Abstract
Sol i 2 is a potent allergen in Solenopsis invicta venom, and most humans exhibit reactivity to it. The Sol gem 2 allergen found in the venom of the Thai tropical fire ant Solenopsis geminata was analysed in the present study. The protein was present in higher amounts than other proteins, as determined by SDS-PAGE, and presumably has allergenic properties similar to those of Sol i 2. Sol gem 2 molecular weight is 28 and 15 kDa, respectively, under non-reducing and reducing conditions, indicating that its native form is a dimer. LC-MS/MS analysis confirmed its similarity to Sol i 2. The mono/dimeric form of Sol gem 2 was determined to be relevant by proteomic approach and immunoblotting. An anti-Sol gem 2 antibody was produced in mice, with a titer greater than 1:800 according to the Western blotting analysis. The Sol gem 2-neutralising activity of this antibody was determined in crickets. The paralytic dose 50 (PD50) of crude S. geminata venom was elevated from 0.18 mg/g of body weight to more than 0.90 mg/g of body weight after preincubation with antibody at a ratio of 1:1. These results suggest that Sol gem 2 plays an important role in mediating the effects of the piperidine derivatives in the venom.
allergy; ant venom; hymenoptera; immunoreactivity
ORIGINAL PAPER
Characterization of the allergen Sol gem 2 from the fire ant venom, Solenopsis geminata
Sukprasert SI; Uawonggul NII; Jamjanya TIII; Thammasirirak SI; Daduang JIV; Daduang SI
IProtein and Proteomics Research Group, Department of Biochemistry, Faculty of Science, Khon Kaen University, Khon Kaen, Thailand
IIFaculty of Liberal Arts and Science, Nakhon Phanom University, Nakhon Phanom, Thailand
IIIDepartment of Entomology, Faculty of Agriculture, Khon Kaen University, Khon Kaen, Thailand
IVDepartment of Clinical Chemistry, Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, Thailand
Correspondence toCorrespondence to:Sakda Daduang Protein and Proteomics Research Group Department of Biochemistry, Faculty of Science Khon Kaen University Khon Kaen, 40002, Thailand Phone/fax: +66 43 342 911 E-mail: sakdad@kku.ac.th
ABSTRACT
Sol i 2 is a potent allergen in Solenopsis invicta venom, and most humans exhibit reactivity to it. The Sol gem 2 allergen found in the venom of the Thai tropical fire ant Solenopsis geminata was analysed in the present study. The protein was present in higher amounts than other proteins, as determined by SDS-PAGE, and presumably has allergenic properties similar to those of Sol i 2. Sol gem 2 molecular weight is 28 and 15 kDa, respectively, under non-reducing and reducing conditions, indicating that its native form is a dimer. LC-MS/MS analysis confirmed its similarity to Sol i 2. The mono/dimeric form of Sol gem 2 was determined to be relevant by proteomic approach and immunoblotting. An anti-Sol gem 2 antibody was produced in mice, with a titer greater than 1:800 according to the Western blotting analysis. The Sol gem 2-neutralising activity of this antibody was determined in crickets. The paralytic dose 50 (PD50) of crude S. geminata venom was elevated from 0.18 mg/g of body weight to more than 0.90 mg/g of body weight after preincubation with antibody at a ratio of 1:1. These results suggest that Sol gem 2 plays an important role in mediating the effects of the piperidine derivatives in the venom.
Key words: allergy, ant venom, hymenoptera, immunoreactivity.
INTRODUCTION
Venomous insects such as bees, wasps, hornets, yellow jackets and fire ants belong to the order Hymenoptera. These insects have a stinging apparatus at the tail end of their abdominal segment and still comprise a serious problem in tropical climates. Every year, a large number of people are stung by these insects (1). The stings are capable of delivering between 100 ng (fire ants) and 50 µg (bees) of venom (2, 3). The venom is injected from a gland through the stinger and is used to catch prey and to defend against predators. The venom of these insects is composed of various biologically active peptide and protein components, some of which can cause toxicity or anaphylaxis in humans (4).
Fire ants (Solenopsis sp.) are members of the Formicidae family. The medically important aggressive species are S. invicta, S. richteri, S. geminata and S. saevissima (5, 6). The venom components of these ants and their immunoreactivity have been extensively studied. Approximately 95% of fire ant venom consists of water-insoluble piperidine alkaloids, and approximately 0.1% of its weight (10 to 100 ng of protein in 0.04 to 0.11 µL per sting) is protein (7). The toxicity of the venom is thought to be due to solenopsins and methyl-, alkyl- or alkenyl-substituted piperidines (7-9). The venom has cytotoxic, insecticidal, antibiotic and antimicrobial properties as well (7, 9-19). The protein components of venom, which are well-known allergens, are believed to play important roles in accelerating the spread of solenopsins to nearby cells and tissues. Solenopsins are believed to be responsible for reactions to S. invicta stings in humans. Allergic symptoms appear in the form of sterile pustules, urticaria, edema and dermal necrosis in the absence of IgE responses; anaphylactic shock and death may occur in rare cases (20, 21).
