Abstracts
Xyliphius anachoretes, a new species of aspredinid catfish is described from the Tocantins-Araguaia River system. Xyliphius anachoretes is diagnosed by the presence of six developed retrorse serrae on posterior border of pectoral-fin spine, presence of papillae on the lower lip bearing minute branches, and only two dorsal procurrent rays. Comments about the informativeness of character-state variation among Xyliphius species and aspredinid related genera are furnished. Also, a brief discussion about conservation status of the new taxon is made.
Xyliphius; Serra da Mesa Dam; Brazilian shield
Xyliphius anachoretes, uma nova espécie de bagre aspredinídeo é descrita para o sistema Tocantins-Araguaia. Xyliphius anachoretes é diagnosticado pela presença de seis serras retrorsas desenvolvidas na borda posterior do espinho da nadadeira peitoral, presença de papilas no lábio inferior apresentando minúsculas ramificações, e somente dois raios pró-correntes dorsais. Comentários sobre a informação contida na variação de estados de caráter entre as espécies de Xyliphius são fornecidos. Além disso, uma breve discussão sobre o estado de conservação do novo táxon é feita.
A new species of Xyliphius, a rarely sampled banjo catfish (Siluriformes: Aspredinidae) from the rio Tocantins-Araguaia system
Carlos A. FigueiredoI,II; Marcelo R. BrittoII
INúcleo de Gestão Ambiental, Instituto de Biociências, Universidade Federal do Estado do Rio de Janeiro. Av. Pasteur, 458, sala 512-F, 22290-240 Rio de Janeiro, RJ, Brazil. carlos.figueiredo@gmail.com
IIDepartamento de Vertebrados, Museu Nacional/Universidade Federal do Rio de Janeiro. Quinta da Boa Vista, s/n, 20940-040 Rio de Janeiro, RJ, Brazil. mrbritto2002@yahoo.com.br
ABSTRACT
Xyliphius anachoretes, a new species of aspredinid catfish is described from the Tocantins-Araguaia River system. Xyliphius anachoretes is diagnosed by the presence of six developed retrorse serrae on posterior border of pectoral-fin spine, presence of papillae on the lower lip bearing minute branches, and only two dorsal procurrent rays. Comments about the informativeness of character-state variation among Xyliphius species and aspredinid related genera are furnished. Also, a brief discussion about conservation status of the new taxon is made.
Key words: Xyliphius, Serra da Mesa Dam, Brazilian shield.
Xyliphius anachoretes, uma nova espécie de bagre aspredinídeo é descrita para o sistema Tocantins-Araguaia. Xyliphius anachoretes é diagnosticado pela presença de seis serras retrorsas desenvolvidas na borda posterior do espinho da nadadeira peitoral, presença de papilas no lábio inferior apresentando minúsculas ramificações, e somente dois raios pró-correntes dorsais. Comentários sobre a informação contida na variação de estados de caráter entre as espécies de Xyliphius são fornecidos. Além disso, uma breve discussão sobre o estado de conservação do novo táxon é feita.
Introduction
The banjo catfish genus Xyliphius was established by Eigenmann (1912) to include a single species, X. magdalenae Eigenmann, from río Magdalena basin, Girardot, Colombia. That description was based on a single specimen, 32.0 mm SL. Later, four species were described in a short time frame: X. lepturus and X. melanopterus from western headwaters of río Bobonaza, upper Amazon basin (Orcés, 1962), X. barbatus (Alonso de Arámburu & Arámburu, 1962), and X. lombarderoi (Risso & Risso, 1964), from río Paraná, in Argentina. A sixth species, X. kryptos, was described by Taphorn & Lilyestrom (1983), from río Aricuaisá, lago de Maracaibo basin, Venezuela. Further collections, though small, expanded the distribution of species of Xyliphius beyond their type localities (Cala, 1977). Currently, the six nominal species of Xyliphius are known from northern South America in the río Magdalena system (X. magdalenae), lago de Maracaibo (X. kryptos), and western headwaters of the Amazonas basin and río Orinoco (X. lepturus and X. melanopterus), and from southern South America in the río de La Plata system (X. barbatus and X. lombarderoi) (Taphorn & Lilyestrom, 1983; Galvis et al., 1997; Calviño & Castello, 2008).
