Abstracts
The aim of this study, utilizing RAPD techniques, was to determine the genetic variability of Salminus brasiliensis groups collected at passage ladders of the hydroelectric plants (HEP) Canoas I and Canoas II - Paranapanema River (Brazil), as well as to estimate the population structure through different parameters of genetic diversity. The data obtained allowed us to conclude that S. brasiliensis of the Canoas Complex has a moderate index of genetic variability ( > 42.00%) when compared to that of other migratory fish species. All genetic diversity analyses (distance = 0.015 and genetic identity = 0.985, F ST =0.018, AMOVA) were signs of low genetic differentiation, and they led to the clustering of S. brasiliensis from Canoas I and Canoas II. This suggests that the species is genetically structured as a single population. Some findings indicate that this population of S. brasiliensis comes from the Capivara Reservoir (Canoas I downstream), probably fed by the Tibagi and Cinzas Rivers. Literature data denote that after fish transposition by passage ladders of the Canoas Complex, the migratory species are not concluding the reproductive cycle. This mechanism, therefore, could be one more impact factor causing the depletion in downstream recruitment, which could in medium and long term be compromising the natural S. brasiliensis population in the middle Paranapanema River.
Fish transposition; Geneticdiversity; RAPD
O objetivo desse estudo, utilizando a técnica de RAPD, foi estimar a variabilidade genética de grupos de Salminus brasiliensis coletados nas escadas de transposição das hidroelétricas de Canoas I e Canoas II - rio Paranapanema (Brasil), bem como estimar a estrutura populacional através de diferentes parâmetros de diversidade genética. Os dados obtidos permitiram concluir que S. brasiliensis do Complexo Canoas tem um índice moderado de variabilidade genética ( > 42.00%) quando comparado com valores de outras espécies de peixes migradoras. Todas as análises de diversidade genética (distância = 0,015 e identidade genética = 0,985, F ST =0,018, AMOVA) foram indicativas de baixa diferenciação genética, e conduziram ao agrupamento de S. brasiliensis proveniente das escadas de transposição de Canoas I e Canoas II, sugerindo que essa espécie está geneticamente estruturada como uma única população. Alguns dados indicam que essa população de S. brasiliensis é proveniente do Reservatório de Capivara (jusante de Canoas I), provavelmente mantida pelos rios Tibagi e das Cinzas. Dados da literatura indicam que após a transposição das escadas para peixes do Complexo Canoas, as espécies migradoras não estão concluindo o ciclo reprodutivo, esse mecanismo, portanto, pode ser mais um fator de impacto causando a depleção no recrutamento a jusante o que pode a médio e longo prazo comprometer a diversidade genética da população de S. brasiliensis no médio rio Paranapanema.
Fish passage ladders from Canoas Complex Paranapanema River: evaluation of genetic structure maintenance of Salminus brasiliensis (Teleostei: Characiformes)
Carla Martins LopesI; Fernanda Simões de AlmeidaI; Mário Luís OrsiII; Sandro Geraldo de Castro BrittoIII; Rodolfo Nardez SirolIII and Leda Maria Koelblinger SodréI
ILaboratório de Marcadores Moleculares em Peixes, Departamento de Biologia Geral, Centro de Ciências Biológicas, Universidade Estadual de Londrina, Rodovia Celso Garcia Cid PR445 Km 380 Campus Universitário, Cx. postal 6001, 86051990 Londrina, PR, Brazil. leda@uel.br
IIDepartamento de Biologia Animal e Vegetal, Centro de Ciências Biológicas, Universidade Estadual de Londrina, Rodovia Celso Garcia Cid PR445 Km 380 Campus Universitário, Cx. postal 6001, 86051990 Londrina, PR, Brazil
IIIGerência de Meio Ambiente Duke Energy International Geração Paranapanema, Rodovia Chavantes/Ribeirão Claro, Km 10, 18970000 Chavantes, SP, Brazil
ABSTRACT
The aim of this study, utilizing RAPD techniques, was to determine the genetic variability of Salminus brasiliensis groups collected at passage ladders of the hydroelectric plants (HEP) Canoas I and Canoas II Paranapanema River (Brazil), as well as to estimate the population structure through different parameters of genetic diversity. The data obtained allowed us to conclude that S. brasiliensis of the Canoas Complex has a moderate index of genetic variability (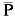 > 42.00%) when compared to that of other migratory fish species. All genetic diversity analyses (distance = 0.015 and genetic identity = 0.985, FST =0.018, AMOVA) were signs of low genetic differentiation, and they led to the clustering of S. brasiliensis from Canoas I and Canoas II. This suggests that the species is genetically structured as a single population. Some findings indicate that this population of S. brasiliensis comes from the Capivara Reservoir (Canoas I downstream), probably fed by the Tibagi and Cinzas Rivers. Literature data denote that after fish transposition by passage ladders of the Canoas Complex, the migratory species are not concluding the reproductive cycle. This mechanism, therefore, could be one more impact factor causing the depletion in downstream recruitment, which could in medium and long term be compromising the natural S. brasiliensis population in the middle Paranapanema River.