The protein components in S. invicta venom contain four major allergic proteins, Sol i 1 to 4, with molecular weights of approximately 37, 28, 26, and 20 kDa, respectively (3). These proteins induce specific IgE responses in allergic victims. Sol i 1 has phospholipase A1B activity and shares some immunologic cross-reactivity with phospholipases in vespid venoms (22). Sol i 2 comprises approximately half to two-thirds of the venom protein content and is not immunologically cross-reactive with other Hymenoptera venoms (23). Sol i 3 is a member of the antigen 5 family but does not exhibit consistent immunologic cross-reactivity with Ves v 5, which shares 44% sequence identity (23, 24). Sol i 4 comprises 8% to 10% of the most basic protein components (isoelectric point, 10.08). It has 35% identity to Sol i 2 but does not cross-react immunologically (25).
Anaphylaxis due to S. invicta venom has been reported in the USA and Spain, some cases of which have been fatal (26, 27). In vitro studies demonstrated that the patient was sensitized to the Sol i 2 allergen (27). However, there is no specific and effective drug for the treatment the allergic reactions in these patients. Epinephrine is the drug of choice in the treatment of anaphylaxis. Allergen immunotherapy has proven to be an extremely effective form of treatment for individuals at risk of anaphylaxis. Immunotherapy with whole-body extracts of S. invicta has been previously used for the diagnosis and treatment of IgE-mediated allergic reactions and is widely used by allergists in the USA (28). However, whole-body extracts have been reported to cause nonspecific reactions and large local reactions at high doses (29, 30). In addition, the specificity of venom immunotherapy depends on the species. However, monoclonal antibodies have been produced in mice that recognize the species-specific determinants on both Sol i 2 and Sol i 3 (31, 32).
The Thai tropical fire ant S. geminata is widely spread throughout all areas in Thailand, and these ants are commonly found in houses and fields. They are aggressive and will attack children and farmers, sometimes causing anaphylactic shock. In this study, the Sol gem 2 allergen in S. geminata venom was identified and its allergenic properties were characterized to better understand the allergic reaction with the goal of developing a vaccine or immunotherapies.
MATERIALS AND METHODS
Fire ants and Venom Collection
Adult S. geminata workers were collected from suburban areas in Khon Kaen City, Khon Kaen Province, Thailand. The species was identified by Assoc. Prof. Dr. Decha Wiwatwitaya, Department of Forest Biology, Faculty of Forestry, Kasetsart University. Venom from the tips of the stingers was collected with capillary tubes under a stereomicroscope and stored at 20ºC in PBS buffer until use.
Protein Quantification
The amount of protein was determined by the method of Bradford (33) using bovine serum albumin as standard.
Polyacrylamide Gel Electrophoresis (PAGE) of Crude Venom
One-dimensional SDS-PAGE was performed following standard method using a 13% (w/v) separating gel and a 4% (w/v) stacking gel. Phosphorylase B (97 kDa), bovine serum albumin (66 kDa), chicken ovalbumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20 kDa) and α-lactalbumin (14.4 kDa) were used as standards. After samples were applied to the gel, the proteins were resolved at 150 V for one hour. The gels were stained with Coomassie brilliant blue R-250 (CBB).
For two-dimensional polyacrylamide gel electrophoresis (2D-PAGE), the crude venom protein (80 µg) was mixed with a rehydration solution containing 7 M urea, 2 M thiourea, 2% CHAPS, DTT 7 mg/2.5 mL, 2% IPG buffer and 1% bromophenol blue to give a total volume of 125 µL, and this solutions was applied to IPG dry strips (pH 3-11 NL, GE Healthcare).
After rehydration for 12 hours, isoelectric focusing (IEF) was carried out by applying 500 V for 30 minutes, 1000 V for 30 minutes and 5000 V for 1.4 hours at 50 mA/strip. After focusing, the strip was soaked for 15 minutes in reduction solution, followed by 15 minutes in alkylation solution. The SDS-PAGE step was performed using a 13% (w/v) polyacrylamide gel at a constant current of 20 mA. The gel was stained with 0.1% colloidal Coomassie brilliant blue G-250 or silver nitrate. After staining, the gel was scanned using a flatbed scanner, and data were analyzed using ImageMaster 2D Platinum version 5.0 (GE Healthcare, Sweden). The identities of the proteins were determined by mass spectrometry.
