Abstract
Meglumine antimoniate (MA) and sodium stibogluconate are pentavalent antimony (SbV) drugs used since the mid-1940s. Notwithstanding the fact that they are first-choice drugs for the treatment of leishmaniases, there are gaps in our knowledge of their toxicological profile, mode of action and kinetics. Little is known about the distribution of antimony in tissues after SbV administration. In this study, we evaluated the Sb content of tissues from male rats 24 h and three weeks after a 21-day course of treatment with MA (300 mg SbV/kg body wt/d, subcutaneous). Sb concentrations in the blood and organs were determined by inductively coupled plasma-mass spectrometry. In rats, as with in humans, the Sb blood levels after MA dosing can be described by a two-compartment model with a fast (t1/2 = 0.6 h) and a slow (t1/2 >> 24 h) elimination phase. The spleen was the organ that accumulated the highest amount of Sb, while bone and thyroid ranked second in descending order of tissues according to Sb levels (spleen >> bone, thyroid, kidneys > liver, epididymis, lungs, adrenals > prostate > thymus, pancreas, heart, small intestines > skeletal muscle, testes, stomach > brain). The pathophysiological consequences of Sb accumulation in the thyroid and Sb speciation in the liver, thyroid, spleen and bone warrant further studies.
pentavalent antimonials; thyroid; liver; leishmaniases; Glucantime; pharmacokinetics
Although it is a metalloid for which no natural biological function has been identified so far, antimony has a long history of medicinal uses. In the XVI century, Paracelsus, a famous alchemist and physician and one of the pioneers of iatrochemistry, was especially fond of antimony and prescribed medicines based on its salts for a number of morbid conditions (Haldar et al. 2011Haldar AK, Sen P, Roy S 2011. Use of antimony in the treatment of leishmaniasis: current status and future directions. Mol Biol Int 2011: 571242.). During the following two centuries, antimony-based drugs became the centre of a dispute between Galenic school doctors and iatrochemists and the medical use of antimony was banned in France and other countries. In the early-XX century, antimony-based drugs made a remarkable return to physicians’ therapeutic armamentarium thanks to their efficacy in treating some parasitic diseases. In 1912, Gaspar Vianna reported that he had achieved a complete clinical cure for mucocutaneous leishmaniasis with a course of intravenous (i.v.) injections of tartar emetic [antimony potassium tartrate (APT)] (Vianna 1912Vianna G 1912. Tratamento da leishmaniose tegumentar por injeções intravenosas de tártaro emético. Anais do VII Congresso Brasileiro de Medicina e Cirurgia 4: 426-428.). A few years later, in Italy, Di Cristina and Caronia (1915) successfully treated children afflicted with visceral leishmaniasis by injecting repeated doses of tartar emetic (Di Cristina & Caronia 1915). Shortly thereafter, in Sudan, after confirming previous reports that i.v. injections of tartar emetic could cure cutaneous (“oriental sore”) and visceral (kala-azar) forms of leishmaniases, John Christopherson noticed that this antimonial drug was also effective against both urinary and intestinal schistosomiases (Christopherson 1918Christopherson JB 1918. The successful use of antimony in bilharziosis. Lancet 7: 325-327., 1923Christopherson JB 1923. The curative dose of antimony tartrate in schistosomiasis (bilharzia disease). Br Med J 2 (3287): 1254-1255.). Since then and until the advent of praziquantel in the 1970s, trivalent antimonial drugs remained as one of the most effective therapeutic approaches for schistosomiasis.
As far as leishmaniasis therapy is concerned, tartar emetic and other SbIII-based drugs were replaced by sodium stibogluconate (SSG) (Pentostam®) and meglumine antimoniate (MA) (Glucantime®), less toxic SbV drugs that were introduced in the market in mid 1940s (Haldar et al. 2011Haldar AK, Sen P, Roy S 2011. Use of antimony in the treatment of leishmaniasis: current status and future directions. Mol Biol Int 2011: 571242.).
The effective dosing schedules for antimony-based drugs in leishmaniasis and schistosomiasis were established decades before their complex kinetics were partially elucidated. The first kinetic investigations showed that patients excreted most of the antimony via urine within a few hours of injection of SbIII or SbV drugs (Goodwin & Page 1943Goodwin LG, Page JE 1943. A study of the excretion of organic antimonials using a polarographic procedure. Biochem J 37: 198-209., Otto et al. 1947Otto GF, Maren TH, Brown HW 1947. Blood levels and excretion rates of antimony in persons receiving trivalent and pentavalent antimonials. Am J Hyg 46: 193-211.). A clear picture of Sb kinetics, however, came to light only in 1988, when Chulay et al. (1988)Chulay JD, Fleckenstein L, Smith DH 1988. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans R Soc Trop Med Hyg 82: 69-72. reported that most of the Sb administered by a single intramuscular (i.m.) injection of SbV is rapidly eliminated (t½ = 2.02 h) so that only residual concentrations are found in the blood 24 h after drug administration. During a 30-day course of injections of SbV spaced 24 h apart, however, these nadir Sb blood levels gradually rose. According to Chulay et al. (1988)Chulay JD, Fleckenstein L, Smith DH 1988. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans R Soc Trop Med Hyg 82: 69-72., their data on Sb blood levels could be described by a two-compartment kinetic model the slow elimination phase of which had a half-life of 76 h. Based on the foregoing information, the authors speculated that the slow elimination phase was related to the in vivo conversion of SbV into SbIII, a bio-reduction that, according to them, could contribute to the toxicity often noted in long-term SbV therapy. Further studies in humans and rhesus monkeys using more sensitive analytical methods, suggested that Sb elimination could be even slower, with a terminal elimination half-life longer than 30 days (Miekeley et al. 2002Miekeley N, Mortari SR, Schubach AO 2002. Monitoring of total antimony and its species by ICP-MS and on-line ion chromatography in biological samples from patients treated for leishmaniasis. Anal Bioanal Chem 372: 495-502., Friedrich et al. 2012Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75.). Moreover, data on speciation of Sb in monkeys’ plasma one and nine days after a 21-day treatment course with MA indicated that the proportion of SbIII in nadir plasma levels of Sb markedly increased with time during the slow elimination phase, a finding that is consistent with the hypothesis that SbIII becomes a major Sb species during the terminal elimination phase (Friedrich et al. 2012Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75.).
Despite the recent advances in the knowledge of antimonial drug kinetics, little is known about the distribution of Sb into tissues of individuals treated with SbIII or SbV compounds. The same holds true for organ distribution of Sb following exposures through occupationally and environmentally relevant routes (i.e., oral, dermal or inhalation routes).