Only Friel (1994) has completed a phylogenetic analysis of Aspredinidae. He recognized Xyliphius as the sister group of the tribe Hoplomyzontini, composed of the genera Hoplomyzon Myers, Micromyzon Friel, Dupouyichthys Schultz and Ernstichthys Fernández-Yépez. A formal diagnosis of the genus Xyliphius has not been published in a peerreviewed journal, but all species described so far share the following exclusive features among aspredinids (Friel, 1994): eyes highly reduced; premaxilla toothless and displaced lateral to mesethmoid; row of fleshy papillae projecting anteriorly from lower lip; unculi and unculiferous tubercles flattened; lamina of pterotic rounded; and lateral end of posterohyal expanded. Other characters not unique to this taxon but useful for identification include: anterior nare opening with fleshy papillae; coronomeckelian bone absent; Meckel's cartilage with high ascending process; and pre-axial serrations absent.
Extensive fish surveys of the upper rio Tocantins system from 1996-2003 yielded a single specimen of Xyliphius from the mouth of the rio Preto. Two years later (2005), another single, small specimen (25.6 mm SL) of the genus was discovered in the rio Araguaia basin, nearly 195 air km from the first locality. Examination of both specimens confirms their conspecificity and distinctiveness from nominal Xyliphius. This new species described herein is the first Xyliphius from lower Amazon basin and first record of the genus for the central portion of Brazilian shield.
Despite seven years of intensive collection efforts during fish surveys of the Serra da Mesa Dam from the same region where the holotype was collected, no other specimens were found. Specimens of another aspredinid species, however, Bunocephalus cf. aleuropsis were extensively collected in the same region and during the same period. This possibly indicates different life histories of these two aspredinids on the rio Tocantins-Araguaia system. Accordingly, at least in Venezuela, Xyliphius live buried in the sand and perhaps gravel of swift flowing streams, whereas Bunocephalus are typically found in slow moving streams, often among leaf litter (anonymous reviewer, pers. comun.).
Material and Methods
Morphometric and meristic data were taken following Stewart (1985), plus prepectoral length, posterior internareal distance, length between anterior and posterior nares, length between posterior nare and orbit. Meristic data of paired fins were collected from both sides of the body. Number of oral papillae on lower jaw was counted along its entire border. Pectoral-spine serrae, procurrent caudal-fin rays and vertebral counts were taken from digital radiographs of the holotype, obtained by a Faxitron digital specimen radiography system model MX20-DC44. Vertebral counts include only free centra, with the compound caudal centrum (preural 1 + ural 1) counted as a single element. Osteological terminology follows Friel (1995), except that parieto-supraoccipital is used instead of supraoccipital (Arratia & Gayet, 1995). Specimens not available for direct observation were examined through photographs and/or radiographs. Institutional abbreviations are according to Ferraris (2007), plus CZUT-IC, Colección Zoológica, Universidad del Tolima, Colômbia; DU, Duke University, Vertebrate Collection, Durham, North Carolina, U.S.A.; OV, G. Orcés Villagomez particular collection, Quito, Ecuador.
Xyliphius anachoretes, new species Fig. 1
Holotype. MNRJ 31923, 88.4 mm SL, Brazil, Goiás, rio Tocantins below the dam of Serra da Mesa Hydropower Plant, in a marginal pond at the mouth of rio Preto, an affluent of the right bank of rio Tocantins (currently under water of the Cana Brava Hydropowerplant reservoir), 13º37'51''S 48º07'01''W, collected in daylight, 3Dec 1996, D. F. Moraes (Equipe Ímpar). Paratype. MZUSP 89546, 25.6 mm SL, Brazil, Goiás, municípios de Crixás/Santa Terezinha de Goiás, rio Crixás-açu, under bridge at the state road GO-465, 14º26'26''S 49º42'37''W, 28 Jul 2005, Equipe CBE.
Diagnosis. Xyliphius anachoretes is diagnosed from its congeners, except X. magdalenae, by the presence of six developed retrorse serrae along posterior border of pectoral spine (vs. 7-9 in X. melanopterus and X. barbatus, 8 in X. lombarderoi, 8-10 in X. lepturus and X. kryptos). Xyliphius anachoretes differs from X. magdalenae by the lower number of total free vertebrae (31 vs. 33-36).
Xyliphius anachoretes is further distinguished, except from X. kryptos, by papillae of lower lip bearing minute branches (vs. large branches in X. lepturus, X. barbatus, X. lombarderoi, and X. melanopterus, or unbranched papillae in X. magdalenae) (Fig. 2 ); and, except from X. magdalenae and X. kryptos, by the presence of 22 or 24 papillae on lower lip (vs. 25-28 in X. lepturus, 27-29 in X. melanopterus, 27-30 in X. barbatus, and 28 in X. lombarderoi). Besides, Xyliphius anachoretes is also promptly distinguished by the lower number of dorsal procurrent rays (two) from X. lepturus (four or five), X. melanopterus (three), and X. magdalenae (four).
