Abstract
Immune response to leptospirosis is mainly humorally mediated, and involves opsonization of leptospires for phagocytosis by macrophages and neutrophils. However, some aspects are still unknown. For a more detailed analysis of the cellular immune response to leptospirosis infection, trials were carried out in order to determine the hydrogen peroxide and nitric oxide (H2O2 and NO) production stimulated or not by Interferon-gamma. The participation of some specific cytokines, such as Tumor Necrosis Factor-alpha (TNF-alpha); Interferon-gamma (IFN-gamma); Interleukin-6 (IL-6); and Interleukin-4 (IL-4), in the immunopathology of this infection was also investigated. For this purpose, we analyzed the supernatant from peritoneal macrophage cell culture and the splenic cells of mice genetically selected as High (H) and Low (L) antibody producers, and inbred Balb/c mice infected with Leptospira interrogans serovar icterohaemorrhagiae. The IL-6 production varied from release peaks to inhibition in H, L, and Balb/c mice. Similar behavior was observed for IL-4, produced only by H and Balb/c mice. The three strains presented constant and elevated production of TNF-alpha until day 14, suggesting its effective participation in the initial phase of the infection. Meanwhile, all the three strains presented a constant and irregular IFN-gamma production, with release peaks between the 7th and 14th days in L mice. The H and Balb/c mice strains presented a higher tendency to Th2 response pattern, whereas L mice tended towards Th1 response.
leptospirosis; cytokines; cellular immunity
ORIGINAL PAPER
Role of cytokines, NO, and H2O2 on the immunopathology of Leptospirosis in genetically selected mice
Marinho M.I; Langoni H.II; Oliveira S. L.III; Lima V. M. F.IV; Peiró J. R.IV; Perri S. H. V.I; Carreira R.II
IDepartment of Animal Health and Production, Araçatuba School of Dentistry, FOA, São Paulo State University, UNESP, Araçatuba, São Paulo, Brazil
IIDepartment of Animal Health, São Paulo State University, UNESP, Botucatu, São Paulo, Brazil
IIIInstitute of Biosciences, São Paulo State University, UNESP, Botucatu, São Paulo, Brazil
IVDepartment of Clinics, Surgery and Animal Reproduction, Araçatuba School of Dentistry, FOA, São Paulo State University, UNESP, Araçatuba, São Paulo, Brazil
Correspondence Correspondence to M. Marinho Rua Clóvis Pestana, 793 Bairro D. Amélia, 16050-690, Araçatuba, São Paulo, Brasil E-mail: mmarinho@fmva.unesp.br
ABSTRACT
Immune response to leptospirosis is mainly humorally mediated, and involves opsonization of leptospires for phagocytosis by macrophages and neutrophils. However, some aspects are still unknown. For a more detailed analysis of the cellular immune response to leptospirosis infection, trials were carried out in order to determine the hydrogen peroxide and nitric oxide (H2O2 and NO) production stimulated or not by Interferon-gamma. The participation of some specific cytokines, such as Tumor Necrosis Factor-a (TNF-a); Interferon-gamma (IFN-g); Interleukin-6 (IL-6); and Interleukin-4 (IL-4), in the immunopathology of this infection was also investigated. For this purpose, we analyzed the supernatant from peritoneal macrophage cell culture and the splenic cells of mice genetically selected as High (H) and Low (L) antibody producers, and inbred Balb/c mice infected with Leptospira interrogans serovar icterohaemorrhagiae. The IL-6 production varied from release peaks to inhibition in H, L, and Balb/c mice. Similar behavior was observed for IL-4, produced only by H and Balb/c mice. The three strains presented constant and elevated production of TNF-a until day 14, suggesting its effective participation in the initial phase of the infection. Meanwhile, all the three strains presented a constant and irregular IFN-g production, with release peaks between the 7th and 14th days in L mice. The H and Balb/c mice strains presented a higher tendency to Th2 response pattern, whereas L mice tended towards Th1 response.
Key words: leptospirosis, cytokines, cellular immunity.