> 42.00%) when compared to that of other migratory fish species. All genetic diversity analyses (distance = 0.015 and genetic identity = 0.985, FST =0.018, AMOVA) were signs of low genetic differentiation, and they led to the clustering of S. brasiliensis from Canoas I and Canoas II. This suggests that the species is genetically structured as a single population. Some findings indicate that this population of S. brasiliensis comes from the Capivara Reservoir (Canoas I downstream), probably fed by the Tibagi and Cinzas Rivers. Literature data denote that after fish transposition by passage ladders of the Canoas Complex, the migratory species are not concluding the reproductive cycle. This mechanism, therefore, could be one more impact factor causing the depletion in downstream recruitment, which could in medium and long term be compromising the natural S. brasiliensis population in the middle Paranapanema River.
Key words: Fish transposition, Geneticdiversity, RAPD.
RESUMO
O objetivo desse estudo, utilizando a técnica de RAPD, foi estimar a variabilidade genética de grupos de Salminus brasiliensis coletados nas escadas de transposição das hidroelétricas de Canoas I e Canoas II rio Paranapanema (Brasil), bem como estimar a estrutura populacional através de diferentes parâmetros de diversidade genética. Os dados obtidos permitiram concluir que S. brasiliensis do Complexo Canoas tem um índice moderado de variabilidade genética (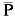 > 42.00%) quando comparado com valores de outras espécies de peixes migradoras. Todas as análises de diversidade genética (distância = 0,015 e identidade genética = 0,985, FST =0,018, AMOVA) foram indicativas de baixa diferenciação genética, e conduziram ao agrupamento de S. brasiliensis proveniente das escadas de transposição de Canoas I e Canoas II, sugerindo que essa espécie está geneticamente estruturada como uma única população. Alguns dados indicam que essa população de S. brasiliensis é proveniente do Reservatório de Capivara (jusante de Canoas I), provavelmente mantida pelos rios Tibagi e das Cinzas. Dados da literatura indicam que após a transposição das escadas para peixes do Complexo Canoas, as espécies migradoras não estão concluindo o ciclo reprodutivo, esse mecanismo, portanto, pode ser mais um fator de impacto causando a depleção no recrutamento a jusante o que pode a médio e longo prazo comprometer a diversidade genética da população de S. brasiliensis no médio rio Paranapanema.
> 42.00%) quando comparado com valores de outras espécies de peixes migradoras. Todas as análises de diversidade genética (distância = 0,015 e identidade genética = 0,985, FST =0,018, AMOVA) foram indicativas de baixa diferenciação genética, e conduziram ao agrupamento de S. brasiliensis proveniente das escadas de transposição de Canoas I e Canoas II, sugerindo que essa espécie está geneticamente estruturada como uma única população. Alguns dados indicam que essa população de S. brasiliensis é proveniente do Reservatório de Capivara (jusante de Canoas I), provavelmente mantida pelos rios Tibagi e das Cinzas. Dados da literatura indicam que após a transposição das escadas para peixes do Complexo Canoas, as espécies migradoras não estão concluindo o ciclo reprodutivo, esse mecanismo, portanto, pode ser mais um fator de impacto causando a depleção no recrutamento a jusante o que pode a médio e longo prazo comprometer a diversidade genética da população de S. brasiliensis no médio rio Paranapanema.
Introduction
Over the last 50 years, the Paranapanema River, which has its headwater in the Paranapiacaba Mountains (Atlantic Forest) and flows into the Paraná River to the west, has suffered enormous human intervention with the building of 10 hydroelectric plants, forming a system of reservoirs in cascade. The hydroelectric plants (HEP) of Canoas I and Canoas II located in the middle of the Paranapanema River form the Canoas Complex, where their respective reservoirs (Canoas I and Canoas II) were built in 1998. The banks of both reservoirs are occupied by pastures, with scarce remnants of riparian vegetation and where marginal lakes are absent (Dias, 2003). They are the only HEPs of this river where fish passage ladders were built, which began operating in November of 2000 with the aim of minimizing the impact of suppressing the maintenance of natural stocks of migratory fish species (Britto & Sirol 2005). Downstream from HEP Canoas I, there is the Capivara Reservoir formed by the HEP Escola Mackenzie (22º32'S; 51º22'W) in 1975 and upstream from the Canoas II Reservoir is the HEP Lucas Nogueira Garcez (Salto Grande) (23º10'S; 49º 13'W) constructed in 1958 (Duke Energy, 2003).