Description. Morphometric data presented in Table 1. Head depressed, roughly triangular with bluntly rounded snout in dorsal view. Skull ornamentation inconspicuous. Dorsal profile straight from tip of snout to origin of caudal fin, except for shallow convexity from posterior tip of parietosupraoccipital to posterior base of dorsal fin. Ventral profile convex from tip of snout to pectoral girdle region, then straight to pelvic-fin origin, shallowly concave from pelvics to analfin origin, ascending obliquely along anal-base and finishing straight along caudal peduncle. Body depressed anteriorly, becoming gradually compressed towards caudal fin. Entire body banjo-shaped in dorsal/ventral view, greatest width at cross-section through region just anterior to pectoral-spine insertions.
Eye very small, diameter 1.5% (holotype) or 2.4% (paratype) of head length, without free orbital margin; located dorsally in anterior half of head. Two nares, anterior one tubular with four separate papillae along its lateral (lower) margin, located at anterior edge of snout; outermost papillae smaller than internal ones. Posterior nare as small opening located at midpoint between anterior nare and eye. One maxillary barbel on side of snout, inserted just above rictus. Maxillary barbel reaching pectoral-fin base. Two mental barbels, both smaller than maxillary. Inner mental barbel mental barbel, separated from it by distance greater than smallest, its length shorter than half-length of outer mental inner mental barbel length. Mouth subterminal, wider than barbel. Inner mental barbel located just posterior to mouth snout with 22 (paratype) or 24 (holotype) fleshy, finger-like opening, and at distance of one-quarter of mouth width from papillae along border of lower lip. Almost all papillae the midventral axis of head. Outer mental barbel half the length same size and weakly dendritic with one to three minute of maxillary barbel, reaching or slightly surpassing branches (Fig. 2b). Anterior margin of snout with shallow transverse through mouth opening when adducted anteriorly. medial notch (Fig. 2b). Gill opening small, restricted to Outer mental barbel located on transverse posterior to inner valvular slit, covered by fleshy skin flap on ventral surface fifth pore located just in front of posterior naris. Two mesial pores, corresponding to epiphyseal and parietal branches, respectively. Four pores of the infraorbital branch of laterosensory system; anteriormost dorsally close to rictus. Five pores of the preoperculomandibular branch; anteriormost pore ventrally close to rictus. Lateral line with 17/18 pores on left and right sides, respectively. Lateral line not extending onto caudal fin.
Total number of vertebrae 31, precaudal vertebrae 11, caudal vertebrae 20. Six pairs of ribs, first pair articulated with parapophyses of first free (sixth) centrum, and slightly larger than others. Ribs gradually decreasing in size caudally.
Dorsal fin I,3,i; roughly triangular, located slightly anterior to mid-standard length. Dorsal spinelet absent. Dorsal spine (second dorsal lepidotrichia) feeble. Anal fin ii,4,i; roughly semi-ovoid, its origin just posterior to tip of longest ray of i,4/4,i; dorsal and ventral procurrent caudal-fin rays 2 and 4, respectively.
Color in alcohol. Ground color of body and head brown, ventral region slightly lighter than dorsal. Irregular light brown middorsal stripe on head from tip of snout to transverse through pectoral-fin origins; stripe widest in interorbital region. All barbels yellowish light brown. Two elongate, irregular, light brown blotches along dorsal midline of body: anterior one small, just in front of dorsal-fin base, posterior one large, extending from base of last dorsal-fin ray to vertical through midlength of anal-fin base. All fin rays and membranes dark brown; borders yellowish white.
Distribution. Xyliphius anachoretes is currently known only from two localities, one in the upper rio Tocantins (type locality) and the second from the upper rio Araguaia (Fig. 4).
Ecological notes. Both specimens were captured by day in shallow waters. Due to its diminished eyes and rough skin somewhat free from underlying muscles, together with its extreme rarity in collections, we speculate that Xyliphius anachoretes is a cryptic fossorial species.
Etymology. The specific epithet of Xyliphius anachoretes is from the Greek anachoretes, meaning ''one that retired from the world'', hermit, recluse, in allusion to the rarity of the only two specimens known, each one found alone and far apart. An adjective.