INTRODUCTION
Immunity to leptospirosis is mainly humorally mediated, and involves opsonization of leptospires for phagocytosis by macrophages and neutrophils (15). Macrophages are able to ingest and kill both virulent and saprophytic leptospires, which are opsonized by homologous IgG. Consequently, the leptospires ability to escape phagocytosis could be considered as an important virulence factor, responsible for their in vivo invasiveness, in addition to resistance to complement activity (13).
Cytokines are immunomodulatory proteins that help organize the immune response to diverse inflammatory processes, including infection. Interleukin-6 (IL-6), tumor necrosis factor-a (TNF-a), and transforming growth factor-b1 (TGF-b1) are cytokines that have been shown to play a role in the immune response against intracellular pathogens in murine and human models (6). Circulatory levels of TNF-a have been detected in patients with leptospirosis (8). Therefore, an association between the presence of TNF-a and the disease severity and mortality among patients with leptospirosis was found. The release of TNF-a by macrophages during leptospirosis plays a dual role in the host response to infections. Although there is a strong evidence of its deleterious role in patients with sepsis, there are also increasing signs that it plays a pivotal role in the host defense (17). As experimental model, we used High (H) and Low (L) antibody producer mice, obtained by bi-directional selective breeding, from genetically heterogenous populations, characterized by the normal distribution for high or low antibody production, initially developed by Biozzi et al. (3).
MATERIALS AND METHODS
Mice and Inoculum
One hundred and thirty-five mice, male and female, age ranging from 4 to 8 weeks, were divided into 3 equal groups according to the strains Low (L) and High (H) antibody producer (selection IV-A), and Balb/c. The animals were infected with 106 viable Leptospira interrogans serovar icterohaemorrhagiae (strain n° 11437) via intraperitoneal route. Mice of the control groups were intraperitoneally inoculated with 1 ml of Fletcher medium. The Leptospira sample was provided by the Fundação Oswaldo Cruz (FIOCRUZ) Laboratory, maintained in semi-solid Fletcher medium, and quantified according to the Faine's technique (9).
Sacrifice
Mice were sacrificed by cervical dislocation, after bleeding from the orbital plexus for serum collection, on days 2, 4, 7, 10, 14, 21, 28, and 35 after infection.
Macrophage activation
Peritoneal macrophages were obtained from the mice peritoneal cavity. Briefly, 10 ml of iced PBS (phosphate buffered solution) was injected into the upper part of the mice abdomen. Then the abdominal cavity was massaged and the peritoneal fluid was reaspirated into the same syringe. This procedure was repeated three times. Peritoneal suspension was stored in plastic tubes maintained in ice, and centrifuged for 10 minutes at 1500 rpm. The cell suspension was then reset in Complete Medium for Cell Culture (CMCC). For subsequent count, 50 µl from each peritoneal suspension was incubated with a solution containing 0.45 µl of 0.02% neutral red at 37ºC for 10 min. Macrophages were identified by dye, counted in a Neubauer chamber, and adjusted to a final concentration of 2x106 macrophages/ml CMCC. Supernatants (0.1 ml/well) were added in triplicate in 96-well, flat-bottomed, microtitre plates (Corning). Then, 10 µl/ml of E. coli lipopolysaccharide (Sigma Co., USA) was added to each well. After 2 hours incubation in 5%CO2 at 37ºC, non-adherent cells were removed by washing with RPMI-1640 medium.
Hydrogen Peroxide production
The H2O2 production was determined according to the Pick and Keisari technique (1980), modified by Pick and Misel (1981). Results were expressed in nanomoles (nmol) of H2O2 per 2x106 cells. Optical density (OD) was compared with a standard curve of known H2O2 concentrations, varying from 2.0 to 0.5 nM. Samples were run in quadruplicate.
Nitrite production
The NO production by macrophages was measured by the colorimetric method, based on the Griess reagent (11). Results were expressed in nanomoles (nmol) of NO per 2x106 cells, by comparing OD with a standard curve of known NO2 concentrations, varying from 200 to 0.39 nM.
