Abstracts
The present study analyzed the length-weight relationship and some aspects of the reproductive biology of Anableps anableps from the mouth of the Maracanã River, in the Brazilian state of Pará. The specimens were collected using two 30 m-long gillnets with 15 and 20 mm mesh size, as well as a 1 m-diameter hand net with a 10 mm mesh. A total of 865 specimens were collected, from which an adult sex ratio of 2.12 females per male was recorded (χ² = 13.07; p<0.05). Females presented positively allometric growth, whereas males were negatively allometric. In the additional analyses, the gonads of 371 female specimens were observed microscopically. Gonadal development was classified in three stages: immature (5.0%), maturing (12.0%), and mature (83.0%). Embryonic development was classified in five phases, according to the size of the embryo and the vitelline sac. Mean fecundity was 12 eggs/embryos per female (range: 1-37 eggs/embryos). A significant relationship was recorded between the standard length of females and ovaries weight (R² = 0.257; p < 0.001), and the number of embryos carried (R² = 0.573; p < 0.001). Mean body length of females at initial sexual maturation (L50) was estimated at 11.7 cm. The species reproduced throughout the year.
"Four-eyed" fish; Reproduction; Viviparity
O presente estudo analisou a relação peso-comprimento e uma série de aspectos da biologia reprodutiva de Anableps anableps da foz do rio Maracanã, no estado brasileiro do Pará. Os espécimes foram coletados por meio de duas redes de emalhar de 30 m de comprimento, com malhas de 15 e 20 mm, e com um puçá com um metro de diâmetro, com malha de 10 mm. Foram capturados 865 espécimes, os quais foram analisados para estabelecer a razão sexual, que foi de 2,12 fêmeas por macho (χ² = 13,07; p<0,05). As fêmeas apresentaram crescimento alométrico positivo, enquanto que os machos foram alométricos negativos. Nas análises adicionais, as gônadas de 371 fêmeas foram observadas ao microscópio. O desenvolvimento gonadal foi classificado em três estádios: imaturo (5,0%), em maturação (12,0%) e maturo (83,0%). O desenvolvimento embrionário foi classificado em cinco fases, de acordo com o tamanho do embrião e do saco vitelino. A fecundidade média foi de 12 ovos / embriões por fêmea (variando de 1-37 ovos / embriões). Uma relação significativa foi verificada entre o comprimento padrão das fêmeas, o peso de seus ovários (R ² = 0,257, p < 0,001), e o número de embriões (R ² = 0,573, p < 0,001). O tamanho médio da primeira maturação sexual (L50) para as fêmeas foi estimado em 11,7 cm. A espécie se reproduz durante todo o ano.
Reproductive characteristics and the weight-length relationship in Anableps anableps (Linnaeus, 1758) (Cyprinodontiformes: Anablepidae) from the Amazon Estuary
Valéria de Albuquerque OliveiraI; Nelson Ferreira FontouraII; Luciano Fogaça de Assis MontagI
IUniversidade Federal do Pará (UFPA), Instituto de Ciências Biológicas, Laboratório de Ecologia e Zoologia dos Vertebrados. Rua Augusto Corrêa, 01, Guamá, P. O. Box 479, 66075-110 Belém, PA, Brazil. valeria_a_o@yahoo.com.br; montag@ufpa.br
IIPontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Faculdade de Biociências, Departamento de Biodiversidade e Ecologia. Av. Ipiranga, 6681, P. O. Box 1429, 90619-900 Porto Alegre, Rs, Brazil. nfontoura@pucrs.br
ABSTRACT
The present study analyzed the length-weight relationship and some aspects of the reproductive biology of Anableps anableps from the mouth of the Maracanã River, in the Brazilian state of Pará. The specimens were collected using two 30 m-long gillnets with 15 and 20 mm mesh size, as well as a 1 m-diameter hand net with a 10 mm mesh. A total of 865 specimens were collected, from which an adult sex ratio of 2.12 females per male was recorded (χ² = 13.07; p<0.05). Females presented positively allometric growth, whereas males were negatively allometric. In the additional analyses, the gonads of 371 female specimens were observed microscopically. Gonadal development was classified in three stages: immature (5.0%), maturing (12.0%), and mature (83.0%). Embryonic development was classified in five phases, according to the size of the embryo and the vitelline sac. Mean fecundity was 12 eggs/embryos per female (range: 1-37 eggs/embryos). A significant relationship was recorded between the standard length of females and ovaries weight (R² = 0.257; p < 0.001), and the number of embryos carried (R² = 0.573; p < 0.001). Mean body length of females at initial sexual maturation (L50) was estimated at 11.7 cm. The species reproduced throughout the year.
Key words: "Four-eyed" fish, Reproduction, Viviparity.