Discussion
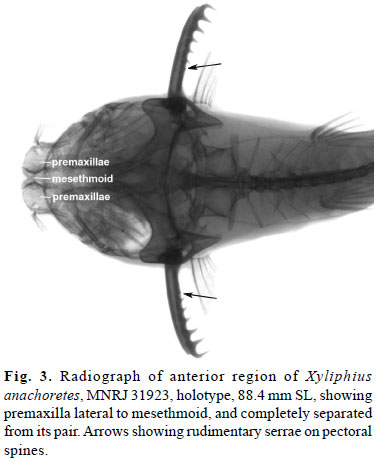
At least three synapomorphies of Xyliphius (Friel, 1994) are observed in the new taxon: 1) premaxillae separated from one another and displaced completely lateral to mesethmoid (Fig. 3); 2) row of fleshy papillae projecting anteriorly from lower lip (Fig. 2); and 3) unculi and unculiferous tubercles flattened. However, several anatomical features obtained through radiographs and external morphology show variation among Xyliphius species. The row of fleshy papillae on lower lip, unique to Xyliphius, is separable into at least two characters among representatives of the genus: the number of papillae, and the degree of development of its branches. Xyliphius anachoretes shares with X. magdalenae and X. kryptos the presence of 22 or 24 papillae on lower lip (recorded specimens of X. kryptos with 22, 24, and 27 papillae; Taphorn & Lilyestrom, 1983), which is the lowest count among their congeners (i.e., 25-28 in X. lepturus, 27-30 in X. barbatus, 28 in X. lombarderoi, and 27-29 in X. melanopterus). Although counts of the new species and X. kryptos might suggest variation related to size, these values are still the lowest of the genus. Furthermore, in the present study, such a variation was not observed in other examined species. Records of a size series with large SL range of X. barbatus (44.5-93.5 mm SL, Calviño & Castello, 2008: 56) reveal count numbers (2830) markedly higher than the range of X. anachoretes and X. kryptos. In the original description of X. melanopterus, Orcés (1962) recorded 24 papillae on the lower lip of the holotype (p.53), which was the only specimen available at that time. That count differs from our examination of the holotype. However, in the same page, Orcés (1962) remarked that the lower lip has 24 papillae, except by one or two on the rictus (p.53: ''Labio inferior con 24 papilas ramificadas, salvo una o dos de las rictales''). Adding the rictal papillae (1+2) to the 24 counted by Orcés equals exactly the number (27) observed herein.
The lack of material makes any inferences concerning the direction of change (increase/decrease on the number of lower-lip papillae) too speculative and premature, mainly due to absence of comparative information in other aspredinids. However, an additive character-state hypothesis could be tested against relevant ontogenetic sequences when they are available. This assertion could also be applied to the degree of development of lower-lip papillae branches. Among Xyliphius species, it is possible to observe four conditions of this character: unbranched papillae (X. magdalenae; Fig. 2a); papillae bearing minute branches (X. kryptos and X. anachoretes; Fig. 2b); papillae bearing developed branches (X. melanopterus; Fig. 2c); and papillae bearing many large branches (X. barbatus, X. lombarderoi and X. lepturus; Fig. 2d). Also, lack of ontogenetic sequences does not allow the formulation of a testable hypothesis.
Another character that helps to diagnose Xyliphius anachoretes, except from X. magdalenae, is the presence of six developed retrorse serrae along posterior border of pectoral-fin spine. A proximal rudiment of serrae (detectable only on x-ray imaging; Fig. 3, arrow) was not considered in the count. Orcés (1962: 53, and table 1) recorded six/seven serrae on pectoral spine for X. melanopterus (p. 53: ''Espina pectoral con 6/7 denticulaciones espiniformes en su borde posterior, inclusive una rudimentaria, cercana a la base''). However, the diagnosis in the same page reported six to eight serrae on pectoral spine (p. 53: ''(...) 6 a 8 denticulaciones tras la espina pectoral''). Examination of the holotype of X. melanopterus as reported herein reveals seven/eight serrae hooks on the left and right pectoral spine, respectively, plus a proximal minute serrae in each one, perceived with the help of a thin nail. In all specimens examined and for which x-ray images were taken, except X. magdalenae (USNM 120224), there was an extra rudiment of serrae not detected on manual examination, but only through x-ray imaging. Since there are no available x-ray images of the holotype of X. melanopterus, it is not possible to discard the presence of an additional rudimentary serrae. Xylihius magdalenae showed variation on the number of developed serrae on pectoral spine among specimens examined, ranging from four to nine, seemingly related to body length. However, this correlation was not observed in X. anachoretes, the two known specimens show six serrae each and have sizes of 88.4 mm SL (holotype), and 25.6 mm SL (paratype). Despite of this, it is worthy to note that this variation observed in X. magdalenae comprehends a smaller size range sample (32.0-73.0 mm SL) than X. anachoretes type series.