Protein Enzymatic Digestion
The excised spots were washed twice with MilliQ water and destained with 50 mM ammonium bicarbonate and 50% methanol and dehydrated with 100 % ACN. Samples were digested with trypsin solution (20 ng trypsin in 50% ACN/10 mM ammonium bicarbonate) overnight at 37ºC. The resulting peptides were extracted by washing the gel pieces three times with 30 µL of 50% ACN/0.1% formic acid. The supernatants containing the peptides were then vacuum-dried in an incubator at 40ºC for 3 to 4 hours and stored at 20ºC until mass spectrometry analysis.
Protein Identification by Liquid Chromatography Coupled with Mass Spectrometry (LC-MS/MS)
The digested peptides were separated by nanoscale LC using a NanoAcquity system (Waters Corp., USA) equipped with a Symmetry C18 (5 µm, 180-µm x 200-µm) Trap column and a BEH130 C18 (1.7 µm, 100-µm x 100-µm) analytical reversed phase column (Waters Corp., USA) at a flow rate of 350 mL/minute. Water and acetonitrile, both containing 0.1% formic acid, were used as solvents A and B, respectively. All samples were analyzed in triplicate. The analysis of tryptic peptides was performed using a SYNAPTTM HDMS mass spectrometer (Waters Corp., UK).
All analyses were performed using positive nanoelectrospray ion mode. The time-of-flight analyzer of the mass spectrometer was externally calibrated with [Glu1] fibrinopeptide B from m/z 50 to 1600 with the acquisition lock mass corrected using the monoisotopic mass of the doubly charged precursor of [Glu1] fibrinopeptide B. The quadrupole mass analyzer was adjusted such that ions from m/z 200 to 1990 were efficiently transmitted. Bioworks 3.2 software (Thermo Electron, USA) was used to process the data and convert it into a Mascot Generic File.
Database Searching
Peptides were identified using a local Mascot server (Matrix Science, USA) and the following search parameters: a specified trypsin enzymatic cleavage with one possible missed cleavage; +/ 0.6 Da mass tolerances for MS/MS; a peptide tolerance of 1.2 Da; 1+, 2+, 3+ ions; methionine oxidation variable modification; carbamidomethyl (C) fixed modification; monoisotopic mass; and 20 responses.
Molecular Mass Determination
The matrix-assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-TOF MS) technique was used to obtain the molecular mass of the crude venom protein. The MALDI mass spectra were recorded on an Ultraflex III TOF/TOF mass spectrometer (Bruker Daltonik, GmbH, Germany) equipped with a 2 GHz LeCroy digitiser and a 337 nm N2 laser. Fifty shots were used per position, and overall 3000 shots were summed up. A 1 µL aliquot of the sample solution was spotted with 1 µL of a sinapinic acid matrix solution (10 mg sinapinic acid in 1 mL of 50% acetonitrile containing 0.1% trifluoroacetic acid). The ProteoMassTM Peptide & Protein MALDI-MS Calibration Kit (Sigma, USA) was used as a control standard. The instrumental parameters were as follows: positive polarity; acceleration voltage, 25.13 kV; IS/2, 23.58; focusing lens voltage, 6.03 kV; extraction delay, 100 ns; and detection range, 5-50 kDa. The program used to generate mass lists was Compass 1.2 for flex-series, ServicePack 1, flexAnalysis Version 3.0 (Build 92).
Amino Acid Sequence Determination
Crude venom proteins were separated on a non-reducing Tris-glycine SDS-PAGE gel. The protein bands were electrotransferred onto a polyvinylidene difluoride (PVDF) membrane (Problot, Applied Biosystems, USA). The Sol gem 2 band was cut out and sent for automated Edman degradation analysis at the Scientific Equipment Center, Prince of Songkla University, as described previously by Thammasirirak et al. (34).