RESUMO
O presente estudo analisou a relação peso-comprimento e uma série de aspectos da biologia reprodutiva de Anableps anableps da foz do rio Maracanã, no estado brasileiro do Pará. Os espécimes foram coletados por meio de duas redes de emalhar de 30 m de comprimento, com malhas de 15 e 20 mm, e com um puçá com um metro de diâmetro, com malha de 10 mm. Foram capturados 865 espécimes, os quais foram analisados para estabelecer a razão sexual, que foi de 2,12 fêmeas por macho (χ² = 13,07; p<0,05). As fêmeas apresentaram crescimento alométrico positivo, enquanto que os machos foram alométricos negativos. Nas análises adicionais, as gônadas de 371 fêmeas foram observadas ao microscópio. O desenvolvimento gonadal foi classificado em três estádios: imaturo (5,0%), em maturação (12,0%) e maturo (83,0%). O desenvolvimento embrionário foi classificado em cinco fases, de acordo com o tamanho do embrião e do saco vitelino. A fecundidade média foi de 12 ovos / embriões por fêmea (variando de 1-37 ovos / embriões). Uma relação significativa foi verificada entre o comprimento padrão das fêmeas, o peso de seus ovários (R ² = 0,257, p < 0,001), e o número de embriões (R ² = 0,573, p < 0,001). O tamanho médio da primeira maturação sexual (L50) para as fêmeas foi estimado em 11,7 cm. A espécie se reproduz durante todo o ano.
Introduction
The genus Anableps encompasses three species: Anableps anableps (Linnaeus, 1758), A. dowi Gill, 1861, and A. microlepis Müller & Troschel, 1844, which range from Central America to northern South America, with A. dowi being restricted to the Pacific Ocean (Miller, 1979).
The members of this genus are the largest viviparous fishes of the order Cyprinodontiformes, with body lengths of up to 35 cm (Burns & Flores, 1981). These animals are also characterized by their prominent eyes, which are divided horizontally by an opaque membrane, with each half having its own retina, hence the common English name "four-eyed" fish. This ocular structure permits the simultaneous surveillance of both the aquatic and aerial environments, permitting the efficient exploration of shallow and shoreline habitats (Miller, 1979; Oliveira et al., 2006).
A number of studies have focused on the ocular physiology of these species (Schwassmann & Kruger, 1965; Oliveira et al., 2006), although few data are available on their ecology or general biology. However, studies of feeding ecology have been conducted in the Pacific, where Miller (1979) focused on all three Anableps species, and in Brazil, on A. anableps (Brenner & Krumme, 2007). In addition, some population parameters, such as the weight-length relationship, and variation in the condition factor and the sex ratio, have been analyzed in Brazilian A. anableps in Maranhão (Ribeiro & Castro, 2003), and in the Curuçá estuary, in Pará (Ikeda et al., 2005).
The reproductive biology of the species has been the subject of a number of studies in Central America. Turner (1938) described the adaptations of the ovaries and embryos of A. anableps for viviparity, while Burns & Flores (1981) studied the reproductive biology of A. dowi in El Salvador. Knight et al. (1985) conducted a more general study of the follicular placenta and embryo growth in Anableps, and Burns (1991) described the development of the testes and gonopods of the males of A. dowi in the El Tamarino estuary. In Brazil, the only study available is the one of Nascimento & Assunção (2008), who analyzed the reproductive ecology of A. anableps and A. microlepis on Marajó Island in Pará. However, none of these studies of the reproductive biology of A. anableps has focused on features such as body size at sexual maturity, female investment in reproduction, the relationship between size and the sex ratio, the polyphasic weight-length relationship, or between-sexes variation in the condition factor.
Anableps anableps is a typical resident of estuarine environments, and according with Carvalho-Neta & Castro (2008) the whole of the biological cycle of the species reproduction, growth, and feeding - occurs within estuarine habitats. Even though A. anableps is relatively abundant in the region of the Amazon Estuary, and can be differentiated from other fish species by its distinct behavior, few studies of its biology or ecology have been conducted in this environment. Given this, the present study describes important aspects of the reproductive biology of the species in the mouth of the Maracanã River in Pará. This study focused on sex ratio, weight-length relationship, and the condition factor in males and females, as well as embryo development, mean fecundity, size at initial gonadal maturity, and the breeding season.
Material and Methods
The A. anableps specimens analyzed in the present study were collected from the mouth of the Maracanã River, in the Brazilian state of Pará (00º46'03"S, 47º27'12"W), east of the estuary of the Amazon (Fig. 1). The region's climate is equatorial hot and humid, or category Af in Köppen's classification, with mean temperatures of around 27ºC. Annual precipitation is approximately 2000 mm, distributed mainly in the first half of the year (Costa et al., 2009).