Molokhia and Smith (1969)Molokhia MM, Smith H 1969. Tissue distribution of trivalent antimony in mice infected with Schistosoma mansoni. Bull World Health Organ 40: 123-128. measured (by neutron activation analysis) the Sb content of tissues of Schistosoma mansoni-infected mice at different time intervals (0.5 h, 8 h, 24 h, 2, 4, 7 and 15 days) after a single intraperitoneal (i.p.) injection of a SbIII drug (tartar emetic or sodium antimony 2,3 mesodimercapto-succinate, Astiban®). The authors found the highest levels of Sb in the liver and spleen, followed by alimentary tract organs (colon, duodenum and stomach), 30 min after treatment. Levels of Sb were similarly low in all tissues of mice euthanised on post-treatment day 4 and thereafter.
Recently, Borborema et al. (2013)Borborema SET, Osso Jr JA, de Andrade Jr HF, do Nascimento N 2013. Biodistribution of meglumine antimoniate in healthy and Leishmania (Leishmania) infantum chagasi-infected BALB/c mice. Mem Inst Oswaldo Cruz 108: 623-630. determined the proportion of injected radioactive Sb (122Sb and 124Sb radioisotopes produced in neutron-irradiated Glucantime®) in tissues of Leishmania infantum chagasi-infected (BALB/c) mice treated with an i.p. injection of MA. Mice treated with SbV were euthanised at post-treatment time intervals ranging from 3 min to three days. The highest % of Sb injected activities (IA) was found in the liver 30 min after the MA injection (61% and 47.5% in non-infected and infected mice, respectively). According to the authors, measurable activities of Sb radioisotopes were also detected in spleen, intestines, stomach and kidneys, while no accumulation of radioactive Sb was noted in the brain, lungs, heart or uterus.
The foregoing studies shed some light on the tissue distribution of Sb after single doses of SbIII or SbV drugs. Two additional studies determined Sb levels in organs of mice and rats exposed to APT for longer periods. Poon et al. (1998)Poon R, Chu I, Lecavalier P, Valli VE, Foster W, Gupta S, Thomas B 1998. Effects of antimony on rats following 90-day exposure via drinking water. Food Chem Toxicol 36: 21-35. exposed rats to APT orally (drinking water) for 90 days and described that levels of Sb in tissues [measured by inductively coupled plasma (ICP) emission spectrometry] were dose-related and followed a descending order of concentrations from liver and spleen to brain and adipose tissue [red blood cells (RBC) >> spleen, liver > kidneys > brain, fat > plasma]. Dieter et al. (1991)Dieter MP, Jameson CW, Elwell MR, Lodge JW, Hejtmancik M, Grumbein SL, Ryan M, Peters AC 1991. Comparative toxicity and tissue distribution of antimony potassium tartrate in rats and mice dosed by drinking water or intraperitoneal injection. J Toxicol Environ Health 34: 51-82. exposed B6C3F1 mice and F344 rats to APT through the drinking water for 14 days and by i.p. injections every other day (a dosing schedule intended to minimise local mesenteric inflammation) for 90 days. The authors found dose-related concentrations of residual Sb in the blood, liver, kidney, spleen and heart of rats and in the liver and spleen of mice.
As far as the authors are aware, except for a previous study from our laboratory in rhesus monkeys infected with Leishmania braziliensis, residual levels of Sb in different tissues after a treatment course with SbV drugs had not been investigated yet. This study was undertaken to provide data on the kinetics and tissue distribution of Sb in rats treated with a 21-day course of MA.
MATERIALS AND METHODS
Animals - Male Wistar rats from the Oswaldo Cruz Foundation (Fiocruz) breeding stock were used in this study. Upon arriving at the laboratory animal quarters, approximately 80 day-old rats were individually housed in standard plastic cages with stainless steel cover lids and pinewood shavings as bedding. Animals were kept under controlled environmental conditions (12 h light:12 h dark cycle, lights on from 08:00 am-08:00 pm, temperature 22ºC ± 1ºC, relative humidity approximately 70%) throughout the study. All rats were given free access to a pelleted diet for rats and mice (CR1 Nuvital, Nuvilab Ltd, Brazil) and tap water. Experiments were conducted in accordance with Brazilian animal protection and welfare laws and the study protocol was cleared by the Ethical Committee on the Use of Laboratory Animals of Fiocruz.
Treatment - MA (Glucantime®, Sanofi-Aventis Farmacêutica Ltd, Brazil) was administered by i.v. injections (penis vein) or by subcutaneous (s.c.) injections on the back skin of the rat. MA is a poorly characterised drug that is produced by the reaction of SbV with N-methyl-D-glucamine. Evidence has been provided that up to 4 N-methyl-D-glucamine hydroxyls are coordinated with each antimony atom (Roberts et al. 1998Roberts WL, McMurray WJ, Rainey PM 1998. Characterization of the antimonial antileishmanial agent meglumine antimonate (glucantime). Antimicrob Agents Chemother 42: 1076-1082.). According to the manufacturer, each ampoule (5 mL) of Glucantime® contains 425 mg N-methyl MA/mL or 85 mg SbV/mL. Traces of SbIII are commonly found in pharmaceutical formulations of MA. The content of SbIII in MA ampoules varies between lots of the drug and very different concentrations (up to 10 mg/mL) have been reported in the literature. Total Sb, SbV and SbIII concentrations were determined in the Glucantime® lot used in this investigation and also in ampoules of additional lots. Levels of total Sb in ampoules from the lot used in this study was 90.1 mg/mL while the concentration of SbIII [measured by hydride generation-ICP-mass spectrometry (MS) as described by Miekeley et al. (2002)Miekeley N, Mortari SR, Schubach AO 2002. Monitoring of total antimony and its species by ICP-MS and on-line ion chromatography in biological samples from patients treated for leishmaniasis. Anal Bioanal Chem 372: 495-502.] was 3.2 mg/mL or 3.5% total Sb, while the concentration of Sbv [(Sb-total) - (SbIII)] was 86.9 mg/mL. In ampoules from two additional lots of Glucantime® (not used in this study) Sb concentrations were 85 mg/mL and 87.6 mg/mL, 3.8% and 3.9% of which as SbIII (Friedrich et al. 2012Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75.).
The injected doses were 75 mg SbV/mg/kg body wt (single dose, i.v.) or 300 mg SbV/kg body wt/d (s.c.) and injection volumes were 0.88 mL/kg body or 3.5 mL/kg body wt/d, respectively. A vehicle-only treated control group (n = 6) received s.c. injections (1.76 mL/kg body wt/d) of the vehicle (potassium metabisulfite, 1.6 mg/mL and sodium sulfite, 0.18 mg/mL). In a preliminary test, six animals were injected intravenously with a single dose of MA (75 mg SbV/mg/kg body wt) to evaluate the fast elimination phase of Sb kinetics in the male rat. In a subsequent experiment, 12 rats were treated by the s.c. route with a dose of MA as high as 300 mg SbV/kg body wt/d for 21 days. Half of the MA-treated rats were euthanised 24-h after the last dose of MA while the remaining animals were euthanised 21 days later. A third experiment (6 MA-treated and 3 vehicle-control rats) was performed to evaluate the extent to which residual Sb blood levels declined after the end of a 21-d course of treatment with MA (300 mg SbV/kg body wt/d, s.c.) when a longer post-treatment time interval (105 days) was examined. Rats were euthanised by carbon dioxide inhalation.