Proliferation
To evaluate the T cell activity in the different groups, spleen cell cultures were used. The cultures were prepared by removing the mice spleens, placing them on Petri dishes (20 x 100 mm) containing 5 ml of RPMI-1640 medium (Sigma Chemical Co., USA), and crushing them with the aid of a pestle and a nylon sieve. The material was gathered and centrifuged in plastic tubes (20 x 2000 mm) for 10 min at 1500 rpm. Right after, supernatants were collected and the cell core was loosened by homogenization with the addition of 1 ml sterile distilled water so that hemolysis could occur. After homogenization, 10 ml of RPMI-1640 medium was added to the solution, which was centrifuged for 10 min at 1500 rpm. Supernatants were collected and the cells were resuspended in 1 ml RPMI-1640 medium supplemented with 10% fetal bovine serum (Nutricel) enriched with 1% L-glutamine (Sigma Chemical Co., USA). The final concentration was adjusted to 2x106 cells/ml. Then, 0.1 ml of this suspension was added in 96-well flat-bottomed plates (Corning). The cells were cultivated in presence or absence of the mitogen Concanavalin-A (Con-A; Calbiochem) and incubated at 37ºC in 5% CO2. After 24 and 48 hours incubation, supernatants were collected and stored in aliquots at -70ºC for subsequent detection of cytokines, Interleukin-4 and Interferon-gamma.
TNF-a , IFN-g , IL-4, and IL-6 determination
Levels of TNF-a, IFN-g, IL-4, and IL-6 in the cell cultures supernatants were determined by ELISA, as described by Haagmans et al. (12), according to the manufacturer's instructions (Pharmigen). A standard curve was obtained by using preparations with known concentrations of mouse recombinant cytokines (rTNF-a, rIFN-g, rIL-4, and rIL-6).
Statistical analysis
Serologic titers obtained from the Balb/c and the genetically selected mice (H and L mice strains) were compared by non-parametric Kruskal-Wallis test for independent variables. In order to compare the cytokine means, we used analysis of variance, with repetition of means, and the Student-Newman-Keuls test for multiple comparisons, with p<0.05 (18).
RESULTS
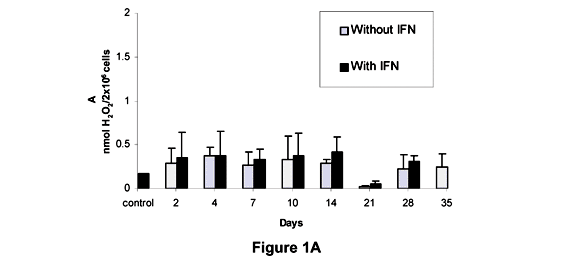
Figure 1 shows the levels of H2O2 produced by the peritoneal macrophages from L, H and Balb/c mice and their respective controls, with and without the addition of IFN-g. In L mice, the H2O2 levels in absence of IFN-g were significantly different from controls on the 2nd, 4th, 7th, 14th, and 28th days; while in the presence of INF-g, H2O2 levels were significantly different from control only on the 2nd, 14th, and 35th days (Figure 1A).
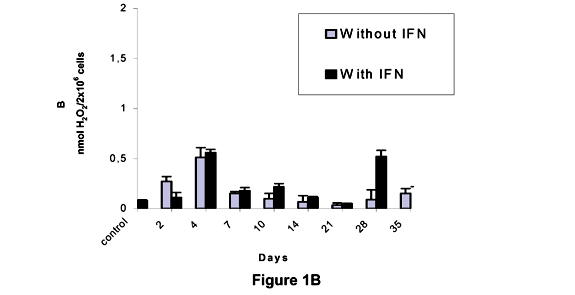
We can see in Figure 1B that the production of H2O2 by the macrophages of H mice was not significantly different from that of controls during the whole study; results were only significantly different between controls.
The levels of H2O2 produced by the macrophages of Balb/c mice without addition of IFN-g were different from those of controls on the 2nd and 35th days after inoculation, whereas with addition of IFN-g the results were significantly different between controls on the 4th, 28th, and 35th days of the experiment (Figure 1C).