Polyclonal Antibody Production
Crude venom proteins were separated by SDS-PAGE, and the band of protein proposed to be Sol gem 2 was excised from the gels and frozen at 70ºC. The gel was dried by lyophilization and then ground into a fine powder. The powder was rehydrated in 1-2 mL of PBS buffer (135 mM NaCl, 1.5 mM KH2PO4, 2.5 mM KCl and 8.0 mM Na2HPO4, pH 7.4). This protein suspension was emulsified with an equal volume of Freund's complete adjuvant (Sigma, USA). Anesthetized mice were subcutaneously immunized with approximately 100 µL of the emulsion and were boosted at 2-week intervals with the emulsion prepared from the gel suspension and Freund's incomplete adjuvant. After the fourth booster injection, blood was collected by retro-orbital plexus bleeding using a 100 µL micropipette coated with 1 U/mL heparin (35). The blood was maintained at 4ºC after collection. The serum was collected by centrifugation at 10,000 x g for ten minutes, and then the supernatant containing the antiserum was pooled and used for enzyme-linked immunosorbent assays and Western blotting.
Enzyme-Linked Immunosorbent Assay (ELISA)
Fire ant venom was diluted to 20 µg/mL in carbonate buffer, pH 9.5. The ELISA was carried out in a 96-well microtiter plate (Nalge Nunc International, Denmark). Each well of a 96-well polystyrene plate was coated with 50 µL of 20 µg/mL diluted venom (as antigen) and incubated at 4ºC overnight. The unbound antigen was washed with TBST [TBS: 10 mM Tris-HCl, pH 8.0, and 150 mM NaCl containing 0.05% (v/v) Tween-20]. The wells were blocked with 100 µL blocking solution [5% (w/v) skimmed milk in TBST] for 1 hour at 37ºC.
After three rinses with TBST, 50 µL of the serum was diluted serially (1:100, 1:500, 1: 2500, 1: 12500, 1:62500, v/v) in blocking solution and then incubated in each well for one hour at 37ºC. After subsequently washing with TBST, the plate was incubated with 50 µL of conjugated anti-mouse IgG linked with alkaline phosphatase at 37ºC for one hour. After washing with TBST again, the plate was further washed with TBS three times. Bound antiserum was detected using freshly prepared chromogenic substrate (1 mg/mL of r-nitrophenyl phosphate, 100 mM Tris-HCl, pH 9.5, 100 mM NaCl and 50 mM MgCl2). The absorbance was measured at 405 nm using an ELISA plate reader.
Western Blotting
After electrophoresis, the gel was placed into a blotting apparatus, and the proteins were electrotransferred to a nitrocellulose membrane for one hour. The membrane was incubated with blocking solution (5% nonfat dry milk in TBST buffer). It was also incubated with an anti-Sol gem 2 antibody diluted in blocking solution and with goat anti-mouse IgG linked with alkaline phosphatase. The blotted protein bands or spots were detected using an alkaline phosphatase substrate kit (GE Healthcare, Sweden).
Paralytic Dose 50 (PD50) Assay
To determine the PD50, crickets (Gryllus sp.) were used. The venom activity was quantified by injecting crickets in the abdomen with PBS buffer (pH 7.4), crude venom or crude venom that had been preincubated with antibody for 30 minutes. Every test used six crickets and was performed in triplicate. After 30 minutes, non-paralyzed crickets were counted, and the PD50 value was estimated. The PD50 was defined as the amount of venom that could paralyze 50% of the venom-injected crickets; crickets that could not turnover from the overturned position were considered paralyzed (36).
RESULTS AND DISCUSSION
PAGE Analysis and Protein Identification of Crude Venom
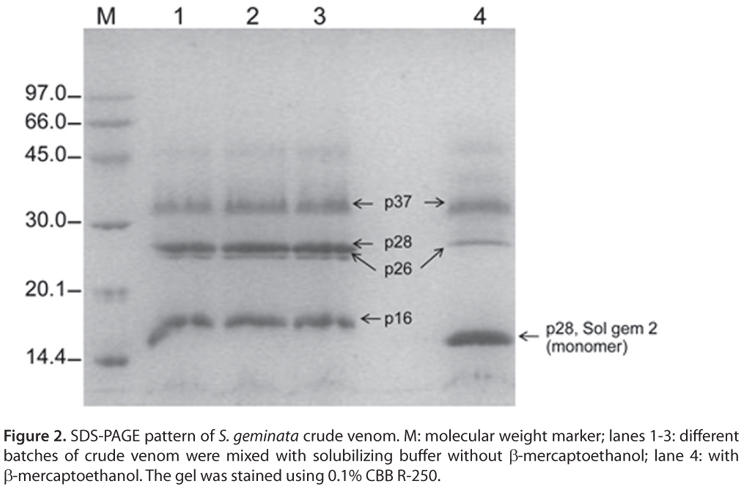
Venom (approximately 0.01 µL) was collected from each stinger at the end of the metasoma (Figure 1). The total protein concentration in the venom varied from 3 to 12 µg/µL. The crude venom proteins were separated by SDS-PAGE with or without β-mercaptoethanol (Figure 2). Under non-reducing conditions, there were four major bands of approximately 37.0 (p37), 28.0 (p28), 26.0 (p26) and 16.0 (p16) kDa, and there were three major bands of approximately 37.0, 28.0 and 15 kDa under reducing conditions.