The construction of fish passage ladders at some reservoirs is one of the mitigating measures employed according to laws implemented in Brazil. Although there is no question as to the ability of many migratory species to ascend the ladders and reach the reservoir, the selectivity of the passage ladders of the dam has been the subject of studies conducted. Some indicators show that these works would have dubious effectiveness in the preservation or conservation of the stocks in a series of dams (Borghetti et al.,1994; Agostinho et al., 2002; Agostinho & Gomes, 2005).
The interruption of the migratory routes of these species, fragmenting the environment of initial development, spawning and growth is, in large part, responsible for the virtual disappearance of large migratory species in the upper stretches of the Paraná River basin (Agostinho et al., 2002).
The construction of hydroelectric plants destroys habitats and increases the geographic fragmentation for many fish species. Reduction in gene flow may even change the ratio of components of diversity between and within populations (Vrijenhoek, 1998). Currently, populations of freshwater fishes are distributed in isolated places which can minimize gene flow between them, leading to processes of genetic differentiation (Hilsdorf & Petrere, 2002). Therefore, evaluations of the genetic structure of populations provide a contemporary genetic picture of populations permitting the extraction of important information for the elaboration of management plans (Perez Sweeney et al., 2003).
The species Salminus brasiliensis (Cuvier, 1816)belongs to the order Characiformes, and family Characidae (Lima et al., 2003). They are migratory over long distances, especially for food and reproducing, rheophilic fish (spawningrun fish). These fish have a reproductive cycle, external fecundation and total spawning and do not demonstrate care for their offspring (Godoy, 1975; Duke Energy, 2003). According to Barbieri et al. (2001) and Lima et al. (2003), this species is also highly abundant in the largest hydrographic basins of South America, where it is very common in recreational and commercial fishing.
Studies utilizing molecular markers have been applied in the identification of the diversity and genetic structure of species of Neotropical fishes, with the aim of not only exploring characteristics of economic interest but also the preservation of evolutionarily significant units for the maintenance of this biodiversity (Torres et al., 2004).
Random amplified polymorphic DNA (RAPD) analysis is a technique based on the polymerase chain reaction (PCR) amplification of discrete regions of the genome with short oligonucleotide primers of arbitrary sequence (Williams et al., 1990). The method is simple and quick to perform, and most importantly, no a priori knowledge of the genetic makeup of the organism in question is required. In fishes, RAPD has been successfully used in population structure analysis (for example: Prioli et al., 2002; Sekine et al., 2002; Almeida et al., 2003; Hatanaka & Galetti, 2003; Ali et al., 2004; Leuzzi et al., 2004; Matoso et al., 2004; Sofia et al., 2006; Oliveira et al., 2006).
The aims of this study were: (i) to investigate the genetic variability and structure of Salminus brasiliensis groups that migrate by way of the passage ladders at HEPs Canoas I and Canoas II in different spawning runs, and (ii) to determine if the groups belong to a single population or are from a shoal with a differentiated genetic structure. The results promise to be useful for the fishery management, aquaculture and stock conservation of this species.
Materials and Methods
Collection of specimens
Specimens of S. brasiliensis were collected in the fish passage ladders of the HEPs of Canoas I ( 22º 56'S; 50º 31'W) and Canoas II (22º 56'S; 50º 15'W), in the middle Paranapanema River (Fig. 1) in the period of March, 2003 to March, 2005, always with respect to the spawning run period which includes the beginning of September of one year to the end of March of the following year. For better understanding the collection, specimens were grouped according to spawning period: March of 2003, spawning A; from November of 2003 to March of 2004, spawning B; November of 2004 to March of 2005, spawning C. In HEP of Canoas I were present spawning A and C (17 and 24 specimens, respectively), and in HEP of Canoas II spawning B and C (19 and 27 specimens, respectively).
The adipose fin was obtained from each specimen and stored in alcohol 70% at 20ºC for later extraction of DNA. Most of the specimens collected were released in the same location, and some were labeled and preserved in the Zoology Museum of the Londrina State University, Brazil.
DNA extraction and RAPD analysis
DNA was extracted from the fin of fish following the procedure described by Almeida et al. (2001). DNA concentration was determined in a Dyna Quant200 fluorimeter (Hoefer).
For RAPD screening, 60 decamer oligonucleotides (Operon Technologies Ltd.) were used as random primers, of which 12 were selected (OPX 1, 6, 7, 9, 12, 13; OPAM 7, 9, 10, 11, 13; OPC 2) that produced a good number of amplified bands and patterns of reproducible fragments.