The Maracanã is a meandering river with a number of tributaries, the largest of which is the Caripi River, on its western bank. The main channel of the Maracanã varies in depth from 15 to 20 m (Costa et al., 2009), and reaches its highest levels between April and February, and its lowest in September and October (Costa et al., 2009). During the present study period (2009), salinity concentrations varied between 5‰ and 35‰.
Specimens were collected each month between September, 2008 and August, 2009. Given the diurnal habits of the species, and its relative vulnerability at low tide, the collection of specimens was carried out during the diurnal low tide.
Specimens were captured using two 30 m-long gillnets with 15 mm and 20 mm meshes, and a 1 m-diameter hand net with a 10 mm mesh. For the analysis of the sex ratio, weight-length relationship, and the condition factor, the maximum possible number of specimens captured per month was analyzed. For all other analyses, monthly samples of ±30 individuals were used.
Following collection, all specimens were sexed, weighed and measured (standard length, L). Sexing was based on the presence of the gonopod, a modified anal fin, in the males (Turner, 1950). Standard length was measured using a ruler with a precision of 0.5 cm. Specimens were weighed to the nearest 0.1 g using a digital scale. Gonads were weighed on an analytical scale with a precision of 0.001 g.
The gonads were removed through a longitudinal incision between the urogenital opening and the head. The gonads were weighed fresh, and then conserved in 10% formaldehyde for 24 hours, before being preserved in 70% ethanol.
Voucher specimens were deposited at the fish collection of the Museu Paraense Emílio Goeldi (MPEG 16581, MPEG 16582, MPEG 16583, MPEG 16584, MPEG 16585, MPEG 16586, MPEG 16751 e MPEG 16752), in Belém, Pará State, Brazil.
The sex ratio (females to males) was calculated for each monthly sample, and its deviation from equality was tested using Chi-square (Sokal & Rohlf, 1981), with Yates' correction.
The weight-length relationship was adjusted in order to identify possible differences between the sexes. This relationship was described for females and males using the equation W = a.Lb, where W = total weight; L = standard length; a = coefficient of proportionality, and b = coefficient of allometry. The proportional residuals ([observed weight - expected weight]/observed weight) were then plotted in relation to length, in order to evaluate a possible trend for polyphasic growth (Bervian et al., 2006). Proportional residuals were used instead absolute values in order to avoid bias derived from heterocedasticity. To test possible trends in the residuals, linear and polynomial regressions were applied between the residuals and standard lengths. When a trend was identified, a polyphasic weight-length relationship was applied following Bervian et al. (2006):
W= f(a).Lf(b)
f(a) = a1+ (a2-a1) . [1+ e Rsc.(L Psc)]-1
f(b) = b1+ (b2-b1) . [1+ e Rs.c(L Psc)]-1
where W = total weight; L = standard length; a1 = coefficient of proportionality in phase 1; a2 = coefficient of proportionality in phase 2; Rsc = Stanza changing rate; Psc = Stanza changing point; b1 = coefficient of allometry in phase 1, and b2 = coefficient of allometry in phase 2. Adjustment of the equations was carried out in the Solver routine of Microsoft Office Excel® 2007, with the adjusted model being based on the lowest sum of squared proportional residuals.
In addition, for each gender and growth Stanza, Student's t test was used to determine whether the "b" value recorded for each sex was significantly different from 3, at the 5% significance level (Zar, 1999). This analysis is based on the fact that, when b = 3, growth is isometric, i.e., length increases at the same rate as weight, whereas when b is significantly different from 3 (higher or lower), growth is allometric (weight and length change at different rates).
The allometric condition factor was estimated separately for each sex, based on the average coefficient of allometry (b), using the equation K= W/Lb, where K = the condition factor; W = total weight; L = standard length, and b = the average coefficient of allometry for the weight-length relationship.
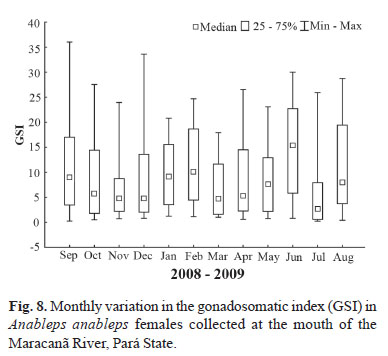
The different stages of gonadal maturation in the females were identified according to Vazzoler (1996). The gonadosomatic index (GSI) was calculated for each female, in order to establish the gonadal maturation curve, and thus identify the breeding season. The index is given by GSI= GW/W x 100, where GSI = gonadosomatic index; GW = gonad weight, and W = total weight. The monthly variation in the GSI values was also tested using the Kruskal-Wallis analysis of variance, with α = 0.05.