The four native masses of S. geminata venom proteins (p37, p28, p26, p16) parallel those of the allergens Sol i 1, Sol i 2, Sol i 3 and Sol i 4 in S. invicta venom (3). Thus, the allergens from S. geminata venom were designed Sol gem 1, Sol gem 2, Sol gem 3 and Sol gem 4. Interestingly, no p28 band could be detected under reducing conditions (Figure 2, lane 4). Based on this result, we hypothesized that the 28/15 kDa band in the SDS-PAGE gel, which corresponded to the allergen Sol gem 2, most likely similar to allergen Sol i 2.
According to a previous report, allergen Sol i 2 is a major allergen in S. invicta venom with a native MW of 28 kDa. In addition, Sol i 2 can be easily cleaved in half by proteolysis (3). Sol i 2 is less stable than Sol i 3 and has a natural acid cleavage site, and therefore Sol i 2 can be destroyed in some whole-body extracts (31). The native form of Sol i 2 is a homodimer linked by a single disulfide bridge (24). The x-ray structure of Sol i 2 revealed that each identical monomer of Sol i 2 contains seven cysteines, six of which form three intramolecular disulfide bridges that stabilize its structure. The seventh cysteine (Cys22) links two monomers by a disulfide bridge (37).
As shown in Figure 2, Sol gem 2 was present at higher concentrations than other proteins. Therefore, we further characterized Sol gem 2. Under reducing condition (Figure 2, lane 4), the Sol gem 2 band co-migrated with the Sol gem 4 band, suggesting that these two proteins might have similar structures. Sol i 2 and Sol i 4 are members of a unique protein family (3, 38, 39). Sol i 4 is structurally related to Sol i 2, with 118 amino acids and no carbohydrate modifications (40).
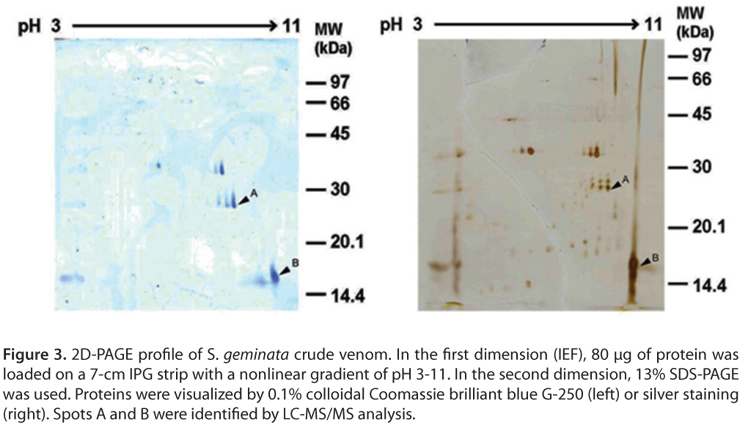
To identify and clarify the mono/dimeric form of Sol gem 2, a proteomic approach was used in this study. About 5 to 6 major protein spots were observed after colloidal Coomassie brilliant blue G-250 staining (Figure 3, left panel), whereas more than 20 spots were found after silver staining (Figure 3, right panel). Based on SDS-PAGE results, the candidate Sol gem 2 spots, spot A and B, with pI/Mw values of approximately 7.98/9.77 and 27/16 kDa, respectively, were trypsinized and subsequently identified using LC-MS/MS (Table 1).
Spot A was similar to allergen Sol i 3, whereas spot B was similar to allergen Sol i 2 from S. invicta. This result obviously indicated that spots A and B are different proteins; therefore, the protein in spot A was Sol gem 3, whereas the protein in spot B was Sol gem 2. These MS results agree with the SDS-PAGE results. Furthermore, the monomeric vs. dimeric form of Sol gem 2 was also clarified by this approach. The protein identification experiments also revealed that Sol gem 2 was similar to three other fire ant allergens, Sol g 4, Sol r 2 and Sol i 4. These results indicate that Sol gem 2 exhibits some homology with related proteins in other Solenopsis venoms.
In addition, Sol gem 2 belongs to the allergenic protein family. Other proteins similar to Sol i 2 have been reported in other Solenopsis species, including S. richreti (Sol r 2), S. geminata (Sol g 2) and S. saevissima (Sol s 2) (41). Sol i 2 showed 78.1% sequence homology with Sol r 2 and 71.4% with Sol s 2 and Sol g 2 (40).