Antimony determination in biological matrices - Levels of Sb in biological matrices (whole blood, plasma and tissues) were determined by ICP-MS as described in detail elsewhere (Miekeley et al. 2002Miekeley N, Mortari SR, Schubach AO 2002. Monitoring of total antimony and its species by ICP-MS and on-line ion chromatography in biological samples from patients treated for leishmaniasis. Anal Bioanal Chem 372: 495-502., Friedrich et al. 2012Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75.).
An ELAN DRC II (PerkinElmer Sciex, USA) instrument equipped with a Meinhard nebuliser and a cyclonic spray chamber (Glass Expansion, Australia) was used. Antimony measured isotopes 121Sb, 123Sb and 103Rh were employed as internal standards. To determine total Sb by solution nebulisation ICP-MS, whole blood and plasma samples were analysed after digestions with two-fold sub-boiled distilled HNO3 and adequate dilution (1:10 or 1:100) with deionised water (18 MΩ cm minimum resistivity, MilliQ, Millipore, USA). Tissue samples were lyophilised and wet-ashed with HNO3-H2O2 in closed polyethylene tubes essentially as previously reported (Miekeley et al. 2002Miekeley N, Mortari SR, Schubach AO 2002. Monitoring of total antimony and its species by ICP-MS and on-line ion chromatography in biological samples from patients treated for leishmaniasis. Anal Bioanal Chem 372: 495-502.). The diluted digest was then analysed by ICP-MS in the quantitative external calibration method.
The accuracy of the method was checked by the analysis of a reference material (bovine whole blood provided by the Adolpho Lutz Institute, Brazil) and a tissue (liver) from a control individual. The Sb concentration in these samples was below the limit of detection (LOD). The samples were spiked with 10 µg/L and 60 µg/L of Sb and recoveries were between 96-99.7% (10 µg/L) and between 101.9-102.8% (60 µg/L), respectively. The relative standard deviation was below 5%. The repeatability (calculated as r = t(n-1, 1-α).√2.s) was 1.8 µg/L and 10.9 µg/L for the spikes of 10 µg/L and 60 µg/L, respectively. The LOD of the method were 0.5 ng Sb/g for plasma and whole blood and 1 ng Sb/g for tissues, while limits of quantification (LOQ) were 1.7 ng Sb/g for plasma and whole blood and 3.3 ng Sb/g for tissues. The analytical solutions were prepared from SbV (KSb(OH)6, p.a. Merck) and SbIII (C4H4KO7Sb + 0.5H2O, p.a. Merck) stock solutions (1,000 mg/L) in water.
Blood sampling - Venous blood samples (0.5 mL) were taken from the tail vein prior to MA treatment (d 0) and immediately before injections on treatment days 1, 5, 9, 13, 18 and on post-treatment days 1, 4, 8, 12, 16 and 21. Na-heparin was used as anticoagulant and plasma was separated by centrifugation (2,400 g for 15 min). Whole blood and plasma samples were distributed into polyethylene tubes and kept at -20ºC until further use.
Statistical analysis - Data were evaluated by ANOVA and Dunnett’s test, by the Student t test or, when results did not fit a normal distribution, by the Kruskal-Wallis test followed by the Mann-Whitney U test. In any case, a difference was considered significant for p < 0.05. Descriptive statistics, statistical inference tests and linear regression were performed using SPSS (v.11) or a Graph Pad Prism 45 software.
RESULTS
Blood levels of Sb after single and multiple doses of MA - Fig. 1A depicts the time course of changes in whole blood levels of Sb after a single i.v. (bolus) injection of MA (75 mg SbV/kg body wt) given to male rats. The sharp fall in Sb blood concentrations (t1/2 = 0.6 h) indicates that almost all Sb given as MA was cleared from the body within 6-12 h of drug injection. Nonetheless, a closer look at Sb terminal elimination phase (Fig. 1A, insert) reveals that, after attaining concentrations as low as 2 µg/g or less within 6 h of administration, further elimination proceeds very slowly so that 24 h after an i.v. injection of MA, nadir levels of Sb are found in the blood. Similar fast elimination phases of Sb with very low residual levels 24 h after MA administration were also observed when a SbV drug is given to rats by the s.c. route (Miranda et al. 2006Miranda ES, Miekeley N, De-Carvalho RR, Paumgartten FJ 2006. Developmental toxicity of meglumine antimoniate and transplacental transfer of antimony in the rat. Reprod Toxicol 21: 292-300.). In this study, we did not examine the fast elimination following s.c. administration, but Fig. 2 shows the increase in nadir blood levels of Sb (measured 24 h after a previous injection) during the treatment course period and the slow decline of Sb concentrations thereafter. As shown in Fig. 2, upon repeated administration (by the s.c. route) of 24 h spaced doses of MA (300 mg SbV/kg body wt/d, s.c.), nadir levels of Sb steadily rose so that at the end of a 21-day course of treatment, Sb attained levels as high as 35-40 µg/g in whole blood. Blood levels of Sb in rats euthanised one day after the last dose of MA (on day 22) did not differ from the levels of this metalloid in the blood of rats euthanised 21 days later (on day 42). In a subsequent experiment, six rats were treated with MA (300 mg SbV/kg body wt/d, s.c.) for 21 days and their Sb blood levels were measured on the day following the last dose of MA (day 22) and 105 days later (day 126). The results showed that after almost three months post-treatment, Sb blood levels fell modestly from 51.0 ± 7.3 µg/g (day 22) to 35.6 ± 4.6 µg/g (day 126). Sb levels in whole blood of control (untreated and vehicle only-treated) rats remained undetected or close to the LOQ.
: time course of antimony (Sb) concentrations (µg/g) in the blood (whole blood) of male rats (n = 6) treated intravenously with a single dose (75 mg SbV/kg body wt) of meglumine antimoniate (MA) [insert: a magnified view of nadir Sb levels at post-injection intervals longer than 6 h (terminal elimination phase)]; B: linear plot of decline in Sb blood levels during the fast elimination phase. Data are shown as natural logarithm of Sb concentration (µg/g) in the blood vs. time after MA administration.
: time course of nadir concentrations of antimony (µg/g) in the blood from male rats (n = 6) treated with meglumine antimoniate (MA) (300 mg SbV/kg body wt, subcutaneous) during 21 consecutive days. Blood samples were taken from the tail vein 24 h after a prior MA administration. Levels of Sb in the blood of untreated controls (n = 3) and of rats treated with the vehicle only (n = 6) (not shown) were below the limit of quantification of the method.
