Abstracts
Bile acids are potent olfactory and gustatory stimulants for fish. Electro-olfactogram recording was used to test whether the olfactory epithelium of pintado catfish Pseudoplatystoma corruscans is specifically sensitive to bile acids, some of which have been hypothesized to function as pheromones. Five out of 30 bile acids that had been pre-screened for olfactory activity in fish were selected. Cross-adaptation experiments demonstrated that sensitivity to bile acids is attributable to at least 3 independent classes of olfactory receptor sites. The taurocholic acid (TCA) and taurochenodeoxycholic acid (TCD) were the most potent compounds. By using avoidance/preference tests, we found that P. corruscans prefers water containing TCA. Bile acids are discriminated by olfactory epithelium of pintado, supporting that these compounds could function as pheromones.
Behavior; Electro-olfactogram (EOG); Olfaction; Preference test
Os ácidos biliares são potentes estimulantes olfatórios e gustatórios em peixes. Registros em eletro-olfactograma foram usados para testar se o epitélio olfatório de Pseudoplatystoma corruscans, pintado, é sensível aos ácidos biliares, alguns dos quais têm sido propostos como feromônios. Foram selecionados cinco de uma lista de trinta ácidos biliares previamente testados em atividade olfatória em peixes. Testes de adaptação cruzada demonstraram que a sensibilidade aos ácidos biliares se dá por 3 classes independentes de sites de receptores olfatórios. O ácido taurocólico (TCA) e o ácido tauroquenodesoxicólico (TCD) foram os compostos mais potentes. Em testes de evasão/preferência, P. corruscans prefere água contendo o ácido TCA. Os ácidos biliares são discriminadas por epitélio olfatório de pintado, evidenciando que estes compostos podem funcionar como feromônios.
Introduction
Bile acids are known to act as potent odorants for teleosts (Hara, 1994Hara, T. J. 1994. The diversity of chemical stimulation in fish olfaction and gustation. Reviews in Fish Biology and Fisheries, 4: 1-35.; Zhang et al., 2001Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.; Giaquinto & Hara, 2008Giaquinto, P. C. & T. J. Hara. 2008. Discrimination of bile acids by the rainbow trout olfactory system: evidence as potential pheromone. Biological Research, 41: 33-42.), and conspecific bile fluid has been shown to have pheromonal activity for some fish species (Vermeirssen & Scott, 2001Vermeirssen, E. L. M. & A. P. Scott. 2001. Male priming pheromone is present in bile as well as urine of female rainbow trout. Journal of Fish Biology, 58: 1039-1045.; Huertas et al., 2007Huertas, M., P. C. Hubbard, A. V. M. Canario & J. Cerda. 2007. Olfactory sensitivity to conspecific bile fluid and skin mucus in the European eel Anguilla anguilla (L.). Journal of Fish Biology, 70: 1907-1920.). Two lines of evidence suggest that the odorants contained in the bile fluid are likely to be mainly bile acids. Firstly, the bile fluid is a concentrated source of bile acids (normally 25-50 mM) where the concentration may reach 300-400 mM after starvation (Karlaganis et al., 1989Karlaganis, G., S. E. Bradley, J. L. Boyer, A. K. Batta, G. Salen, B. Egestad & J. Sjövell. 1989. A bile alcohol sulfate as a major component in the bile of the small skate (Raja erinacea). Journal of Lipid Research, 30: 317-322.; Grosell et al., 2000Grosell, M., M. J. O'Donnell & C. M. Wood. 2000. Hepatic versus gallbladder bile composition: in vivo transport physiology of the gallbladder in rainbow trout. American Journal of Physiology, 278: 1674-1684.). Secondly, many species of fishes have been shown to have a high olfactory sensitivity to bile acids (Doving et al., 1980Doving, K. B, R. Selset & G. Thommesen. 1980. Olfactory sensitivity to bile acids in salmonid fishes. Acta Physiologica Scandinavica, 108: 123-131.), thus, it is reasonable to suppose such acids act as chemical signal for fish communication.