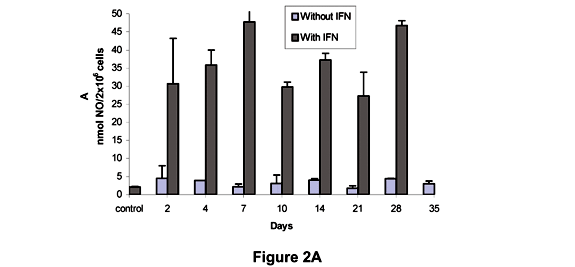
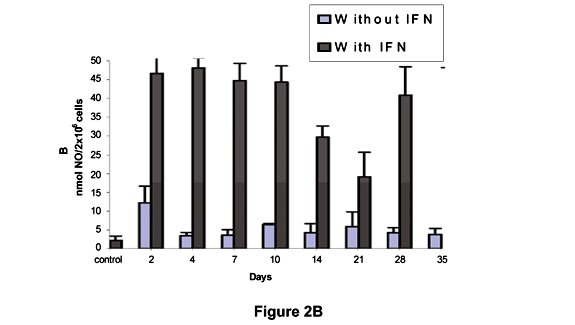
Figure 2A shows that the levels of NO produced by L mice in the absence of IFN-g differed significantly from those produced in the presence of IFN-g at all moments. In H mice, a significant difference in NO levels was detected after 2 days compared to control in the absence of IFN-g. However, when IFN-g was present NO levels were significantly higher than in controls at all times (Figure 2B). As shown in Figure 2C, the NO production without IFN-g was significantly different from controls on the 4th and 28th days in Balb/c mice. High NO levels were observed after incubation with IFN-g in relation to control, but not with the same intensity as in L and H mice.
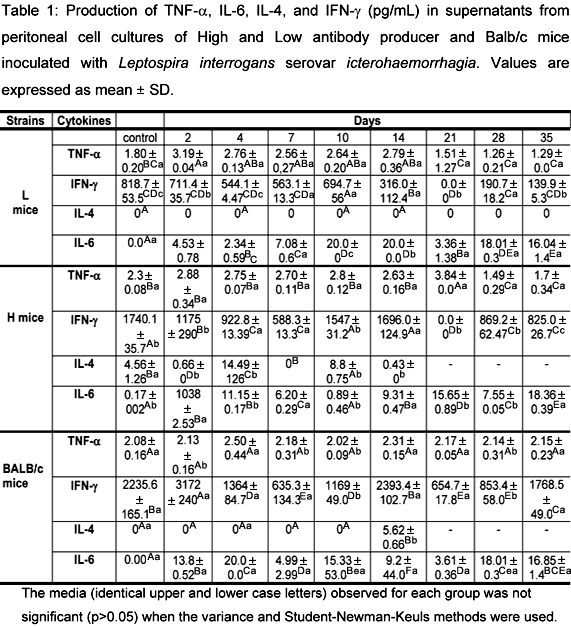
TNF-a, IL-6, IL-4, and IFN-g determination
The production of TNF-a, IL-6, IL-4, and IFN-g by cell cultures of peritoneal macrophages from L, H, and Balb/c mice over 48 hours is demonstrated in Table 1. Analysis of the TNF-a results showed that there were no statistical differences between H and L mice, whereas differences were observed in the three groups when compared to their controls. When L and Balb/c mice were compared, the values were different on the 2nd, 10th, and 28th days after infection. The results differed between H and Balb/c mice on the 2nd, 7th, 10th, and 28th days after inoculation. In L mice, there was a difference on the 2nd day in relation to control. On the 28th and 35th days, these levels were lower than control. No significant differences in TNF-a levels were observed between Balb/c and H mice.
These data confirmed the presence of high levels of TNF-a as a result of Leptospira infection. Production of TNF-a was detected in all groups of mice during the first days of infection, with a marked decline after the 14th day. Balb/c mice showed a constant and elevated production of this cytokine during the experiment.