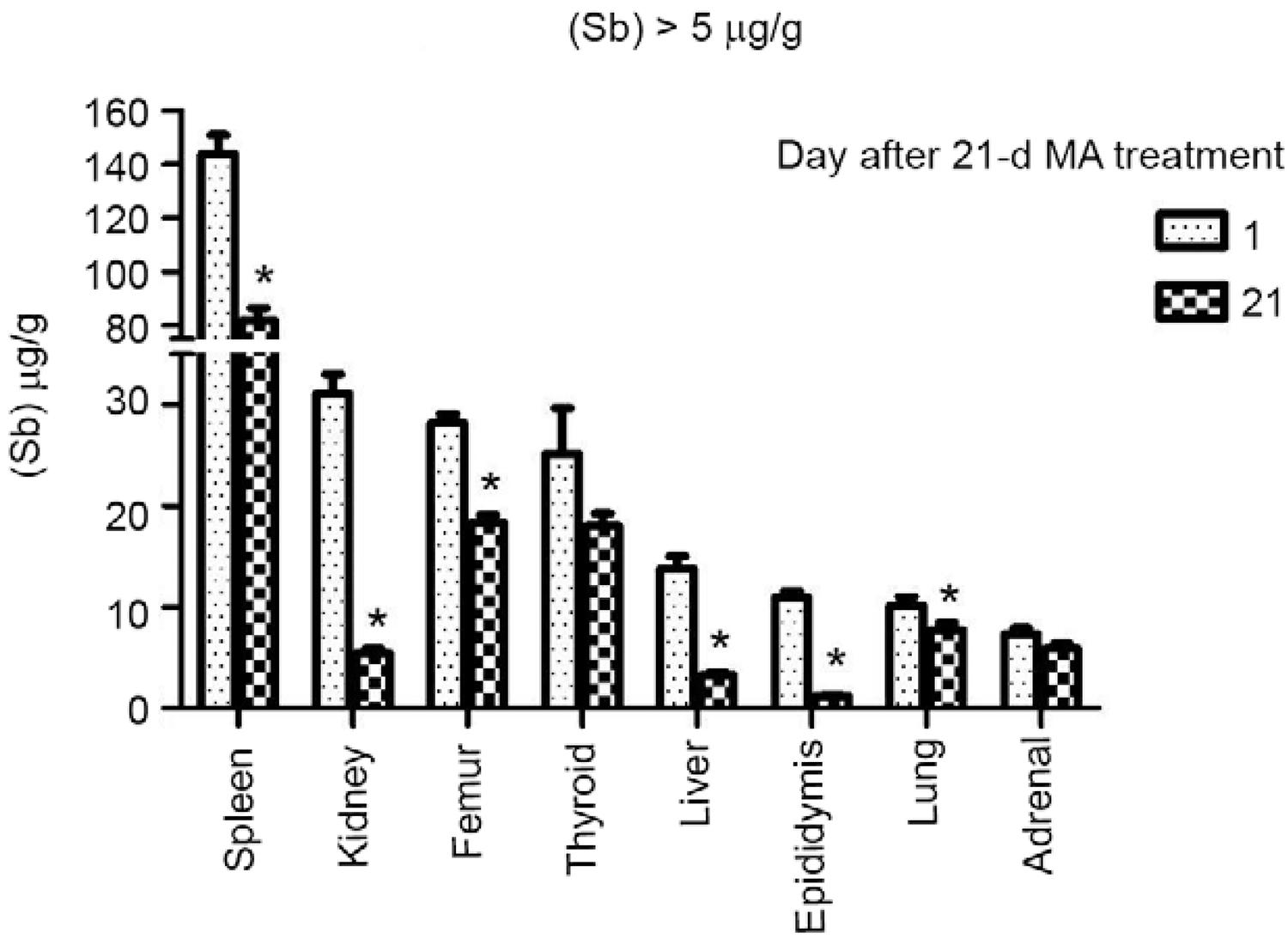
Residual levels of Sb in tissues after a 21-day course of treatment with MA - Tissue concentrations of Sb were determined in rats killed 24 h after the last dose of MA and in a second group of animals killed 21 days after treatment discontinuation (Table I). Fig. 3 shows the distribution of Sb in the spleen, kidneys, femur, thyroid, liver, epididymis, lungs and adrenals, all of which presented Sb levels higher than 5 µg/g at the end of treatment. The spleen ranked first among the tissues with the highest levels of Sb at the end of treatment. Although declining markedly over a three-week post-treatment period, levels of Sb in the spleen were still the highest among all tissues on day 42 (Table I). The kidneys, bones (femur) and thyroid gland ranked second in a descending order of Sb content in tissues at the end of MA administration period (Table I). In contrast to the bones and thyroid, the Sb levels which exhibited a small reduction, kidney levels showed a drastic decline during the three post-treatment weeks. The liver, epididymis, lungs and adrenals showed intermediate levels of Sb at the end of treatment (Table I). On day 42, levels of Sb in the liver and epididymis were markedly lower than the levels on the day after the last dose of MA, while levels in lungs and adrenals were modestly reduced. Fig. 4 presents tissues that showed the lowest concentrations of Sb (< 5 µg/g) after a course of treatment with MA. The tissues with low (< 5 µg/g) Sb concentrations on day 22 that exhibited a further reduction of metalloid content within the next three weeks were as follows: prostate, thymus, small intestine, skeletal muscle, testes and stomach. The tissues which did not exhibit a discernible change in Sb levels between days 22-42 were pancreas, heart and brain (Table I).
: tissues the residual antimony levels of which were higher than 5 µg/g. Sb content (µg/g, dry wt) was determined by inductively coupled plasma-mass spectrometry in rats killed 24 h (n = 6) and 21 days (n = 6) after a 21-d treatment with meglumine antimoniate (MA) (300 mg SbV/kg body wt/d, subcutaneous). Asterisks mean a decrease (Student t test, p < 0.05) of Sb concentration within three weeks of the end of MA administration.
: tissues the residual antimony levels of which were lower than 5 µg/g. Sb content (µg/g, dry wt) was determined by inductively coupled plasma-mass spectrometry in rats killed 24 h (n = 6) and 21 days (n = 6) after a 21-d treatment with meglumine antimoniate (MA) (300 mg SbV/kg body wt/d, subcutaneous). Asterisks mean a decrease (Student t test, p < 0.05) of Sb concentration within three weeks of the end of MA administration
Whole blood and plasma Sb levels 105 days after the end of treatment with MA are shown in Table II. The huge difference between whole blood and plasma concentrations of Sb is consistent with the notion that at this terminal elimination phase RBCs account for almost all Sb contained in the blood. It is of note that levels of antimony in the blood and plasma of untreated and vehicle-only treated rats were extremely low or below the quantification and/or detection limits.