The amino acid residues at the N-terminus of Sol gem 2 were determined to be ANEELKIIRK, a sequence that has 80% identity with that of the N-terminus of Sol i 2 (24, 38). This result indicated that Sol gem 2 most likely shares high homology with allergen Sol i 2.
Finally, the molecular masses of the crude venom proteins were determined by MALDI-TOF mass spectrometry (Figure 4). Five species were detected. They were 10791.911, 13273.435, 14766.537, 24107.693 and 32866.583 Da. No native mass of approximately 28 kDa for Sol gem 2 was detected. We hypothesized that Sol gem 2 was cleaved by proteases in the venom because no protease inhibitor was added when the venom was collected. Likewise, Sol i 2 is less stable than the others (37).
Thus, we propose that the 13273.435 Da species represents the monomeric form of Sol gem 2. In addition, the masses of 14766.537, 24107.693 and 32866.583 Da corresponded to Sol gem 4, Sol gem 3 and Sol gem 1, respectively, in S. geminata venom. The molecular masses in S. geminata venom differ from the masses obtained by PAGE analysis due to the different levels of glycosylation.
Characterization of the Allergenic Properties of Sol gem 2
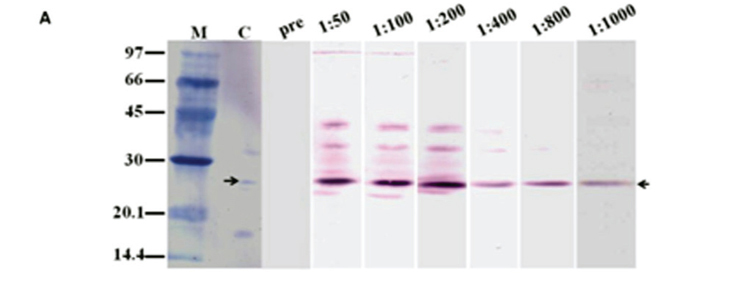
To determine the antigenic properties of Sol gem 2, an anti-Sol gem 2 antibody was produced in mice. The antibody titers were higher than 1:2500 according to the results of the ELISA (data not shown), suggesting that Sol gem 2 is highly allergenic. The Western blotting analysis demonstrated that the antibodies specifically bound to Sol gem 2 in its native form (Figure 5 A) and cleaved form (Figure 5 B and C) that lacks the dimerising cysteine.
These results apparently showed that the produced antibody had the highest specificity for Sol gem 2. However, the antibody cross-reacted with Sol gem 3 when high dilutions of sera were used (1:50, 1:100 and 1:200 dilutions). Interestingly, the anti-Sol gem 2 antibody did not show cross reactivity with Sol gem 4 either its native and denatured form. These results agree with previously reported results for Sol i 2 and Sol i 4, which exhibited 35% sequence homology but no antibody cross-reactivity (25).
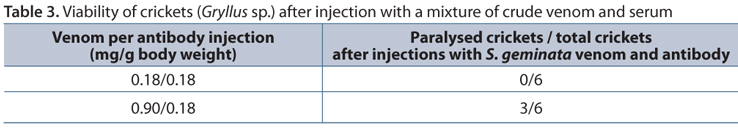
Anti-Sol gem 2 antisera were investigated in a neutralization test in crickets. The PD50 of crude S. geminata venom in crickets was approximately 0.089 mg/g body weight (Table 2). The crickets were injected with PBS as a mock control and incubated with a mixture of crude venom and serum for 30 minutes. The results showed that the PD50 increased to more than 0.90 mg/g body weight (Table 3).
This result suggests that antibody was remarkably able to neutralize Sol gem 2. The anti-Sol gem 2 antibody was able to elevate the PD50 from 0.18 to more than 0.90 mg/g body weight. This finding could eventually lead to the development of immunotherapies for the prevention of allergic reactions after red fire ant stings.
The other issue that was inevitably discussed concerned the actions of piperidine derivatives. As commonly known, piperidine derivatives are the main active components that paralysis prey. In addition, these derivatives can cause severe allergic reactions in humans. This experiment clearly showed that Sol gem 2 has important roles in the action of these alkaloid piperidines.
ACKNOWLEDGMENTS
This work was supported by the "TRF-CHE Research Grant for Mid-Career University Faculty", jointly funded by the Thailand Research Fund (TRF) and the Commission on Higher Education (CHE), Ministry of Education, fiscal years 2007-2009, with additional support from the "CHE grant fund under the program Strategic Scholarships for Frontier Research Network for the Ph.D. Program Thai Doctoral degree (CHE-PhD-THA-SUPV)" to S.S. from the office of the Commission on Higher Education, Thailand, with additional support from the Khon Kaen University Research Fund, fiscal years 2007-2010.