Amplification reactions were performed in a total volume of 15 mL containing 10 ng of template DNA, 0.33 mM of primer, 0.25 mM dNTP (Invitrogen), 3.53 mM MgCl2, 0.75 U of Taq DNA polymerase (EMBRAPA, Brazil), using the reaction buffer supplied. Control reactions were run containing all components except genomic DNA. Also, to avoid distortion of the results due to problems regarding reproducibility (Williams et al., 1990), only reproducible patterns were included in the data analysis.
DNA amplifications were carried out in a thermal cycler (MJ Research PTC100), and the amplification protocol consisted of 4 min at 92 °C followed by 40 cycles of 40 s at 92 °C, 1.5 min at 40 °C, and 2 min at 72 °C. The last round of amplification was followed by an additional extension at 72 °C for 5 min.
Samples of 15 mL of amplification products were assayed by electrophoresis on 1.4% agarose gels with TBE buffer (0.89 M Tris, 0.89 M boric acid, 1 mM EDTA, pH 8.3) diluted 1:20 (v:v), run at 3V.cm1 and stained with ethidium bromide. Agarose gel images were documented under UV light, using the Kodak Electrophoresis Documentation and Analysis System (EDAS) 290.
The RAPD marker profiles were determined by direct comparison of the amplified electrophoretic profiles of the DNA from each individual, and each band was analyzed as a binary variable (band presence or absence).
Comparative electrophoretic analysis was performed for the purpose of obtaining comparative data between the populations of S. brasiliensis from the two passage ladders (Canoas I versus Canoas II), considering spawning A and C from the passage ladder of Canoas I as a single group of S. brasiliensis (CI) and spawning B and C from Canoas II as group CII, where each group comprised respectively 21 and 27 specimens of S. brasiliensis chosen randomly.
The RAPD technique produces dominant multilocus markers. Statistical methods developed for codominant markers have been modified for use with RAPD markers. For the purpose of this study, each locus was treated as a twoallele system, with only one of the alleles per locus being amplifiable by PCR. It was also assumed that marker alleles from different loci did not comigrate to the same position on a gel, and that populations were under HardyWeinberg equilibrium (Lynch & Milligan, 1994).
Statistical analysis
The following parameters were calculated with the software TFPGA 1.3 (Miller, 1997) and using the correction described by Lynch and Milligan (1994): genetic variability estimated from the proportion of polymorphic loci (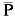 ), using the 95% criterion, genetic identity (I), and distance (D) (Nei, 1978).
), using the 95% criterion, genetic identity (I), and distance (D) (Nei, 1978).
The software Arlequin version 3.0 (Excoffier et al., 2005) was utilized to determine the distribution of genetic differentiation by means of the estimates of FST (Weir & Cockerham, 1984) among populations, and for analysis of molecular variance (AMOVA) (Excoffier et al., 1992). The significance of these tests was verified by the method of random permutations, with 1000 and 10000 permutations, respectively.
The magnitude of genetic differentiation among the groups of S. brasiliensis, was determined utilizing the scale proposed by Wright (1978), where FST values of 0 to 0.05 indicate little genetic differentiation, 0.05 to 0.15 moderate, 0.15 to 0.25 high, and above 0.25 very high.
Results
As described previously, the collections of the specimens were carried out on different dates. Therefore, there was the possibility of existing genetic differentiation among these groups, in case they originated from S. brasiliensis shoals with different genetic structure. To verify this notion, a comparative analysis was performed between the two groups (A, C) of S. brasiliensis collected in the passage ladders of Canoas I and those (B, C) from Canoas II. The results (Table 1) obtained in group comparisons (D = 0.006, I = 0.994 and FST 0.014; p<0.05) and AMOVA revealed that the specimens of S. brasiliensis collected in the passage ladder of Canoas I, could be considered as a single group. The same could be inferred for the two groups from Canoas II (D = 0.008, I = 0.992 and FST 0.012; p<0.05) (Table 1). Thus, the subsequent analyses were carried out considering the two larger groups referred to as CI and CII, respectively.
The analysis of RAPD electrophoretic profiles yielded 192 loci that could be utilized in the comparative genetic study of groups CI and CII. The values determined for genetic variability were 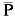 = 42.19% for CI and 44.79% for CII.
= 42.19% for CI and 44.79% for CII.
Analysis of molecular variance (AMOVA) (Table 2) showed that the major part of the genetic variation of the groups of S. brasiliensis from CI and CII is contained within each group (98.22%) and not between the groups (1.78%). Reinforcing this idea, the FST value calculated (0.018; p<0.05) indicates little genetic differentiation between the specimens of S. brasiliensis from CI and CII, corroborated as well by the estimates of genetic identity (0.982) and distance (0.015) (Table 2).