DISCUSSION
Data provided by this study indicated that, in male rats treated with MA, a decline of Sb levels in the blood can be described by a two-compartment kinetic model with fast (t1/2 = 0.6 h) and slow elimination phases. Similar bi-exponential declines in Sb blood concentration over time had been described for pregnant and non-pregnant female rats treated with MA (Miranda et al. 2006Miranda ES, Miekeley N, De-Carvalho RR, Paumgartten FJ 2006. Developmental toxicity of meglumine antimoniate and transplacental transfer of antimony in the rat. Reprod Toxicol 21: 292-300.) and also for dogs (Tassi et al. 1994Tassi P, Ormas P, Madonna M, Carli S, Belloli C, De Natale G, Ceci L, Marcotrigiano GO 1994. Pharmacokinetics of N-methylglucamine antimoniate after intravenous, intramuscular and subcutaneous administration in the dog. Res Vet Sci 56: 144-150.), mice (Nieto et al. 2003Nieto J, Alvar J, Mullen AB, Carter KC, Rodríguez C, San Andrés MI, San Andrés MD, Baillie AJ, González F 2003. Pharmacokinetics, toxicities and efficacies of sodium stibogluconate formulations after intravenous administration in animals. Antimicrob Agents Chemother 47: 2781-2787.), hamsters (Radwan et al. 2007Radwan MA, Al Jaser MH, Al Rayes ZR 2007. The effects of induced diabetes and cutaneous Leishmania infection on the pharmacokinetics of antimony in hamsters. Ann Trop Med Parasitol 101: 133-142.), non-human primates (Friedrich et al. 2012Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75.) and humans (Chulay et al. 1988Chulay JD, Fleckenstein L, Smith DH 1988. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans R Soc Trop Med Hyg 82: 69-72., Miekeley et al. 2002Miekeley N, Mortari SR, Schubach AO 2002. Monitoring of total antimony and its species by ICP-MS and on-line ion chromatography in biological samples from patients treated for leishmaniasis. Anal Bioanal Chem 372: 495-502.) treated with SbV drugs by parenteral routes.
There is a paucity of data on the distribution of Sb into different tissues and on their elimination from the body after exposure to organic antimony compounds or even to inorganic antimony. As mentioned in the introduction of this article, Borborema et al. (2013)Borborema SET, Osso Jr JA, de Andrade Jr HF, do Nascimento N 2013. Biodistribution of meglumine antimoniate in healthy and Leishmania (Leishmania) infantum chagasi-infected BALB/c mice. Mem Inst Oswaldo Cruz 108: 623-630. has recently described the distribution of labelled Sb (122Sb, 124Sb) into some BALB/c mouse tissues at different time intervals after a single i.p. injection of MA. A study by Molokhia and Smith (1969)Molokhia MM, Smith H 1969. Tissue distribution of trivalent antimony in mice infected with Schistosoma mansoni. Bull World Health Organ 40: 123-128. also reported the distribution of Sb into a variety of mouse tissues following a single i.p. administration of SbIII schistosomicidal drugs. The foregoing studies described the distribution of Sb after single injections of antimonial drugs. There are only a few studies on the tissue distribution of residual Sb after prolonged exposures through drinking water or repeated injections of SbIII organic compounds (e.g., tartar emetic) (Dieter et al. 1991Dieter MP, Jameson CW, Elwell MR, Lodge JW, Hejtmancik M, Grumbein SL, Ryan M, Peters AC 1991. Comparative toxicity and tissue distribution of antimony potassium tartrate in rats and mice dosed by drinking water or intraperitoneal injection. J Toxicol Environ Health 34: 51-82., Poon et al. 1998Poon R, Chu I, Lecavalier P, Valli VE, Foster W, Gupta S, Thomas B 1998. Effects of antimony on rats following 90-day exposure via drinking water. Food Chem Toxicol 36: 21-35.). As far as the authors are aware, except for a report on Sb levels in organs from rhesus monkeys treated with MA (Friedrich et al. 2012Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75.), no study has provided data on Sb distribution into tissues after a course of treatment with SbV drugs.
The marked disproportion between Sb levels in whole blood and in plasma 105 days after the end of treatment of with MA (Table II) is consistent with the hypothesis that, during the terminal slow elimination phase, Sb is found inside RBCs with very little in plasma. Friedrich et al. (2012)Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75. reported that, in monkeys that received i.m. injections of MA, the ratio of the Sb concentration in the plasma to the Sb concentration in RBC [(Sb)plasma/(Sb)RBC] was > 1, but progressively diminished with time in the fast elimination phase (e.g., 6 and 12 h post-dosing). An inverse ratio (< 1), however, was noted in the slow elimination phase (e.g., 24-h and longer post-treatment time intervals). Along the same line, a study by Quiroz et al. (2009)Quiroz W, De Gregori I, Basilio P, Bravo M, Pinto M, Lobos MG 2009. Heavy weight vehicle traffic and its relationship with antimony content in human blood. J Environ Monit 11: 1051-1055. found that the levels of Sb-total in the blood of workers occupationally exposed to Sb in the air (vehicle emissions) were higher in the RBCs than in the plasma. In a recent study, Quiroz et al. (2013)Quiroz W, Aguilar L, Barría M, Veneciano J, Martínez D, Bravo M, Lobos MG, Mercado L 2013. Sb(V) and Sb(III) distribution in human erythrocytes: speciation methodology and the influence of temperature, time and anticoagulants. Talanta 115: 902-910. spiked human blood samples (in vitro) with SbIII (APT) and SbV [KSb(OH)6] and demonstrated that both species penetrate the RBC membrane and leave the cell cytoplasm with time.
Many authors believe that some of the SbV that penetrates the erythrocyte is intracellularly reduced to SbIII, a form that is retained within the cell by forming complexes with organic ligands, such as glutathione (GSH) (Haldar et al. 2011Haldar AK, Sen P, Roy S 2011. Use of antimony in the treatment of leishmaniasis: current status and future directions. Mol Biol Int 2011: 571242.). A hypothesis has also been suggested that, while the initial rapid elimination (via urine) is governed by a major pool of Sb (or SbV in the case of MA and SSG) that remained in the extracellular medium (including plasma), the slow terminal phase is governed by an intracellular Sb pool, the mobilisation of which is slow (Friedrich et al. 2012Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75.).