Accepted: July 24, 2012.
Abstract published online: August 7, 2012.
Full paper published online: August 31, 2012.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
FINANCIAL SOURCE: TRF-CHE, CHE-PhD-THA-SUPV from the office of the Higher Education Commission, and Khon Kaen University, Thailand, provided the financial grants.
ETHICS COMMITTEE APPROVAL: The present study was approved by the Animal Ethics Committee of Khon Kaen University based on the Ethics for Animal Experimentation of the National Research Council of Thailand (reference n. 0514.1.12.2/1).
- 1. Fitzgerald KT, Flood AA. Hymenoptera stings. Clin Tech Small Anim Pract. 2006;21(4):194-204.
- 2. Hoffman DR, Jacobson RS. Allergens in hymenoptera venom XII. How much protein in a sting? Ann Allergy. 1984;52(4):276-8.
- 3. Hoffman DR, Dove DE, Jacobson RS. Allergens in Hymenoptera venom. XX. Isolation of four allergens from imported fire ant (Solenopsis invicta) venom. J Allergy Clin Immunol. 1988;82(5 Pt 1):818-27.
- 4. Barnard JH. Studies of 400 Hymenoptera sting deaths in the United States. J Allergy Clin Immunol. 1973;52(5):259-64.
- 5. Hoffman DR. Reactions to less common species of fire ants. J Allegy Clin Immunol. 1997;100(5):679-83.
- 6. Tankersley MS. The stinging impact of the imported fire ant. Curr Opin Allergy Clin Immunol. 2008;8(4):354-9.
- 7. Baer H, Liu TY, Anderson MC, Blum M, Schmid WH, Frank JJ. Protein components of fire ant venom (Solenopsis invicta). Toxicon. 1979;17(4):397-405.
- 8. MacConnell JG, Blum MS, Fales HM. The chemistry of fire ant venom. Tetrahedron. 1971;27(6):1129-39.
- 9. Deslippe RJ, Guo YJ. Venom alkaloids of fire ants in relation to worker size and age. Toxicon. 2000;38(2):223-32.
- 10. Caro MR, Derbes VJ, Jung R. Skin responses to the sting of the imported fire ant (Solenopsis saevissima). AMA Arch Dermatol.1957;75(4):475-88.
- 11. Blum MS, Walker RJ, Callahan PS, Novak AF. Chemical, insecticidal and antibiotic properties of fire ant venom. Science. 1958;128(3319):306-7.
- 12. Adrouny GA, Derbes VJ, Jung RC. Isolation of a hemolytic component of fire ant venom. Science.1959;130(3373):449.
- 13. Blum MS, Callahn PS. Chemical and biological properties of the venom of the imported fire ant (Solenopsis saevissima var. richteri Forel) and the isolation of the insecticidal component. Proc 11th Int Congr Entomol. Vienna.1960;3:290-93.
- 14. Jung RC, Derbes VJ, Burch AD. Skin response to solenamine, a hemolytic component of fire ant venom. Dermatol Trop Ecol Geogr. 1963;15:241-4.
- 15. Jouvenaz DP, Blum MS, MacConnell JG. Antibacterial activity of venom alkaloids from the imported fire ant, Solenopsis invicta Buren. Antimicrob Agents Chemother. 1972;2(4):291-3.
- 16. Read GW, Lind NK, Oda CS. Histamine release by fire ant (Solenopsis) venom. Toxicon.1978;16(4):361-7.
- 17. Lind NK. Mechanisms of action of fire ant (Solenopsis) venoms, I. Lytic release of histamine from mast cells. Toxicon. 1982;20(5):831-40.
- 18. Blum MS. Biocidal and deterrent activities of nitrogen heterocycles produced by venomous myrmicine ants. In: Cutler HG, editor. Biologically Active Natural Products: Potential Use in Agriculture. Washington, DC: American Chemical Society; 1988;438-49.
- 19. Storey GK, Vander Meer RK, Boucias DG, McCoy CW. Effect to fire ant (Solenopsis invicta) venom alkaloids on the in vitro germination and development of selected entomogenous fungi. J Invert Path. 1991;58(1):88-95.
- 20. deShazo RD, Griffing C, Kwan TH, Banks WA, Dvorak HF. Dermal hypersensitivity reactions to imported fire ants. J Allergy Clin Immunol. 1984;74(6):841-7.