Discussion
The fixation index FST serves as a convenient and widely used measure of genetic differences among subpopulations. The identification of the causes underlying a particular value of FST observed in a natural population is often difficult (Hartl & Clark, 1997). The values of FST presented in Table 1 were significant, but indicate little genetic differentiation (<0.05), according to the scale proposed by Wright (1978). Similarly, the results of AMOVA (Table 1) showed that the major part of the genetic variation is contained within (98.63%; 98.81%) the spawning groups and not between the groups (1.37%; 1.19%). The other analyses of genetic diversity (distance and identity) corroborate the data described above and reveal that the specimens of S. brasiliensis collected in the passage ladder of Canoas I, had a genetic structure that indicated they comprised a single group (CI). The same consideration could be applied to the groups of Canoas II (CII).
The values observed for genetic variability () for S. brasiliensis in the clustersCI and C II are considered smaller than those obtained for other migratory species in the same locations, Prochilodus lineatus (74.51% CI and 74.90 % CII; Paula, 2006) and for Leporinus friderici (62.89% CI and 62.64% CII; Ashikaga, 2005).
Paula (2006) utilized RAPD and microsatellite markers in studies of groups of Prochilodus lineatus and showed that the major component of genetic variation is contained within each group (96.76% with RAPD and 99.82% with microsattelite) and not between the groups (3.24% and 0.17%, respectively). Reinforcing this idea, the FST values calculated (0.032, p<0.01; 0.0017 p>0.01) indicated a low genetic differentiation between specimens of P. lineatus from CI and CII, a result similar to that obtained in the present study.
The maintenance of genetic variability is directly related to the population size attributed to a given species. Britto & Sirol (2005) studied fish ladders as a form of management at Canoas Complex for 4 consecutive years (2000 2004), where soon after the opening of the fish passage ladders of the Canoas Complex, collections were carried out for tagging and recapture of fish in the period of November of 2000 to April of 2001. These authors found for this reproductive period a total number of 371 specimens of S. brasiliensis (Canoas I n=25 and Canoas II n=346), which were considered the fourth most abundant species in these locations. After this period, these authors detected a decline of almost 90% in the number of specimens of S. brasiliensis collected.
In view of the above findings and the number of collected specimens of S. brasiliensis, for all of the groups analyzed in the fish passage ladders of both HEP Canoas I and HEP Canoas II, we can notice that there was a reduced number of specimens collected at both places and at any one of the collection dates. It is worth pointing out that both HEP Canoas I and HEP Canoas II began their operations in the year of 1998 and their passage ladders were only open since 2000, the year in which the study of Britto & Sirol was conducted. Therefore, the impact of the dam on the ichthyofauna with regard to fish passage at the time of that study was recent. In the present study, however, the first collections were begun after 5 years of operation of the two hydroelectric plants and 3 years after the opening of the fish transposition ladders for the first time. In other words, the time of exposure to this environment, and thus their impact, was greater in this study than in the one previously mentioned.
According to Agostinho (1995 apud Agostinho & Gomes 2005), "among the species of fish, the population depletion affects mainly the one of larger size, usually of habit migratory, high longevity and small reproductive potential."
The data obtained with RAPD markers allow us to conclude that S. brasiliensis in the CanoasComplex has a moderate index of genetic variability ( > 42 %), maintained by the biological characteristics of this species. All the analyses of genetic diversity (Table 2) indicated little genetic differentiation and led to the clustering of S. brasiliensis of Canoas I and Canoas II. The value of FST (0.018)presented in Table 2 differed significantly but was very small. According to Wright (1978), the different allele frequencies between the groups CI and CII could reflect the sample size and not the existence of distinct gene pools. Similarly the results of AMOVA (Table 2) showed that the major part of the genetic variation is contained within the groups and not between them. Therefore, the data obtained using this molecular marker suggest that the specimens of S. brasiliensis from the passage ladders at Canoas I and Canoas II are genetically structured as a single population.
What is the origin of this population? Some findings indicate that this population of S. brasiliensis comes from the Capivara Reservoir which is downstream from HEP Canoas I and receives on its left bank two large affluents, the Tibagi and Cinzas Rivers. Based on the report by Orsi (2005), after the construction of the dams of Canoas I and Canoas II, only the stretch of the Capivara Reservoir between the Cinzas River and HEP of Canoas I has lotic waters which are needed for sexual stimuli for reproduction of S. brasiliensis. The specimens of S. brasiliensis present in the Capivara Reservoir are mainly from the Cinzas River, which was verified by Orsi (2005). This investigator made seasonal collections in the period of 2001 to 2004 at four different sites in this reservoir, and this species was found only at a site close to the mouth of this river.
After the construction of the Capivara HEP (in 1975), for twentythree years this species of fish had the middle Paranapanema River as a free stretch for migration, which was for a large part lotic. This area compromised this dam and that of Salto Grande (Fig. 1) which was already a natural geographic barrier formed by the falls so named (Sampaio, 1944). This made it possible for the fish in the reproductive stage to be able to migrate, find some propitious locations for spawning and return to the river below as larvae and juveniles. Such was no longer possible after the construction of the HEPs of Canoas I and Canoas II, even with the presence of the passage ladders.