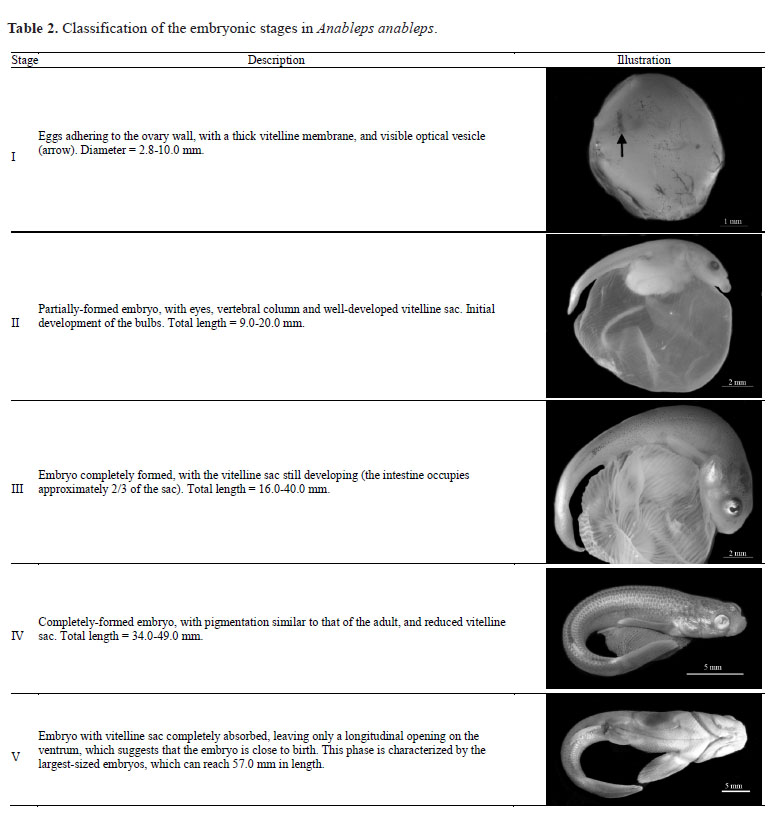
The developmental stages of the embryos collected during the study were also defined, based on the size of the embryo and the vitelline sac. The stages of embryonic development used here were adapted from the scheme proposed by Nascimento & Assunção (2008) and were illustrated using a Leica M205A digital camera and the LAS automounting program.
Fecundity was determined by the number of eggs and embryos counted per female each month. The relationship between the number of eggs/embryos recorded per female and its standard length was analyzed using a simple linear regression, in order to assess the influence of female size on reproductive investment. The log-transformed data for standard length and ovary weight were tested using an ANCOVA (α = 0.05), in which ovary weight was dependent on the months, and standard length was inserted as the covariable, with the null hypothesis that ovary weight was dependent on the month.
Mean size at first gonadal maturation (L50) was estimated based on the frequency of reproductive and non-reproductive females in each 1 cm size class, according to the logistic equation P = A . (1+e(r.(L-L50))-1, where P = the proportion of reproductive individuals or adults in each size class; r = rate of change between non-reproductive and reproductive status; L = standard length (cm); L50 = size at first maturation (cm).
The adjustment of the function and the estimate of the 95% confidence interval were obtained using the Levenberg-Marquardt algorithm in the SPSS® 17.5 program. The mean size at initial sexual maturation corresponds to the standard length at which 50% of the individuals are mature.
Results
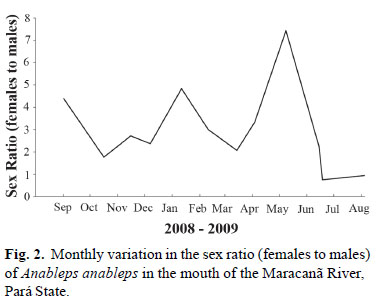
In all, 865 specimens of A. anableps were collected during the 12 months of the study. Of this total, 529 (61.16%) individuals were female, 249 (28.79%) male, and 87 (10.55%) juveniles, for which the sex could not be determined. The sex ratio for the study period as a whole was 2.12 females per male (χ² = 13.07; p<0.05). However, this ratio varied considerably during the course of the study, between a maximum of 7:1 in May, 2009, and a minimum of 1:1 in July, 2009 (Fig. 2).