When data were compared, IL-6 activity was different between controls and H mice. Analysis of the results showed that there were differences between L and H mice controls. The H mice strain presented values that differed statistically from control on days 2, 4, 7, 14, 21, 28 and 35 after inoculation. Balb/c mice presented values that differed statistically from control on all days of experiment. The IL-6 levels varied from 0 to 20 pg/mL. The levels of IL-6 produced by L and H mice were proportionally inverted at most of the moments during this research, except on the 7th and 35th days, when the production of this cytokine was similar in both groups. The IL-4 levels, observed after 24 hours in spleen cell culture, revealed a significant difference between the three groups. Interleukin-4 was not produced by L mice at any time during the experiment. However, it was produced by H and Balb/c mice until the 14th day. Among H mice, values differed from control on days 2, 4, 7, 10, and 14, with peaks of production on the 4th and 10th days. On days 2, 7, and 14, the IL-4 levels were lower than those of control. In Balb/c mice, only on day 14 the IL-4 production was not detected in cellular culture at 48 hours in every mouse. Based on the IFN-g production in all the three groups, we can observe that it was significant in L mice in relation to controls on the 2nd, 7th, 10th, 14th, 21st, 28th and 35th days of the experiment, being null on the 21st day after infection. The H and Balb/c mice strains presented a significant production of IFN-g in relation to controls on the 2nd, 4th, 7th, 21st, 28th and 35th days.
4. DISCUSSION
We can observe that the kinetics of the H2O2 production showed distinct characteristics among L, H and Balb/c mice inoculated with the pathogenic sample. The L mice strain presented an increased production of H2O2 on the 2nd day after inoculation, with a later decrease on the 7th day; reaching a new peak on the 14th day, followed by a sudden decrease. Association of results revealed a proportionally inverted kinetics to the production of antibodies. In H mice, the H2O2 production by peritoneal macrophages showed a positive association with the antibody production at the beginning of infection, as an increased H2O2 production was observed simultaneously with antibody production until the 4th day after inoculation. During the second week, the infection revealed the same kinetics for the production of hydrogen peroxide and metabolites.
Statistically significant differences between means were observed during the experiment; however, these means are not very trustworthy, as they did not reach the minimum values considered correct. This probably occurred as a function of the genetic and environmental variability, affecting H, L, and Balb/c mice. In general, H2O2 levels observed in all the three strains were more evident until the 14th day after infection, but a marked decrease in those levels was seen on the 21st day.
Baeurele et al. (1) reported that, even with quantities of oxygen unable to present satisfactory microbicide effects, H2O2 levels play a role of a secondary messenger in stimulating inflammatory response. Thus, there are strong indices that reactive oxygen and nitrogen intermediates act as second messengers in regulating immune response by increasing the action of genes responsible for the synthesis of certain cytokines. In general, the intermediate oxygen metabolites do not appear to have a relevant part in the control of Leptospira infection, but the hypothesis that they may be aiding the defense process, probably in an indirect manner, cannot be discarded. The NO production by peritoneal macrophages showed similar behavior between the strains at all moments of the experiment, even when submitted to IFN-g.
Nitric oxide has shown to be a good parameter for macrophage activation. Several studies have reported the association between its production and the microbicide activity, particularly against intracellular pathogens (4). On the other hand, microbicide activity can be blocked with the use of specific inhibitors of NO synthesis. Thus, animals resistant to some specific infection liberate high concentrations of this metabolite, and when treated with inhibitors become more susceptible (16). Based on the present study, we cannot affirm that there could be a release of an inhibiting factor for NO production in leptospirosis. Although Leptospira is not an intracellular bacterium, the production of immunosuppressors inhibitors of cellular immune response cannot be discarded.
It was observed that Leptospira infection stimulated a high production of TNF-a in all the genetically selected mice strains. TNF-a is considered an important immunomodulator of host defenses. Alteration of TNF-a levels may produce different results in bacterial sepsis. Unlike sepsis, in which the control of bacterial growth is crucial for a good clinical outcome, in leptospirosis the control of the inflammatory response is the keystone of therapy (17). The L and H mice strains presented modifications in B and T cells, as well as alterations in the macrophages metabolic activity (2, 5, 7). The TNF-a levels were constant and elevated in the first days of infection, also in Balb/c mice, but differed on the 21st day with a marked production by H mice and evident lower levels in L mice. The opposite was observed for IL-6 at the same period of time. Therefore, it can be assured that the differences in the macrophage activity between these mice strains do not interfere with TNF-a synthesis. This is probably due to the fact that TNF-a synthesis occurs in response to the stimuli caused by a pathogenic sample of leptospires during infection.