- 21. Rhoades RB, Stafford CT, James FK Jr. Survey of fatal anaphylactic reactions to imported fire ant stings. Report of the Fire Ant Subcommittee of the American Academy of Allergy and Immnology. J Allergy Clin Immunol. 1989;84(2):159-62.
- 22. Hoffman DR, Sakell RH, Schmidt M. Sol i 1, the phospholipase allergen of imported fire ant venom. J Allergy Clin Immunol. 2005;115(3):611-6.
- 23. Hoffman DR, Dove DE, Moffitt JE, Stafford C. Allergens in Hymenoptera venom. XXI. Cross-reactivity and multiple reactivity between fire ant venom and bee and wasp venoms. J Allergy Clin Immunol.1988;82(5 Pt 1):828-34.
- 24. Hoffman DR. Allergens in Hymenoptera venom XXIV: the amino acid sequences of imported fire ant venom allergens Sol i II, Sol i III, and Sol i IV. J Allergy Clin Immunol. 1993;91(1 Pt 1):71-8.
- 25. Schmidt M, McConnell TJ, Hoffman DR. Immunologic characterization of the recombinant fire ant venom allergen Sol i 3. Allergy. 2003;58(4):342-9.
- 26. Hoffman DR. Fire ant venom allergy. Allergy. 1995;50:535-44.
- 27. Fernández-Meléndez S, Miranda A, García-González JJ, Barber D, Lombardero M. Anaphylaxis caused by imported red fire ant stings in Málaga, Spain. J Investig Allergol Clin Immunol. 2007;17(1):48-9.
- 28. Stafford CT, Rhoades RB, Bunker-Soler AL, Thompson WO, Impson BS. Survey of whole body extract immunotherapy for imported fire ant and other Hymenoptera sting allergy. J Allergy Clin Immunol. 1989:83(6):1107-11.
- 29. Paull BR, Coghlan TH, Vinson SB. Fire ant venom hypersensitivity. I. Comparison of fire ant venom and whole body extract in the diagnosis of fire ant allergy. J Allergy Clin Immunol. 1983;71(5):448-53.
- 30. Stafford CT, Wise SL, Robinson DA, Crosby BL, Hoffman DR. Safety and efficacy of fire ant venom in the diagnosis of fire ant allergy. J Allergy Clin Immunol. 1992;90(4 Pt 1):653-61.
- 31. Hoffman DR, Jacobson RS, Schmidt M, Smith AM. Allergens in Hymenoptera venom. XXIII. Venom content of imported fire ant whole body extracts. Ann Allergy. 1991;66(1):29-31.
- 32. Smith AM, Hoffman DR. Further characterization of imported fire ant venom allergens. J Allergy Clin Immunol. 1992;89:293.
- 33. Bradford MM. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-54.
- 34. Thammasirirak S, Ponkham P, Preecharram S, Khanchanuan R, Phonyothee P, Daduang S, et al. Purification, characterization and comparison of reptile lysozymes. Comp Biochem Physiol C Toxicol Pharmacol. 2006;143(2):209-17.
- 35. Riley V. Adaptation of orbital bleeding technique to rapid serial blood studies. Proc Soc Exp Med. 1960;104:751-4.
- 36. Uawonggul N, Thammasirirak S, Chaveerach A, Arkaravichien T, Bunyatratchata W, Ruangjirachuporn W, et al. Purification and characterization of Heteroscorpine-1 (HS-1) toxin from Heterometrus laoticus scorpion venom. Toxicon. 2007;49(1):19-29.
- 37. Borer AS, Wassmann P, Schmidt M, Hoffman DR, Zhou JJ, Wright C, et al. Crystal structure of Sol i 2: a major allergen from fire ant venom. J Mol Biol. 2012;415(4):635-48.
- 38. Schmidt M, Walker RB, Hoffman DR, McConnell TJ. Nucleotide sequence of cDNA encoding the fire ant venom protein Sol i II. FEBS Lett. 1993;319(1-2):138-40.
- 39. Schmidt M, McConnell TJ, Hoffman DR. Production of a recombinant imported fire ant venom allergen, Sol i 2, in native and immunoreactive form. J Allergy Clin Immunol. 1996;98(1):82-8.
- 40. Hoffman DR. Hymenoptera venom allergens. Clin Rev Allergy Immunol. 2006;30(2):109-28.
- 41. Hoffman DR. Reactions to less common species of fire ants. J Allegy Clin Immunol. 1997;100(5):679-83.
Publication Dates
-
Publication in this collection
14 Sept 2012 -
Date of issue
2012
History
-
Received
15 May 2012 -
Accepted
24 July 2012