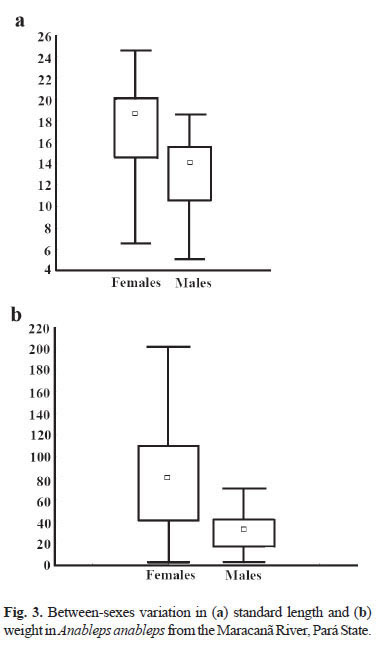
Mean standard length in the females was 18.5 cm, with a maximum of 24.5 cm, whereas in males, the mean length was 14.0 cm, and the maximum 18.5 cm (Fig. 3a). Females weighed 80.0 g, on average, with a maximum recorded weight of 200.7 g, while males had an average weight of 31.3 g and a maximum of 69.7 g (Fig. 3b).
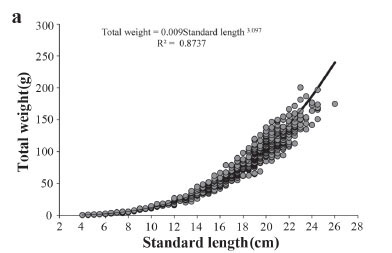
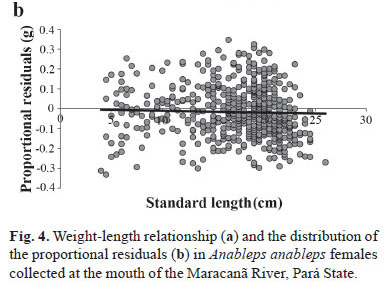
The weight-length relationship in females (a = 0.00992; b = 3.097) indicates a pattern of positive allometric growth (t = 13.3; p<0.05) (Fig. 4a). No significant trend in the proportional residuals was identified (Fig. 4b; p = 0.410), which confirms the adequacy of the regular weight-length relationship for the females.
By contrast, when the residuals of the weight-length relationship in males (Fig. 5a) were plotted, a parabolic trend was identified (Fig. 5b), which indicates that a simple, single-phase model is not an adequate for this sex (p = 0.00652). The estimated parameters for the polyphasic growth model (Table 1) identified a change in the growth pattern at a body length of 8.7 cm. Growth was negatively allometric in both phases, but the coefficient was greater in the first phase, i.e. body length < 8.7 cm (b1=2.79), in comparison with the second phase (b2=2.26). Following adjustment of the polyphasic model, an untrended residual pattern was identified (Fig.5c), indicating the adequacy of the model.
The monthly variation in the allometric condition factor was similar in the two sexes, although the values for males were larger than those for females due to their lower values for the coefficient of allometry. Values of K varied between 0.011 and 0.015 in males, and 0.09 and 0.11 in females (Fig. 6).
Reproductive parameters in females were adjusted from monthly samples of around 30 females (n=371), with standard lengths of between 4.5 cm and 27.0 cm. According to the gonadal stages identified, 83.0 % of the collected females were mature, 12.0% were maturing, and only 5.0% were immature. Mature females predominated throughout the study period, while immature individuals were consistently rare, except for July (Fig. 7).
The standard length of adult females ranged from 11.9 cm to 27.0 cm, with a mean of 18.6 cm. Analysis of the monthly samples indicates that A. anableps reproduces throughout the year, given that mature females were collected in all months, and high gonadosomatic indices were also recorded in most samples (Fig. 8). However, the gonadosomatic index varied significantly among months (H = 31.180; p< 0.05), with significant differences being identified between July and September (p = 0.04), June and March (p = 0.04), and June and July (p = 0.0007). These differences may be related primarily to the increase in reproductive investment observed in July, when the largest number of immature individuals was recorded.
Five embryonic stages were identified (Table 2). All the embryos present in any given ovary were at the same stage of development, which suggests that superfetation is rare or absent in A. anableps. The largest embryo collected during the study had a total length of 57.6 mm, and a weight of 1.732 g.
Between one and 37 eggs or embryos were recorded per mature female (mean = 12). A significant relationship was found between the standard length of females and both the number of embryos (R² = 0.575; n= 231; p < 0.001) and ovary weight (R² = 0.234; n = 314; p< 0.001), which indicates a relationship between the size of a female and its investment in reproduction (Fig. 9), as detected by the ANCOVA, which confirmed the lack of influence of the month on the investment of females in reproduction, with no significant monthly variation in (ln) gonad weight (F = 1.60; DF = 10; p = 0,102). However, there was a highly significant interaction between (ln) gonad weight and the (ln) standard length of the specimens (F = 321.09; DF = 1; p < 0.001).