Estavoyer et al. (8) observed high TNF-a concentrations in the plasma of patients with leptospirosis, and associated these circulating levels with the illness degree. Tajiki et al. (17) attributed a dual function to TNF-a in leptospirosis, since at lower levels this cytokine is a key element in host defense, but at higher levels it is deleterious in patients with sepsis.
In this study, a high variation in the IL-6 production was observed in all the three groups, what is related to the difference in the macrophage activity between these strains.
Champsi et al. (6) showed that IL-6 is produced by macrophages and Natural Killer cells (NK) when exposed in vitro to Mycobacterium avium and may have a role in the infection pathogenesis, suggesting that this cytokine could act as a potent immunosuppressor of the immune response to infection. However, the real role of this cytokine in leptospirosis is not yet clear. Although in literature several IL-6 functions are described (among them its capacity to act on plasmatic cells that influence in the formation of antibodies and stimulate them to produce IL-6 by T cells, acting as co-agents), it is an important cytokine in the acute-phase response in sepsis, and is likely to be involved in the Jarisch-Herxheimer reaction, which in leptospirosis may be responsible for morbidity (10). Previous studies, referring to the lymphocyte activities in this experimental model, have shown certain analogy between the various selections, indicating that modifications in the T and B cells different functions induced by the selective processes occurred in the same manner. However, stimulation of these cells depends on the macrophages accessory function, due to their capacity in efficiently processing and presenting the antigen to the lymphocyte subpopulations.
The IL-4 production by splenic cell supernatants was not detected by the ELISA test at any time during this study. In the 24-hour culture test, the same was observed for the L mice strain. In Balb/c mice, IL-4 was produced only on the 14th day after infection, whereas the H mice strain produced this cytokine at varied intervals. Since IL-4 is an important factor during B cells proliferation and differentiation, in the H mice strain this cytokine could be related to the greater production of antibodies, maintaining thus the multi-specific effect for higher antibody production.
The INF-g production was constant among the strains, however a peak of production was observed from the 10th to the 14th day in the L mice strain. Although IFN-g levels are similar to the controls, its importance could not be noticed at the beginning of the infection. Data analysis revealed a major participation of the immune cell response during the first days of infection. This is true because leptospirosis is an acute disease. These bacteria remained free in the circulatory system at the beginning of the infection, disappearing later. Macrophage activity was hardly noticeable during the experiment. However, there was a response to the infectious stimulus, sometimes shown by the production of reactive oxygen species and other times shown by the different cytokine levels produced. The low macrophage activity observed here is probably due to the fact that leptospires are extracellular bacteria, or it is a consequence of a process that involves cell apoptosis.
Overall, H and Balb/c mice presented a Th2 response pattern, with greater antibody production, higher degree of lesions (unpublished data) and synthesis of IL-4; whereas the L strain presented a Th1 response pattern, with high IFNF-g production and macrophage activity as well as development of lesions (14). The results obtained suggest the necessity of additional research using immunohistochemical techniques to further elucidate immunopathology aspects in Leptospira infection.
ACKNOWLEDGEMENTS
The authors express their thanks to FAPESP, the State of São Paulo Research Foundation, for financial aid. Process 96/0437-5.
Received: March 19, 2004
Accepted: September 9, 2004
Published online: March 3, 2005
- 1 BAEURELE PA., RUPEC RA., PAHL HL. Reactive oxygen intermediates as second messengers of a general pathogen response. Pathol. Biol., 1996, 44, 29-35.
- 2 BANFI E., CINCO M., BELLINI M., SORANZO MR. The role of antibodies and serum complement in the interaction between macrophages and leptospires. J. Gen. Microbiol., 1982, 12, 813-6.