Among radiographed specimens, X. anachoretes presents fewer dorsal procurrent rays than the remaining species examined, X. magdalenae, X. melanopterus, and X. lepturus (two vs. three to five), and the total count of caudalfin rays is lower than all other specimens examined (16 vs. 17 to 21), but one specimen identified as X. melanopterus (FMNH 99483).
A more comprehensive study is needed to shed light on possible ontogenetic and geographic variation within species of the genus. Although currently known from only two specimens, it is timely to provide a scientific description of the new species with material at hand. Xyliphius anachoretes is the first record of the genus from the central portion of the Brazilian shield, and also from basins draining into the lower Amazon. The holotype was sampled during several exhaustive fish surveys in the upper portion of rio Tocantins system between 1996-2003, during the establishment of the Serra da Mesa Dam. Currently, the type locality is underwater in the Cana Brava Hydropower-plant reservoir that began operations in 2002. Considering the impact of transforming a fast-flowing river into a reservoir with still waters, we suspect that the present conditions of this portion of upper Tocantins does not support viable populations of Xyliphius anachoretes at its type locality. Furthermore, increasing demand for watershed spaces by the hydroelectric industry will probably lead to the construction of more dams in several drainages in Central Brazil, mainly rio Tocantins basin, to generate electric energy. This situation leads to alteration of watercourses, and subsequently, the potential loss of many undescribed or endemic species. Thus, documenting new species is a prerequisite for conservation measures. Furthermore, having the species formally named will hopefully stimulate search for additional specimens.
Material examined. Ernstichthys sp.: Brazil, Goiás State: MNRJ 12574, 1, 29.0 mm SL, Minaçu, rio Boa Nova, 13º52'S 48º21'W. Mato Grosso State: MZUSP 37814, 3, 1 c&s, 20.6-21.9 mm SL, Aripuanã, igarapé Ingazeiro, 20 km upstream from mouth of rio Canumã, rio Aripuanã, downstream from Dardanelos, 09º58'S 59º19'W. Hoplomyzon sexpapilostoma: Venezuela, Barinas: MCNG 5376, 1 paratype, c&s, 20.9 mm SL, Apure River drainage, río Masparro at site of Masparro Dam, 08º50'40''N 70º06'00''W. Xyliphius barbatus: Argentina, Santa Fe: MLP 6798, holotype, 92.0 mm SL, río Paraná in Rosario. Xyliphius lepturus: Ecuador, Napo: FMNH 99487, 2, 99.1-99.6 mm SL, río Napo, 10.7 km upstream from the bridge at Coca, in main stream, 0º32'36''S 77º02'54''W; FMNH 99489, 1, 107.1 mm SL, río Aguarico, few km upstream from mouth of río Eno, 0º11'S 76º30'W; FMNH 99491, 1, 115.1 mm SL, río Coca, downstream from río Sardinas confluence, 0º06'0''S 77º12'30''W. Xyliphius magdalenae: Colombia, Girardot: BMNH 1947.7.1.215.216, 2, 35.0-70.0 mm SL, x-rayed; FMNH 56039, holotype, 32.0 mm SL, x-rayed. Tolima, Honda: IAvH-P 4127, 1, 72.0 mm SL, río Magdalena, 05º15'N 74º50'W; USNM 120224, 2, x-rayed, 51.0-73.0 mm SL, CZUT-IC 1288, 1, 72.9 mm SL; Xyliphius melanopterus: Ecuador, Napo: DU T.31.79, 1 c&s, ca. 90.0 mm SL; FMNH 99492, 1, 101.1 mm SL, río Napo near Tiputini, 0º47'0''S 75º33'W; FMNH 99493, 1, 103.2 mm SL, río Aguarico, about 15-20 min downriver from Destacamento Zancudo, 0º33'S 75º27'W; MEPN 3025 (ex-OV 2021), holotype, 114.0 mm SL, bajo río Pucayacu no lejos de su boca en el Bobonaza, cerca de Montalvo, aprox. 02º04'S 76º58'W.