The spleen was the organ that ranked first in a descending order of tissues according to Sb residual content after treatment with MA (Fig. 3). The marked accumulation of Sb in the spleen is possibly explained by some of the organ’s functions, such as to hold a reserve of blood and to remove senescent erythrocytes (Mebius & Kraal 2005Mebius RE, Kraal G 2005. Structure and function of the spleen. Nat Rev Immunol 5: 606-616.). Moreover, erythrophagocytosis by the spleen and liver macrophages and the scavenging of haemoglobin (and haptoglobin-bound haemoglobin) from the circulation by splenic macrophages play a key role in iron recycling (Mebius & Kraal 2005Mebius RE, Kraal G 2005. Structure and function of the spleen. Nat Rev Immunol 5: 606-616.). Antimony speciation and the fate (metabolomics) within the splenic and liver tissues, however, are still obscure questions. Spleen has been reported to be one of the tissues with the highest residual concentrations of Sb in mice treated with a single injection of SbIII (Molokhia & Smith 1969Molokhia MM, Smith H 1969. Tissue distribution of trivalent antimony in mice infected with Schistosoma mansoni. Bull World Health Organ 40: 123-128.) or SbV (Borborema et al. 2013Borborema SET, Osso Jr JA, de Andrade Jr HF, do Nascimento N 2013. Biodistribution of meglumine antimoniate in healthy and Leishmania (Leishmania) infantum chagasi-infected BALB/c mice. Mem Inst Oswaldo Cruz 108: 623-630.) drugs and in rats and mice exposed by the oral or i.p. route to SbIII (tartar emetic) for 90 days (Dieter et al. 1991Dieter MP, Jameson CW, Elwell MR, Lodge JW, Hejtmancik M, Grumbein SL, Ryan M, Peters AC 1991. Comparative toxicity and tissue distribution of antimony potassium tartrate in rats and mice dosed by drinking water or intraperitoneal injection. J Toxicol Environ Health 34: 51-82., Poon et al. 1998Poon R, Chu I, Lecavalier P, Valli VE, Foster W, Gupta S, Thomas B 1998. Effects of antimony on rats following 90-day exposure via drinking water. Food Chem Toxicol 36: 21-35.).
Among all rat tissues examined in this study, the brain (whole brain) had the lowest residual levels of Sb. Friedrich et al. (2012)Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75. also found that, in monkeys treated with MA, the central nervous system (CNS) structures (frontal and occipital lobes, parietal and temporal lobes, mesencephalon, medulla oblongata and cerebellum) were the tissues that exhibited the lowest levels of Sb. The brain also had the lowest residual levels of antimony in rodents treated with a single (Molokhia & Smith 1969Molokhia MM, Smith H 1969. Tissue distribution of trivalent antimony in mice infected with Schistosoma mansoni. Bull World Health Organ 40: 123-128.) or multiple doses of tartar emetic (Dieter et al. 1991Dieter MP, Jameson CW, Elwell MR, Lodge JW, Hejtmancik M, Grumbein SL, Ryan M, Peters AC 1991. Comparative toxicity and tissue distribution of antimony potassium tartrate in rats and mice dosed by drinking water or intraperitoneal injection. J Toxicol Environ Health 34: 51-82., Poon et al. 1998Poon R, Chu I, Lecavalier P, Valli VE, Foster W, Gupta S, Thomas B 1998. Effects of antimony on rats following 90-day exposure via drinking water. Food Chem Toxicol 36: 21-35.). These findings in rodents and non-human primates are consistent with the hypothesis that the blood-brain barrier prevents the penetration of SbIII and SbV into the brain.
A remarkable finding of this study was that the thyroid gland of rats treated with MA accumulated a high content of Sb and that no decline of Sb concentrations occurred in the organ over three post-treatment weeks. In the rat, the levels of Sb in the thyroid gland were higher than the levels in the liver and comparable to levels found in the bones (Fig. 3). Along the same line, Friedrich et al. (2012)Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75. had reported that, in monkeys treated with a low and a standard MA dosage regimen, the thyroid was the analysed tissue with the highest content of Sb. Although the marked accumulation of Sb by the thyroid during treatment with SbIII or SbV drugs remained almost unnoticed in the medical literature, it had already been noted in a few older studies with SbIII compounds. Brady et al. (1945)Brady FJ, Lawton AH, Cowie DB, Andrews HL, Ness AT, Ogden GE 1945. Localization of trivalent radioactive antimony following intravenous administration to dogs infected with Dirofilaria immitis. Am J Trop Med 25: 103-107., for instance, treated dogs infected with Dirofilaria immitis with APT (labelled with 124Sb) and found that while the liver ranked first in Sb content, combined thyroid and parathyroid were the tissues with the second largest accumulation of radioactive Sb. Kramer (1950)Kramer LB 1950. Antimony concentration in the thyroid gland and its effect upon metabolic rate and serum cholesterol level. B Johns Hopkins Hosp 86: 179-181. treated male rabbits with tartar emetic (once a day for 21 days i.v.) and noted that Sb concentrations in the thyroid were appreciably higher than those in any other tissue (kidneys, muscle and spleen) with the exception of the liver. According to Kramer (1950)Kramer LB 1950. Antimony concentration in the thyroid gland and its effect upon metabolic rate and serum cholesterol level. B Johns Hopkins Hosp 86: 179-181., Sb accumulation in the rabbit thyroid was not accompanied by changes of gland function or histology. In human volunteers who received sodium antimony mercapto-succinate (labelled with 124Sb) by the i.v. route, Abdallah and Saif (1962)Abdallah A, Saif M 1962. Trace studies with antimony 124 in man. In GEW Wolstenholme, M O’Connor (eds.), Bilharziasis, Ciba Foundation Symposium, Churchill, London, p. 287-309. noted that the highest radioactivity was recorded in the liver, followed by that in the thyroid and in the heart. Poon et al. (1998)Poon R, Chu I, Lecavalier P, Valli VE, Foster W, Gupta S, Thomas B 1998. Effects of antimony on rats following 90-day exposure via drinking water. Food Chem Toxicol 36: 21-35. did not determine Sb levels in the gland of rats exposed to APT, but they reported some treatment-related histological abnormalities, such as reduced follicle size, increased epithelial height and nuclear vesiculation, all of which are morphological changes that have been interpreted as reflecting a mild adaptive change of thyroid function of minor or no toxicological importance (Lynch et al. 1999Lynch BS, Capen CC, Nestmann ER, Veenstra G, Deyo JA 1999. Review of subchronic/chronic toxicity of antimony potassium tartrate. Regul Toxicol Pharmacol 30: 9-17.). At any rate, localisation of Sb forms within the thyroid tissue and possible influences of Sb accumulation on gland function deserve further studies.
The Sb levels in epididymis and prostate fell markedly over the three post-treatment weeks so that residual levels of Sb in all male reproductive organs (epididymis, prostate and testes) were consistently low three weeks after the end of treatment with MA. The low residual levels of Sb in male reproductive organs is consistent with our previous findings, suggesting that treatment of rats during gestation and lactation periods with MA (doses up to 300 mg SbV/kg body wt/d, s.c.) did not affect offspring sperm parameters and male fertility in adulthood (Coelho 2010Coelho DR 2010. Desenvolvimento somático, neurocomportamental e fertilidade da prole de ratos exposta ao antimoniato de meglumina pela via transplacentária e leite materno, MSc Dissertation, Fiocruz, Rio de Janeiro, 72 pp.).