No significant relationship was found between the number of stage V embryos and either their mean total length (R² = 0.303; n = 22; p = 0.007) or mean total weight (R² =0.277; n = 22; p = 0.011) (Fig. 10), which indicates that embryo size does not decrease as the number of embryos increases. Mean size (standard length) at first gonadal maturation (L50) in females was estimated as 11.7 cm (11.3-12.1) and all of the females larger then 15 cm were classified as adult (Fig. 11).
Discussion
The sex ratio recorded for A. anableps in the mouth of the Maracanã River was similar to that obtained by Brenner & Krumme (2007) in Caeté Bay, also in Pará State (2.2:1), but differed considerably from the ratio of 1:1 recorded at Marajó Island, in the mouth of the Amazon by Nascimento & Assunção (2008). In fish, sex ratios may vary in accordance with a series of factors, such as mortality and population growth rates (Vazzoler, 1996). As these factors may affect the two sexes differentially, shifts in the sex ratio may also represent an adaptation to the availability of dietary resources, leading to a predominance of females when food is abundant (Nikolsky, 1969). This author also proposed that deviations in the sex ratio could be the product of the selective collection of specimens, where males and females occupy different habitats.
Seasonal variation in the sex ratio has been recorded for other cyprinodontiforms, such as Phallotorynus pankalos in the basin of the Paraná River (Súarez et al., 2009), where, once again, a predominance of females was recorded. A similar preponderance of females was recorded in Jenynsia multidentada from the Patos Lagoon in Rio Grande do Sul by Garcia et al. (2004) and Mai et al. (2007). These authors suggested that this deviation may be the result of asymmetric predation due to the unusual reproductive behavior of the species, in which the males spend the majority of their time pursuing females, thereby increasing their vulnerability to predation.
The b values recorded for the weight-length relationship in the females collected in the present study are different from those recorded by Ribeiro & Castro (2003) and Brenner & Krummer (2007), who found values significantly lower than 3 i.e., negative allometric growth -in both males and females.
However, Silva-Júnior et al. (2007) recorded a value of 3.32 for A. anableps from the estuary of the Paciência River, in Maranhão State. Agostinho (1985) has proposed that the coefficients of the length-weight relationship are affected by several factors such as gender, reproductive status, age, level of parasitic infestation, food availability, and the study site or population.
Despite their high investment of energy in reproduction, no change in the weight-length growth pattern was identified in females. By contrast, a polyphasic growth pattern was observed in the males. In the first phase, growth presents negative allomety (b = 2.79), with length increasing at a higher rate than weight, which may reflect the need to attain a minimal length rapidly in order to compete for access to females. At standard lengths above 8.7 cm, the allometric constant decreases further (2.26), suggesting the diversion of energy from weight gain to other processes, such as reproduction.
The low K values obtained for the female A. anableps throughout the study period may be related to the constant need to divert energy to the growth of the embryos, which are nurtured by the mother throughout their development (Knight et al., 1985; Oliveira et al., pers. comm.). This finding is supported by a number of studies, which have demonstrated a positive correlation between the buildup of body fat and the condition factor in fish, in addition to a close relationship (positive or negative) between gonad development and seasonal variation in the K value in these animals (Gomiero & Braga, 2003; Braun & Fontoura, 2004).
The positive relationship recorded between female size and reproductive investment is similar to that recorded for A. dowi in El Salvador (Burns & Flores, 1981), where the number of embryos also varied according to the size of the female. A similar pattern has also been recorded in P. pankalos (Súarez et al., 2009), and J. multidentata (Mai et al., 2007). A positive correlation between female size and the number of embryos has been recorded in a large number of studies of the reproductive biology of fish, given that larger females are able to assign more energy to the development of their offspring (Mai et al., 2007).
The positive relationship observed between the number of embryos in later developmental stages and their weight and length indicates that an increase in the number of embryos entails a reduction in their size. Súarez et al. (2009) propose that the increase in fecundity resulting from that in female size will lower the probability of survival of the females, if the size of the embryos is not reduced.
The five-stage classification of embryonic development in A. anableps presented here differs from that of Turner (1938), which identified eight stages, based on the histological traits of the bulbs (structures in the vitelline sac that play a role in the absorption of nutrients). By contrast, Nascimento & Assunção (2008) identified only four developmental stages in the embryos of A. anableps and A. microlepis, based on the external characteristics of the egg, the size of the embryo and the vitelline sac. In the present study, the classification was based on just two parameters, the size of the embryo and the vitelline sac, following Nascimento & Assunção (2008), due to the fact that these traits are easily observed, can be measured reliably, and provide a clear definition of the different stages of embryonic development.