Therefore, the Capivara Reservoir has conditions for maintaining the population S. brasiliensis in a different manner compared to the reservoirs of Canoas I and Canoas II which do not have lotic waters, large affluents or locations appropriate for reproduction (spawning area, nurseries and feeding areas) (Dias, 2003; Duke Energy, 2003).
The initial objective of this study was to compare the population structure of specimens of S. brasiliensis in the process of migration with those existing in the Capivara Reservoir, outside of the spawning run period, to confirm the origin of the migratory population. During the course of this study, efforts were made to capture specimens in the reservoir but without success.
According to Agostinho et al. (2002), "the degree of interference of a reservoir in this process depends basically on its position in relation to the critical areas of the fish life cycle (spawning area, nurseries and feeding areas). If the upstream stretch of the reservoir is extensive, contains undisturbed spawning sites and has extensive flood areas, it is expected that the migratory species, which remain upstream, would maintain their stocks, with losses in genetic diversity over time. In this case, the objective of fish passages would be only to maintain genetic diversity, with possible damage to the stocks downstream of the dam. In another scenario, the upstream stretch would be short and contain spawning sites without important flood areas. In this case, the stock of large migratory fish would be drastically reduced, with the possibility of being eliminated from the upstream area after some years. Fish passages, in this way, could allow spawning in upstream areas. Eggs and larvae would, however, be conveyed to a reservoir whose waters show low velocity and high transparency, allowing intense predation. The construction of fish passages in the latter case represents an additional source of impact on the success of the reproduction of individuals with the chance of spawning in segments lower than the dam". We believe that this situation occurs in the Canoas Complex.
Britto & Sirol (2005) demonstrated that for the period of 2000 to 2002 there was low capture of icthyoplankton (eggs, larvae and postlarvae) as well as juveniles (2003 to 2004) upstream of the ladders, that is, in the reservoirs of Canoas I and Canoas II, and that the identification the material revealed that it did not belong to any of the migratory species found in the ladders during the monitoring of passage. Therefore, it was concluded that reproductive success of the migratory species identified, including S. brasiliensis, was not consolidated.
Considering the results of the present study with S. brasiliensis and those obtained by Orsi (2005) and by Britto & Sirol (2005), it is possible to devise a diagnostic test for consequences of the construction of fish passage ladders in the Canoas Complex. Initially, a means of ascension of fish to upstream areas suggested that the reproductive cycle of the species was continuing. However, in the short term, this mechanism of passage was compromising its objectives of providing migration for the sake of the reproductive cycle and maintenance of natural stocks. However, based on the results of the present study, the specimens collected at the two passage ladders form a single population whose origin is that of the Capivara Reservoir, making it possible to estimate that in the medium and long term, this mitigating measure that was imposed and implemented can result in a generalized depletion of natural stocks of S. brasiliensis, principally due to recruitment downstream of the Canoas I Reservoir (Capivara Reservoir), despite of the level of genetic variability and reproductive capability of this species. Therefore, the fish passage ladders installed do not compromise the objective of providing sustainability to this ecosystem.
Thus, more important than the functioning of the fish passage ladders of the Canoas Complex would be the preservation of the principal affluent of the Capivara Reservoir, Cinzas and Tibagi Rivers, with the objective of maintaining a constant passage of natural stocks of S. brasiliensis with the genetic characteristics identified in this study. Besides, at least the passage ladder at Canoas I should remain closed during the spring and summer which is the reproductive period of S. brasiliensis and other migratory species, since in that way the fish would migrate only from the Capivara Reservoir to the large affluent where they would find favorable conditions for conclusion of their reproductive cycle.
The maintenance of S. brasiliensis stocks in the reservoirs of Canoas I and Canoas II could be realized by opening the passage ladders outside the spawning run period or by restocking with fingerlings produced from populations of S. brasiliensis present in the Capivara Reservoir. Assisted reproduction would guarantee replacement with replenishing stocks bearing genetic characteristics identified for the natural population of S. brasiliensis belonging to this region of the Paranapanema River (Sirol & Britto, 2005).
Finally, the continuation of these monitoring studies is indispensable so that management actions can be always based on recent scientific findings, since the environment undergoes constant changes, including those due to anthropic impacts.
Acknowledgements
The authors thank the collecting staff from Duke Energy and Universidade Estadual de Londrina, to Dr. Oscar Akio Shibatta for identification of the specimens. This work was financially supported by Duke Energy International, ANEEL (Agência Nacional de Energia Elétrica).
