectively (p>0.05). So, Jiangzhe A. halys venom may be used as a substitute for A. halys venom in tests for antihemorrhagic potency of Jiangzhe A. halys antivenom.
In the slope-ratio assay, both the standard and the test venom showed straight-line and parallel dose-response curves. Slopes measure the change rate of the dependent variable as the independent variable changes, and the greater the slope the steeper the line. Jiangzhe A. halys and A. halys antivenoms showed equal potency against A. halys venom. Their slope ratios were 0.957, which indicates positive relationship. Their log-dosage response curves were linear and parallel (p>0.05) and had common slopes. The efficacy of A. halys and J. A. halys antivenoms against Jiangzhe A. halys venom was almost equal and their slope ratios were 0.348, indicating a positive relationship (p>0.05). Their log-dosage response curves were linear and parallel (p>0.05). We concluded it is possible and useful to utilize Jiangzhe A. halys venom as a standard for testing antihemorrhagic activity since the availability of A. halys venom is limited in Korea.
In addition, antivenom potency of the unknown sample was found to be 300 units/ml by both the current lot release test method in Korea (data not shown) and the newly established method in this study. Thus, we may consider the unknown antivenom tested passes the current specification for antivenom lot release test in Korea using the test methods established in this study, which suggests the possibility of adopting it in the antivenom lot release system in Korea.
ACKNOWLEDGEMENTS
We thank Dr. Motohide Takahashi (National Institute of Infectious Diseases, Japan), Dr. Setsuji Ishida (Tokyo Women's Medical College, Japan), and Dr. Wei Zhu (Shanghai Institute of Biological Products, China) for their valuable collaboration.
Received: August 8, 2005
Accepted: February 10, 2006
Abstract published online: May 5, 2006
Full paper published online: August 31, 2006
Supported by grants from the Korea Food and Drug Administration.
- 1 ARCE V., ROJAS E., OWNBY CL. Preclinical assessment of the ability of polyvalent (Crotalinae) and anticoral (Elapidae) antivenoms produced in Costa Rica to neutralize the venoms of North American snakes. Toxicon, 2003, 41, 851-860.
- 2 DONG-SHIN PHARMACOLOGICAL COMPANY. Dong Shin Salmusa Snake Antivenom: production and quality control. In: COMMERCIAL INTERNET CATALOGUE [serial on-line], 2004, 1158, p.31. [
- 3 FINNY DJ. Statistical method in biological assay. Charles Griffin & Company Ltd, London, 3.ed., 1978, 39-68p, 69-104p.
- 4 KINI R., EVANS HJ. Structural domains in venom proteins: evidence that metalloproteinases and nonenzymatic platelet aggregation inhibitors (disintegrins) from snake venoms are derived by proteolysis from a common precursor, Toxicon, 1992, 30, 265-293.
- 5 KONDO H., KONDO S., IKEZAWA H., MURATAata R. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn. J. Med. Sci. Biol., 1960, 13, 43-51.
- 6 KONDO H., KONDO S., SADAHIRO S., YAMAUCH K., OHSAKA A.,MURATA R. Standardization of antivenine I. A method for determination of antilethal potency of Habu antivenine. Jpn. J. Med. Sci. Biol., 1965a, 18, 101-10.
- 7 MIYAGI H., KATO KI., TAKAHASHI H. Purification of haemorrhagic proteinase from the venom of Agkistrodon caliginosus (Kankoku-Mamushi). Toxicon, 1998, 36, 781-790.
- 8 OMORI T., IWANAGA S., SUZUKI T. The relationship between the hemorrhagic and lethal activities of Japanese Mamushi (Agkistrodon halys blomhoffi) venom. Toxicon, 1964, 2, 1-4.
- 9 REED, LJ., MUENCH H. A simple method of estimating 50% end points. Am. J. Hyg. 1938, 27, 493-497.
Correspondence to:
Publication Dates
-
Publication in this collection
19 Sept 2006 -
Date of issue
2006
History
-
Accepted
10 Feb 2006 -
Received
08 Aug 2005