- 3 BIOZZI G., STITTEI C., MOUTON D., BOUTHILLIER Y., DECREUSEFOND C. Sélection articielle pour la production danticorps chez la souris. Ann. Inst. Pasteur, 1968, 115, 960-5.
- 4 BOOCKVAR KS., GRANGER DL., POSTON RM. Nitric oxid produced mutine listeriosis protective. Infec. Immun., 1994, 62, 421-3.
- 5 CABRERA WH. Mecanismos regulatórios da resposta humoral de camundongos bons e maus respondedores obtidos por seleção genética bidirecional São Paulo: Universidade de São Paulo, Instituto de Ciências Biomédicas, 1993. 81p. [Tese Doutorado]
- 6 CHAMPSI J., YOUNG LS., BERMUDEZ LE. Production of TNF-a, IL-6 and TGF-b, and expression of receptors for TNF-a and IL-6, during murine Mycobacterium avium infection. Immunology, 1995, 85, 547-9.
- 7 COUDERC S., BOUTHILLIER Y., MEVEL JC. Evaluation of T-helper function in lines of mice selected for High or Low antibody production: quantitative inhibition of immune responses by anti-L3T4+monoclonal antibody. Immunol. Lett., 1989, 23, 19-21.
- 8 ESTAVOYER JM., RACADOT E., COVETDIC G., LEROY J., GROSPERRIN L. Tumor necrosis factor in patients with leptospirosis. Rev. Infect. Dis., 1991, 13, 1245.
- 9 FAINE S. Guidelines for the control of leptospirosis Geneva: World Health Organization, 1982. 82-3.
- 10 FRIEDLAND JS., WARREL DA. The Jarisch-Herxheimer reaction in leptospirosis: possible pathogenesis and review. Rev. Infect. Dis., 1991, 13, 207-10.
- 11 GRIESS JP. Bermerkungen zuder Abhandlung der H H: we selyund Benedict "Überlinige Azoverbindungen". Ber Deustch Chem. Ges., 1979, 112, 426-8.
- 12 HAAGMANS BL., VAN DEN EERTWEGH AJ., CLAASSEN E., HORZINEK MC., SCHIJNS VE. Tumor necrosis factor-alpha production during cytomegalovirus infection in immunosuppressed rats. J. Gen. Virol., 1994, 75, 779-87.
- 13 JOHNSON RC., HARRIS VG. Antileptospiral activity of serum II. Leptospiral virulence fdactor. J. Bacteriol., 1967, 93, 513-9.
- 14 MARINHO M., LANGONI H., OLIVEIRA SL., CARREIRA R., PERRI SHV., LUVIZOTO MC. Resposta humoral, recuperação bacteriana e lesões histológicas em camundongos geneticamente selecionados para bons e maus produtores de anticorpos Balb/c, frente à infecção por Leptospira interrogans sorovar icterohaemorrhagiae. Pesq. Vet. Bras., 2003, 23, 5-12.
- 15 MITCHISON M., BULACH DM., VINH T., RAJAKUMAR K., FAINE S., ADLER B. Identification and caracterization of the dTDP-Rhamnose biosynthesis and transfer genes of the lipopolysaccharide-related rfb locus in Leptospira interrogans serovar copenhageni. J. Bacteriol., 1997, 179, 1262-7.
- 16 OLIVEIRA SL., IBAÑEZ OM., MOUTON D., SANTANNA OA., SIQUEIRA M., BIOZZI G. Independent poligenic regulation of quantitative antibody responsiveness and expression of delayed-type hypersensitivity (DTH). Exp. Clin. Immunogenet., 1985, 2, 223-33.
- 17 TAJIKIH SR., SALOMÃO R. Association of plasma levels of tumor necrosis Factor a with severity of disease and mortality among patients with Leptospirosis. Clin. Infec. Dis., 1996, 23, 1177.
- 18 ZAR JH. Biostatistical analysis 3 ed. Upper Saddle River: Prentice-Hall, 1996. 718p.
Correspondence to
Publication Dates
-
Publication in this collection
03 May 2005 -
Date of issue
June 2005
History
-
Accepted
09 Sept 2004 -
Received
19 Mar 2004