Stomach, small intestines and pancreas were among the rat organs that presented the lowest residual content of Sb (< 5 µg/g) after a 21-day course of treatment with MA. Friedrich et al. (2012)Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75. did not measure Sb content in the small intestines, but found that stomach, colon and pancreas were among the analysed monkey tissues that had the lowest concentrations of Sb 55 and 95 days after a 21-day treatment with MA. Molokhia and Smith (1969)Molokhia MM, Smith H 1969. Tissue distribution of trivalent antimony in mice infected with Schistosoma mansoni. Bull World Health Organ 40: 123-128., however, found that colon, duodenum and stomach were, following liver and spleen, the murine tissues with the most elevated Sb levels 0.5 h after a single i.p. injection of tartar emetic. Borborema et al. (2013)Borborema SET, Osso Jr JA, de Andrade Jr HF, do Nascimento N 2013. Biodistribution of meglumine antimoniate in healthy and Leishmania (Leishmania) infantum chagasi-infected BALB/c mice. Mem Inst Oswaldo Cruz 108: 623-630. reported that, in mice treated with a single i.p. injection of irradiated MA (122Sb, 124Sb), % of IA in the small intestines fell from nearly 13% (3 min, non-infected mice) to less than 2% within 24 h of dosing, while in the large intestines % IA was approximately 1% at 0.5 h, rose to approximately 16% at 2 h and fell to approximately 2% at 24 h after drug administration. The authors interpreted the foregoing findings as reflecting a primary elimination of Sb through hepatobiliary excretion after liver processing.
It should be noted, however, that both Borborema et al. (2013)Borborema SET, Osso Jr JA, de Andrade Jr HF, do Nascimento N 2013. Biodistribution of meglumine antimoniate in healthy and Leishmania (Leishmania) infantum chagasi-infected BALB/c mice. Mem Inst Oswaldo Cruz 108: 623-630. and Molokhia and Smith (1969)Molokhia MM, Smith H 1969. Tissue distribution of trivalent antimony in mice infected with Schistosoma mansoni. Bull World Health Organ 40: 123-128. injected a SbV and a SbIII drug, respectively, into the peritoneal cavity, where liver, pancreas, stomach and small and large intestines are located. Under those conditions, the Sb levels determined by the authors may eventually reflect not only the Sb that reached the tissue indirectly via systemic circulation, but also the Sb that was absorbed directly by the tissue at the site of injection. Moreover, Borborema et al. (2013)Borborema SET, Osso Jr JA, de Andrade Jr HF, do Nascimento N 2013. Biodistribution of meglumine antimoniate in healthy and Leishmania (Leishmania) infantum chagasi-infected BALB/c mice. Mem Inst Oswaldo Cruz 108: 623-630. interpretation that during the fast elimination phase (within 12 h of the injection) Sb from MA is cleared primarily through hepatobiliary excretion is at odds with data provided by most studies. In fact, results from several studies are consistent with the notion that during rapid elimination phase, SbIII is excreted via bile and to a lesser extent via urine, whereas the reverse holds true for SbV. Bailly et al. (1991)Bailly R, Lauwerys R, Buchet JP, Mahieu P, Konings J 1991. Experimental and human studies on antimony metabolism: their relevance for the biological monitoring of workers exposed to inorganic antimony. Br J Ind Med 48: 93-97., for instance, demonstrated that after a single i.v. administration of SbIII (APT) to rats, about the same percentage (50%) of the administered Sb was excreted in the urine and faeces, whereas after i.p. injection, about four times more Sb was excreted in the faeces than in the urine. The hepatobiliary transport of SbIII is GSH-dependent. Along this line, it was described that the i.v. administration of APT increased up to 50-fold the biliary excretion of non-protein thiols (mainly GSH) by the rat (Gyurasics et al. 1992Gyurasics A, Koszorús L, Varga F, Gregus Z 1992. Increased biliary excretion of glutathione is generated by the glutathione-dependent hepatobiliary transport of antimony and bismuth. Biochem Pharmacol 44: 1275-1281., Gregus et al. 1998Gregus Z, Gyurasics A, Koszorús L 1998. Interactions between selenium and group Va-metalloids (arsenic, antimony and bismuth) in the biliary excretion. Environ Toxicol Pharmacol 5: 89-99.). Transport of SbV from MA into the bile apparently requires its reduction to SbIII and the intracellular reduction of SbV is promoted by GSH and other thiols found in the cytosol (Ferreira et al. 2003Ferreira C dos S, Martins PS, Demicheli C, Brochu C, Ouellette M, Frézard F 2003. Thiol-induced reduction of antimony(V) into antimony(III): a comparative study with trypanothione, cysteinyl-glycine, cysteine and glutathione. Biometals 16: 441-446.). Based on the foregoing, it seems plausible to think that hepatobiliary excretion plays a major role in the elimination of residual Sb (mainly as SbIII) during the slow terminal elimination phase after a course of treatment with SbV drugs. Nonetheless, Borborema et al. (2013)Borborema SET, Osso Jr JA, de Andrade Jr HF, do Nascimento N 2013. Biodistribution of meglumine antimoniate in healthy and Leishmania (Leishmania) infantum chagasi-infected BALB/c mice. Mem Inst Oswaldo Cruz 108: 623-630. hypothesis that SbV is excreted primarily in the bile during the rapid elimination phase needs to be substantiated by experimental data.
In this study, the bone was the tissue with the second highest Sb concentration after spleen and next to thyroid, while the skeletal muscle ranked among rat tissues with the lowest levels of Sb. In monkeys treated with MA, both bone (femur) and skeletal muscle were among the tissues classified by Friedrich et al. (2012)Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75. as having accumulated “intermediate” levels of Sb. As far as the authors are aware, these are the only two studies that measured Sb residual levels in bone and muscle after a treatment course with MA.
In conclusion, the decline of Sb blood levels with time after a parenteral injection of MA can be described by a two-compartment model, with a fast elimination phase the half-life of which was 0.6 h and a very slow terminal elimination phase, the half-life of which is longer than 24 h. A course of treatment of rats with 24 h spaced doses of MA, therefore, results in a gradual increase of nadir Sb levels in blood, a kinetic behaviour similar to that described for humans, non-human primates, dogs and mice. This kinetic similarity with humans makes the rat a suitable model for studies of SbV distribution in tissues and toxicity. Furthermore, data from this study also showed that during the terminal elimination phase, the highest residual concentrations were found in the spleen, bones, thyroid gland and the liver. The levels in the kidneys were high at the end of treatment but decline sharply within three post-treatment weeks, a fall that is consistent with the notion renal excretion plays a major role in the clearance of Sb in the fast elimination phase. Sb residual levels were particularly low in the brain. The pathophysiological consequences of Sb accumulation in the thyroid gland and the localisation of Sb forms within the liver, thyroid, spleen and bones warrant further studies.
ACKNOWLEDGEMENTS
To Rosangela De-Carvalho (blood sampling) and Rafael CC Rocha (ICP-MS analysis), for the technical assistance. The authors dedicate this paper to late Prof Norbert Fritz Miekeley, who introduced them in the study of kinetics of antimony-based drugs.
REFERENCES
- Abdallah A, Saif M 1962. Trace studies with antimony 124 in man. In GEW Wolstenholme, M O’Connor (eds.), Bilharziasis, Ciba Foundation Symposium, Churchill, London, p. 287-309.