Acknowledgements
We are grateful to E. P. Caramaschi (UFRJ) for providing the holotype of Xyliphius anachoretes collected during the Project: ''Estudos Básicos sobre a Ictiofauna do AHE Serra da Mesa, GO'', supported by the partnership Furnas-Serra da Mesa Energia, through Fundação BIORIO/UFRJ. We thank J. Maldonado-Ocampo (IAvH, MNRJ), O. Oyakawa and F. Lima (MZUSP), Mary Ann Rogers (FMNH), Donald Taphorn (MCNG) for the loan of specimens, and J. Maclaine (BMNH) for kind reception at that institution. S. Raredon (USNM) and R. Barriga (MEPN) kindly provided photographs of Xyliphius magdalenae and X. melanopterus deposited at their respective institutions. We are also indebted to A. Netto-Ferreira (MZUSP) who carefully photographed and took counts of the holotype of X. melanopterus during his visit to MEPN. F. Lima (MZUSP) also photographed the holotype of X. magdalenae during his visit to FMNH. Funds from the All Catfish Species Inventory (NSF DEB-0315963) provided important information in the form of images, access to rare literature and financial support for fieldwork. CAF benefited from CNPq grant number 155636/2006-5. MRB received financial support from CNPq (grants 557321/2005-0, 309711/ 2006-1, 503572/2008-0, 478804/2008-3, and 477414/2009-5) and FAPERJ (grant E-26/170.687/2007).
Literature Cited
Accepted December 18, 2009
Published March 31, 2010
- Alonso de Arámburu, A. S. & R. H. Arámburu. 1962. Una nueva especie de Xyliphius de la Argentina (Siluriformes, Bunocephalidae). Physis, 23: 219-222.
- Arratia, G. & M. Gayet. 1995. Sensory canals and related bones of tertiary siluriform crania from Bolivia and North America and comparison with recent forms. Journal of Vertebrate Paleontology, 15: 482-505.
- Cala, P. 1977. Los peces de la Orinoquia Colombiana: lista preliminar anotado. Lozania, Acta Zoológica Colombiana, 24: 1-21.
- Calviño, P. A. & H. P. Castello. 2008. Sobre un bagre ciego del Río Paraná médio, Xyliphius barbatus Arámburu y Arámburu, 1962 (Siluriformes: Aspredinidae) uma nueva cita em la Argentina y comentarios adicionales. Lãs Ciências, Revista de la Universidad Maimónides, 1: 55-59.
- Eigenmann, C. H. 1912. Some results from an ichthyological reconnaissance of Colombia, South America. Part I. Contributions to Zoology of the Laboratory of Indiana University, 127: 1-27.
- Ferraris, C. J., Jr. 2007. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa, 1418: 1-300.
- Friel, J. P. 1994. A phylogenetic study of the Neotropical banjo catfishes (Teleostei: Siluriformes: Aspredinidae). Unpublished Ph.D. Dissertation, Durham, Duke University, 256p.
- Friel, J. P. 1995. Micromyzon akamai, gen. et sp. nov., a small and eyeless banjo catfish (Siluriformes: Aspredinidae) from the river channels of the lower Amazon basin. Copeia, 1996(3): 641 648.
- Galvis, G., J. I. Mojica & M. Camargo. 1997. Peces Del Catatumbo. Santafé de Bogotá, Sistemas Holograma, 118p.
- Miles, C. 1942. Rediscovery of the Bunocephalid catfish Xyliphius in the Río Magdalenae, Colombia. Stanford Ichthyological Bulletin, 2: 115-117.
- Orcés, V. G. 1962. Dos nuevos peces del genero Xyliphius Ciencias Naturales, 5: 50-54.
- Reis, R. E., S. O. Kullander & C. J. Ferraris, Jr. 2003. Check List of the Freshwater Fishes of South and Central America, Edipucrs, Porto Alegre, 729p.
- Risso, F. J. J. & E. N. P. de Risso 1964. Hallazgo de una nueva especie de Xyliphius en el Parana (Pisces - Aspredinidae). Notas del Museo de Ciencias Naturales del Chaco, 1: 11-16.
- Stewart, D. J. 1985. A review of the South American catfish tribe Hoplomyzontini (Pisces, Aspredinidae), with descriptions of new species from Ecuador. Fieldiana Zoology, 25: 1-19.
- Taphorn, D. C. & C. G. Lilyestrom. 1983. Un nuevo pez del genero Xiliphius (Aspredinidae) de Venezuela. Revista Unellez Ciencia y Tecnología, 1: 43-44.
Publication Dates
-
Publication in this collection
19 Apr 2010 -
Date of issue
Mar 2010
History
-
Received
18 Dec 2009 -
Accepted
31 Mar 2010