Literature Cited
Received January 2007
Accepted May 2007
- Agostinho, A. A. & L. C. Gomes. 2005. O manejo da pesca em reservatórios da bacia do alto rio Paraná: avaliação e perspectivas. Pp.2355. In: Nogueira M. G., R. Henry & A. Jorcin (Eds). Ecologia de Reservatórios: Impactos Potenciais, Ações de Manejo e Sistemas em Cascata. São Carlos, Rima, 459p.
- Agostinho, A. A., L. C. Gomes, D. R. Fernandez & H. I. Suzuki. 2002. Efficiency of fish ladders for a Neotropical ichthyofauna. River Research and Applications, 18: 299306.
- Ali, B. A., TH. Huang, DN. Qin & XMA.Wang. 2004. Review of random amplified polymorphic DNA (RAPD) markers in fish research. Reviews in Fish Biology Fisheries, 14: 443453.
- Almeida, F. S., M. H. P. Fungaro & L. M. K. Sodré. 2001. RAPD and isoenzyme analysis of genetic variability in three allied species of catfish (Siluriformes: Pimelodidae) from the Tibagi river. Journal of Zoology, 253: 113120.
- Almeida F. S., L. M. K. Sodré & E. P. B. Contel. 2003. Population structure analysis of Pimelodus maculatus (Pisces, Siluriformes) from the Tietê and Paranapanema Rivers (Brazil). Genetics and Molecular Biology, 26: 301305.
- Ashikaga, F. Y. 2005. Análise da estrutura genética de Leporinus friderici (Pisces, Characiformes) nas escadas de transposição de peixes das UHEs do Complexo Canoas rio Paranapanema. Unpublished Monograph, Universidade Estadual de Londrina, Londrina. 76p.
- Barbieri, G., F. A. Salles & M. A. Cestarolli. 2001. Reproductive and nutritional dynamics of Salminus maxillosus Valenciennes, 1849 (Pisces, Characidae) at Mogi Guaçu river, state of São Paulo, Brazil. Acta Scientarium, 23: 441444.
- Borghetti, J. R., S. V. G. Nogueira, N. R. B. Borghetti & C. Canzi. 1994. The fish ladder at the Itaipu Binational hydroelectric complex on the Paraná River, Brazil. Regulated Rivers: Research & Management, 9: 127130.
- Britto, S. G. C. & R. N. Sirol. 2005. Transposição de peixes como forma de manejo: as escadas do Complexo Canoas, médio rio Paranapanema, bacia do Alto Rio Paraná. Pp.285304. In: Nogueira M. G., R. Henry & A. Jorcin (Eds). Ecologia de Reservatórios: Impactos Potenciais, Ações de Manejo e Sistemas em Cascata. São Carlos, Rima, 459p.
- Dias, J. H. P. 2003. Distribuição espacial e temporal da ictiofauna do trecho médio do rio Paranapanema e suas relações com as características morfométricas e limnológicas dos compartimentos da bacia. Unpublished Ph. D. Thesis. Universidade Federal de São Carlos, São Carlos. 103p.
- Duke Energy International Geração Paranapanema. 2003. Peixes do Rio Paranapanema. São Paulo, Horizonte Geográfico, 112p.
- Excoffier, L., G. Laval & S. Schneider. 2005. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online, 1: 4750.
- Excoffier L., P. E. Smouse & J. M. Quattro. 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics, 131: 479491.
- Godoy, M. P. 1975. Peixes do Brasil. Subordem Characoidei. Bacia do Rio Mogi Guassu. Pp. 310335. Piracicaba, Franciscana.
- Hartl, D. L. & A. G. Clark. 1997. Principles of Population Genetics. Sunderland, MA, Sinauers Associates, Inc., 542p.
- Hatanaka, T. & P. M. Galetti Jr. 2003. RAPD markers indicate the occurrence of structured populations in a migratory freshwather fish species. Genetics and Molecular Biology, 26: 1925.
- Hilsdorf, A.W. & M. Petrere Jr. 2002. Conservação de peixes na bacia do rio Paraíba do Sul. Ciência Hoje, 30: 6267.
- Leuzzi, M. S. P., F. S. Almeida, M. L. Orsi & L. M. K. Sodré. 2004. Analysis by RAPD of the genetic structure of Astyanax altiparanae (Pisces, Characiformes) in reservoirs on the Paranapanema River, Brazil.Genetics and Molecular Biology, 27: 355 362.
- Lima, f. c. t., L. R. Malabarba, P.A. Buckup, J. F. P. Silva, R. P. Vari, A. Harold, R. Benine, O. T. Oyakawa, C. S. Pavanelli, N. A. Menezes, C. A. S. Lucena, M. C. S. L. Malabarba, Z. M. S. Lucena, R. E. Reis, F. Langeani, L. Cassati, V. A. Bertaco, C. Moreira & P. H. F. Lucinda. 2003. Genera Incertae Sedis in Characidae. Pp. 106156. In: Reis, R. E., S. O. Kullander & C. J. Ferraris, (Eds.). Check List of the Freshwater Fishes of South and Central America. Porto Alegre, Edipucrs, 729p.