- Bailly R, Lauwerys R, Buchet JP, Mahieu P, Konings J 1991. Experimental and human studies on antimony metabolism: their relevance for the biological monitoring of workers exposed to inorganic antimony. Br J Ind Med 48: 93-97.
- Borborema SET, Osso Jr JA, de Andrade Jr HF, do Nascimento N 2013. Biodistribution of meglumine antimoniate in healthy and Leishmania (Leishmania) infantum chagasi-infected BALB/c mice. Mem Inst Oswaldo Cruz 108: 623-630.
- Brady FJ, Lawton AH, Cowie DB, Andrews HL, Ness AT, Ogden GE 1945. Localization of trivalent radioactive antimony following intravenous administration to dogs infected with Dirofilaria immitis Am J Trop Med 25: 103-107.
- Christopherson JB 1918. The successful use of antimony in bilharziosis. Lancet 7: 325-327.
- Christopherson JB 1923. The curative dose of antimony tartrate in schistosomiasis (bilharzia disease). Br Med J 2 (3287): 1254-1255.
- Chulay JD, Fleckenstein L, Smith DH 1988. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans R Soc Trop Med Hyg 82: 69-72.
- Coelho DR 2010. Desenvolvimento somático, neurocomportamental e fertilidade da prole de ratos exposta ao antimoniato de meglumina pela via transplacentária e leite materno, MSc Dissertation, Fiocruz, Rio de Janeiro, 72 pp.
- Di Cristina G, Caronia G 1915. Sulla terapia della leishmaniosi interna. Bulletin de la Société de Pathologie Éxotique 8: 63-65.
- Dieter MP, Jameson CW, Elwell MR, Lodge JW, Hejtmancik M, Grumbein SL, Ryan M, Peters AC 1991. Comparative toxicity and tissue distribution of antimony potassium tartrate in rats and mice dosed by drinking water or intraperitoneal injection. J Toxicol Environ Health 34: 51-82.
- Ferreira C dos S, Martins PS, Demicheli C, Brochu C, Ouellette M, Frézard F 2003. Thiol-induced reduction of antimony(V) into antimony(III): a comparative study with trypanothione, cysteinyl-glycine, cysteine and glutathione. Biometals 16: 441-446.
- Friedrich K, Vieira FA, Porrozzi R, Marchevsky RS, Miekeley N, Grimaldi Jr G, Paumgartten FJ 2012. Disposition of antimony in rhesus monkeys infected with Leishmania braziliensis and treated with meglumine antimoniate. J Toxicol Environ Health A 75: 63-75.
- Goodwin LG, Page JE 1943. A study of the excretion of organic antimonials using a polarographic procedure. Biochem J 37: 198-209.
- Gregus Z, Gyurasics A, Koszorús L 1998. Interactions between selenium and group Va-metalloids (arsenic, antimony and bismuth) in the biliary excretion. Environ Toxicol Pharmacol 5: 89-99.
- Gyurasics A, Koszorús L, Varga F, Gregus Z 1992. Increased biliary excretion of glutathione is generated by the glutathione-dependent hepatobiliary transport of antimony and bismuth. Biochem Pharmacol 44: 1275-1281.
- Haldar AK, Sen P, Roy S 2011. Use of antimony in the treatment of leishmaniasis: current status and future directions. Mol Biol Int 2011: 571242.
- Kramer LB 1950. Antimony concentration in the thyroid gland and its effect upon metabolic rate and serum cholesterol level. B Johns Hopkins Hosp 86: 179-181.
- Lynch BS, Capen CC, Nestmann ER, Veenstra G, Deyo JA 1999. Review of subchronic/chronic toxicity of antimony potassium tartrate. Regul Toxicol Pharmacol 30: 9-17.
- Mebius RE, Kraal G 2005. Structure and function of the spleen. Nat Rev Immunol 5: 606-616.
- Miekeley N, Mortari SR, Schubach AO 2002. Monitoring of total antimony and its species by ICP-MS and on-line ion chromatography in biological samples from patients treated for leishmaniasis. Anal Bioanal Chem 372: 495-502.
- Miranda ES, Miekeley N, De-Carvalho RR, Paumgartten FJ 2006. Developmental toxicity of meglumine antimoniate and transplacental transfer of antimony in the rat. Reprod Toxicol 21: 292-300.
- Molokhia MM, Smith H 1969. Tissue distribution of trivalent antimony in mice infected with Schistosoma mansoni Bull World Health Organ 40: 123-128.
- Nieto J, Alvar J, Mullen AB, Carter KC, Rodríguez C, San Andrés MI, San Andrés MD, Baillie AJ, González F 2003. Pharmacokinetics, toxicities and efficacies of sodium stibogluconate formulations after intravenous administration in animals. Antimicrob Agents Chemother 47: 2781-2787.
- Otto GF, Maren TH, Brown HW 1947. Blood levels and excretion rates of antimony in persons receiving trivalent and pentavalent antimonials. Am J Hyg 46: 193-211.
- Poon R, Chu I, Lecavalier P, Valli VE, Foster W, Gupta S, Thomas B 1998. Effects of antimony on rats following 90-day exposure via drinking water. Food Chem Toxicol 36: 21-35.
- Quiroz W, Aguilar L, Barría M, Veneciano J, Martínez D, Bravo M, Lobos MG, Mercado L 2013. Sb(V) and Sb(III) distribution in human erythrocytes: speciation methodology and the influence of temperature, time and anticoagulants. Talanta 115: 902-910.
- Quiroz W, De Gregori I, Basilio P, Bravo M, Pinto M, Lobos MG 2009. Heavy weight vehicle traffic and its relationship with antimony content in human blood. J Environ Monit 11: 1051-1055.
- Radwan MA, Al Jaser MH, Al Rayes ZR 2007. The effects of induced diabetes and cutaneous Leishmania infection on the pharmacokinetics of antimony in hamsters. Ann Trop Med Parasitol 101: 133-142.
- Roberts WL, McMurray WJ, Rainey PM 1998. Characterization of the antimonial antileishmanial agent meglumine antimonate (glucantime). Antimicrob Agents Chemother 42: 1076-1082.
- Tassi P, Ormas P, Madonna M, Carli S, Belloli C, De Natale G, Ceci L, Marcotrigiano GO 1994. Pharmacokinetics of N-methylglucamine antimoniate after intravenous, intramuscular and subcutaneous administration in the dog. Res Vet Sci 56: 144-150.
- Vianna G 1912. Tratamento da leishmaniose tegumentar por injeções intravenosas de tártaro emético. Anais do VII Congresso Brasileiro de Medicina e Cirurgia 4: 426-428.
-
Financial support: FAPERJ, CNPq, INOVA-ENSP
Publication Dates
-
Publication in this collection
July 2014
History
-
Received
24 Jan 2014 -
Accepted
4 June 2014