The fact that all the embryos found in a given ovary were at the same stage of development suggests that superfetation is uncommon in A. anableps. A similar pattern was recorded in A. dowi from El Salvador by Burns & Flores (1981). The largest embryo recorded in the present study (57.6 mm long and 1.732 g) was somewhat larger than that found in the Saramaca River in Surinam by Knight et al. (1985), where the largest specimen was 51 mm long and weighed 1.49g. While this variation may be related to between-site differences in environmental variables such as the availability of food and predation rates - which may affect the size at which the embryos are liberated by the mother (Vazzoler, 1996) - they may also be the result of sampling effects.
The size of females at initial gonadal maturity has not been estimated in previous studies for Anableps species, which have all been based on the analysis of macroscopic traits. However, Ikeda et al. (2005) reported that the smallest pregnant female A. anableps collected in the estuary of the Caratateua River, Pará, had a total length of 14.9 cm, while body lengths recorded in the sample varied from 5 cm to 24 cm. Similarly, Nascimento & Assunção (2008) reported a mean length of 15.4 cm for adult female A. anableps from Marajó Island. Given this, the present estimate of the size of females at first sexual maturity (L50=11.7 cm) represents an important advance in the study of Anableps biology. Definition of this parameter is essential, not only for the development of management strategies, but also provides important insights into the population dynamics of a species (Begon & Mortimer, 1990).
As in the present study, Anableps is known to reproduce throughout the year at other sites (Miller, 1979; Burns & Flores, 1981; Nascimento & Assunção, 2008). However, Nascimento & Assunção (2008) report reproductive peaks in A. anableps and A. microlepis in January and October. While this differs from the results of the present study, these authors analyzed the monthly mean GSI values, while the present study was based on the analyis of the individual data.
The significant predominance of mature females in the present study suggests that the study area would be appropriate as a breeding area for the species. Population structure, in terms of the distribution of the different developmental phases, may vary across time and space (Vazzoler, 1996), and while a predominance of juveniles may be typical of feeding areas, that of adults characterizes breeding grounds. While the present study represents an important contribution to the scientific knowledge of the reproductive biology of A. anableps, further macro- and microscopic studies of the gonads, and in particular, an analysis of the size of males at initial maturation, should be prioritized in order to provide a more complete understanding of the biology of the different Anableps species.
Acknowledgments
We would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), for support through the Programa Nacional de Cooperação Acadêmica - Ação Novas Fronteiras (PROCAD-NF 2008), and everyone who contributed to this study in some way, especially Alexandre B. Bonaldo for the photographs of the embryos, and the laboratory assistants Fernanda Paz, Louise Perez and Natália Kiss for their help with the analysis of the gonads.
Literature Cited
Submitted October 6, 2010
Accepted September 26, 2011
- Agostinho, A. A. 1985. Estrutura da população, idade, crescimento e reprodução de Rhinelepis aspera (Agassiz, 1829) (Osteichthyes, Loricariidae) do rio Paranapanema, PR.Unpublished Ph. D. Dissertation, Universidade Federal de São Carlos, São Paulo, 229p.
- Begon, M. & M. Mortimer. 1990. Life-history strategies. Pp.115-172. In: Begon, M. & M. Mortimer (Eds). Population ecology. London, Blackwell Scientific Publications, 548p.
- Bervian, G., N. F. Fontoura & M. Haimovici. 2006. Statistical model of variable allometric growth: otolith growth in Micropogonias furnieri (Actinopterygii, Sciaenidae). Journal of Fish Biology, 68: 196-208.
- Braun, A. S. & N. F. Fontoura. 2004. Reproductive biology of Menticirrhus littoralis in southern Brazil (Actinopterygii: Perciformes: Sciaenidae). Neotropical Ichthyology, 2: 31-36.
- Brenner, M. & U. Krumme. 2007. Tidal migration and patterns in feeding of the four-eyed fish Anableps anableps L. in a north Brazilian mangrove. Journal of Fish Biology, 70: 406-427.
- Burns, J. R. 1991. Testis and gonopodium development in Anableps dowi (Pisces: Anablepidae) correlated with pituitary gonadotropic zone area. Journal of Morphology, 210: 45-53.
- Burns, J. R. & J. A. Flores. 1981. Reproductive biology of the cuatro ojos, Anableps dowi (Pisces: Anablepidae), from el salvador and its seasonal variations. Copeia, 1981: 24-33.
- Carvalho-Neta, R. N. F. & A. C. L. Castro. 2008. Diversidade das assembléias de peixes estuarinos da ilha dos Caranquejos, Maranhão. Arquivos de Ciências do Mar, Fortaleza, 41: 48-57.
- Costa, F. F.; Lima, W. N. & J. C. Dias. 2009. Avaliação hidrogeoquímica em áreas selecionadas na bacia hidrográfica do rio Maracanã (Nordeste do Pará). HOLOS Enviroment, 9: 167-182.