- Lynch, M. & B. G. Milligan. 1994. Analysis of population structure with RAPD markers. Molecular Ecology, 3: 9199.
- Matoso, D. A., R. F. Artoni & P. M. Galetti Jr. 2004. Genetic diversity of the small characid fish Astyanax sp., and its significance for conservation. Hydrobiologia, 527: 223225.
- Miller, M. P. 1997. Tools for population genetic analyses (TFPGA) 1.3: A Windows program for the analysis of allozyme and molecular population genetic data. Computer software distributed by author.
- Nei, M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individual. Genetics, 89: 583590.
- Oliveira, A. V., A. J. Prioli, S. M. A. P. Prioli, T. S. Bignotto, H. F. Júlio Jr., H. Carrer, C. S. Agostinho & L. M. Prioli. 2006. Genetic diversity of invasive and native Cichla (Pisces: Perciformes) populations in Brazil with evidence of interspecific hybridization. Journal of Fish Biology, 69: 260277.
- Orsi, M. L. 2005. Caracterização das estratégias reprodutivas na assembléia de peixes do reservatório de Capivara, rio Paranapanema, região sudeste, Brasil. Unpublished Ph. D. Thesis, Universidade Estadual Paulista Júlio de Mesquita Filho, Botucatu. 113p.
- Paula. F. M. 2006. Diversidade genética de Prochilodus lineatus (Pisces, Characiformes) das escadas de transposição de peixes das usinas hidroelétricas do Complexo Canoas rio Paranapanema. Unpublished Ph.D. Dissertation, Universidade Estadual de Londrina, Londrina. 125p.
- PerezSweeney, B. M., F. P. Rodrigues & D. J. Melnick. 2003. Metodologias moleculares utilizadas em genética da conservação. Pp. 343380. In: Cullen, L. J., R. Rudan & C. ValadaresPadua (Eds.). Métodos de Estudos em Biologia da Conservação e Manejo da Vida Silvestre. Curitiba, Editora da Universidade Federal do Paraná, 664p.
- Prioli, S. M. A. P., A. J. Prioli, H. F. Julio Jr., C. S. Pavanelli, A. V. Oliveira, H. Carrer, D. M. Carraro & L. M. Prioli. 2002. Identification of Astyanax altiparanae (Teleostei, Characidae) in the Iguaçu River, Brazil, based on mitochondrial DNA and RAPD markers. Genetics and Molecular Biology, 25: 421430.
- Sampaio, T. 1944. Relato sobre os estudos efetuados nos rios Itapetininga e Paranapanema. Revista do Instituto de Geografia e Geologia, 2: 3081.
- Sekine, S. E., A. J. Prioli, S. M. A. P. Prioli & H. F. Júlio Jr. 2002. Genetic differentiation among populations of Pseudoplatystoma corruscans (Agassiz, 1829) (Osteichthyes, Pimelodidae) isolated by the Guaíra Falls in the Paraná River. Acta Scientiarum, 24: 507512.
- Sirol, R. N. & S. G. Britto. 2005. Conservação e manejo da ictiofauna: repovoamento. Pp.275284. In: Nogueira M. G., R. Henry & A. Jorcin (Eds.). Ecologia de Reservatórios: Impactos Potenciais, Ações de Manejo e Sistemas em Cascata. São Carlos, Rima, 459p.
- Sofia, S. H., C. R. M. Silva, B. A. Galindo, F. S. Almeida, L. M. K. Sodré & C. B. R. Martinez. 2006. Population genetic structure of Astyanax scabripinis (Teleostei, Characidae) from an urban stream. Hydrobiologia, 553: 245254.
- Torres, R. A., D. A. Matoso & R. F. Artoni. 2004. Genética de peixes neotropicais. Biologia molecular de peixes neotropicais. Biologia e Saúde, 10: 2737.
- Vrijenhoek, R. C. 1998. Conservation genetics of freshwater fish. Journal of Fish Biology, 53: 394412.
- Weir, B. S. & C. C. Cockerham. 1984. Estimating F statistics for the analysis of population structure. Evolution, 38: 13581370.
- Wright, S. 1978. Variability within and among natural populations. In: Evolution and the Genetics of Populations. Vol. 4. Pp. 79103. Chicago, The University of Chicago Press.
- Williams, J. G. K., A. R. Kubelik, K. J. Livak, J. A. Rafalski & S. V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research, 18: 65316535.
Publication Dates
-
Publication in this collection
24 July 2007 -
Date of issue
2007
History
-
Accepted
May 2007 -
Received
Jan 2007