- Garcia, A. M., J. P. Vieira, K. O. Winemiller & M. B. Raseira. 2004. Reproductive cycle and spatiotemporal variation in abundance of the one-sided livebearer Jenynsia multidentata, in Patos Lagoon, Brazil. Hydrobiologia, 515: 39-48.
- Gomiero, L. M. & F. M. S. Braga. 2003. Relação peso-comprimento e fator de condição para Cichla cf. ocellaris e Cichla monoculus (Perciformes: Cichlidae) no reservatório de Volta Grande, rio Grande - MG/SP. Acta Scientiarium: Biological Sciences, Maringá, 25: 79-86.
- Ikeda, R. G. P., J. M. B. Silva & S. C. S. Miranda. 2005. Morfometria do tralhoto, Anableps anableps (Linnaeus, 1758), do estuário de Caratateua - Curuçá - Pará. Boletim Técnico-Científico do CEPNOR, 5: 93-103.
- Knight, F. M., J. Lombardi, J. P. Wourms & J. R. Burns. 1985. Follicular placenta and Embryonic Growth of the Viviparous Four-Eyed Fish (Anableps). Journal of Morphology, 185: 131-142.
- Mai, A. C. G., A. M. Garcia, J. P. Vieira & M. G. Mai. 2007. Reproductive aspects o fone-sided livebearer Jenynsia multidentata (Jenyns, 1842) (Cyprinodontiformes) in the Patos Lagoon estuary, Brazil. Pan-American Journal of Aquatic Sciences, 2: 40-46.
- Miller, R. R. 1979. Ecology, habits and of the middle american cuatro ojos, Anableps dowi (Pisces: Anablepidae). Copeia 1979: 82-91.
- Nascimento, F. L. & M. I. S. Assunção. 2008. Ecologia reprodutiva dos tralhotos Anableps anableps e Anableps microlepis (Pisces: Osteichthyes: Cyprinodontiformes: Anablepidae) no rio Paracauari, ilha de Marajó, Pará, Brasil. Boletim do Museu Paraense Emílio Goeldi. Ciências Naturais, 3: 229-240.
- Nikolsky, G. V. 1969. Theory of fish population dynamics. Oliver & Boyd LTD, Edinburh, 323p.
- Oliveira, F. G., J. P. Coimbra, E. S. Yamada, L. F. A. Montag, F. L. Nascimento, V. A. Oliveira, D. L. Mota, A. M. Bittencourt, V. L. Silva, & B. L. S. A. Costa. 2006. Topographic analysis of the ganglion cell layer in the retina of the four-eyed fish Anableps anableps. Visual Neuroscience, 23: 1-8.
- Ribeiro, D. & A. C. L. Castro. 2003. Contribuição ao estudo da dinâmica populacional do tralhoto Anableps anableps (Teleostei: Cyprinodontidae), no município de Bacuri, Estado do Maranhão. Boletim do Laboratório de Hidrobiologia, 16: 21-27.
- Shwassmann, H. O. 1971. Biological rhythms. Pp. 371-428. In: W. S. Hoar & D. J. Randall (Eds.). Fish physiology. Academic Press, New York, vol. 6, 559p.
- Schwassmann, H. O. & L. Kruger. 1965. Experimental analysis of the visual system of the four-eyed fish Anableps microlepis Vision Research, 5: 269-281.
- Silva-Júnior, M. G., A. C. L. Castro, L. S. Soares & V. L. França. 2007. Relação peso-comprimento de espécies de peixes do estuário do rio Paciência da ilha do Maranhão, Brasil. Boletim do Laboratório de Hidrobiologia, 20: 31-38.
- Sokal, R. R. & F. J. Rohlf. 1981. Biometry. New York, W. H. Freeman, 859p.
- Súarez, Y. R., J. P. Silva, L. P. Vasconcelos & W. F. Antonialli-Júnior. 2009. Ecology of Phallotorynus pankalos (Cyprinodontiformes: Poeciliidae) in a firts-order stream of the upper Paraná Basin. Neotropical Ichthyology, 7: 49-54.
- Turner, C. L. 1938. Adaptations for viviparity in embryos and ovary of Anableps anableps Journal of Morphology, 62: 323-349.
- Turner, C. L. 1950. The skeletal structure of the gonopodium and gonopodial suspensorium of Anableps anableps Journal of Morphology, 86: 329-366.
- Vazzoler, A. E. A. M. 1996. Biologia da reprodução de peixes teleósteos: teoria e prática. EDUEM SBI, São Paulo, 169p.
- Zar, J. H. 1999. Biostatistical analysis. 4ş Ed. Prentice Hall. Upper Saddle River, New Jersey, 663p.
Publication Dates
-
Publication in this collection
16 Nov 2011 -
Date of issue
2011
History
-
Accepted
26 Sept 2011 -
Received
06 Oct 2010