Studies using electro-olfactogram showed that bile acids are potent olfactory stimulants for the Arctic grayling (Thymallus arcticus) and Arctic charr (Salvelinus alpinus) (Doving et al., 1980Doving, K. B, R. Selset & G. Thommesen. 1980. Olfactory sensitivity to bile acids in salmonid fishes. Acta Physiologica Scandinavica, 108: 123-131.), and taurocholic acid (TCA) is a potent olfactory stimulus to several salmonids (Hara et al., 1984Hara, T. J., S. Macdonald, R .E. Evan, T. Marui & S. Arai. 1984. Morpholine, bile acids and skin mucus as possible chemical cues in salmonid. Mechanisms of Migration in Fishes. NATO Conference Series, 14: 363-378.; Quinn & Hara, 1986Quinn, T. P. & T. J. Hara. 1986. Sibling recognition and olfactory sensitive in juvenile coho salmon (Oncorhynchus kisutch). Canadian Journal of Zoology, 64: 921-925.; Zhang & Hara, 1991Zhang, C. & T. J. Hara. 1991. Olfactory and gustatory responses to bile salts in salmonids. Pp. 16-22. In: Zhang, C. & T. J. Hara. 1991. Chemical signals in vertebrates. Philadelphia, Pennsylvania.; Hara & Zhang, 1996), and goldfish (Carassius auratus) (Sorensen et al., 1987Sorensen, P. W., T. J. Hara & N. E. Stacey. 1987. Extreme olfactory sensitivity of mature and gonadally-regressed goldfish to a potent steroidal pheromone, 17, 20-dihydroxy-4-pregnen-3-one. Journal of Comparative Physiology, 160: 305-313.). Additionally, extreme olfactory sensitivities to bile acids, coupled with their wide distribution and chemical variations, have been implicated for their role in fish behavior (Doving et al., 1980Doving, K. B, R. Selset & G. Thommesen. 1980. Olfactory sensitivity to bile acids in salmonid fishes. Acta Physiologica Scandinavica, 108: 123-131.; Hara et al., 1984Hara, T. J., S. Macdonald, R .E. Evan, T. Marui & S. Arai. 1984. Morpholine, bile acids and skin mucus as possible chemical cues in salmonid. Mechanisms of Migration in Fishes. NATO Conference Series, 14: 363-378.; Zhang et al., 2001Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.). For instance, behavioral evidence suggests that one of their functions as olfactory stimulants is their role as pheromones for migratory anadromous fishes, some of which appear to recognize and select the odor of conspecifics when choosing spawning streams (Doving et al., 1980Doving, K. B, R. Selset & G. Thommesen. 1980. Olfactory sensitivity to bile acids in salmonid fishes. Acta Physiologica Scandinavica, 108: 123-131.; Stabell, 1992Stabell, O. B. 1992. Olfactory control of homing behavior in salmonids. Pp. 249-270. In: T. J. Hara (Ed.). Fish chemoreception. London, Chapman and Hall.). Recently, it has been shown that bile acid profiles of lake trout (Salvelinus namaycush) are largely influenced by sex and maturation stage (Zhang et al., 2001Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.) and that bile of female Rainbow trout (Oncorhynchus mykiss) contains a pheromone (Vermeirssen & Scott, 2001Vermeirssen, E. L. M. & A. P. Scott. 2001. Male priming pheromone is present in bile as well as urine of female rainbow trout. Journal of Fish Biology, 58: 1039-1045.). Moreover, male-specific bile acids of sea lamprey (Petromyzon marinus) are potent and specific stimulants to the female olfactory organ (Siefkes & Li, 2004Siefkes, M. J. & W. Li. 2004. Electrophysiological evidence for detection and discrimination of pheromonal bile acids by the olfactory epithelium of female sea lamprey (Petromyzon marinus). Journal of Comparative Physiology A, 190: 193-199.). Furthermore, electrophysiological studies demonstrated that an odotopic map of responses to bile acids and amino acids are represented in different regions of olfactory bulbs of chars (Salvelinus alpinus) and graylings (Thymallus thymallys) (Doving et al., 1980Doving, K. B, R. Selset & G. Thommesen. 1980. Olfactory sensitivity to bile acids in salmonid fishes. Acta Physiologica Scandinavica, 108: 123-131.). Behavioral studies in Atlantic salmon (Salmo salar) also showed that intestinal extracts, which are known to contain bile acids, induced strain-specific preference and searching behaviors (Stabell, 1992Stabell, O. B. 1992. Olfactory control of homing behavior in salmonids. Pp. 249-270. In: T. J. Hara (Ed.). Fish chemoreception. London, Chapman and Hall.). Moreover, lake char (Salvelinus namaycush) exhibit preference behaviors towards water containing bile acids (Zhang et al., 1996Zhang, C., S. B. Brown & T. J. Hara. 1996. Olfactory sensitivity and behavioral reactions of lake char to bile acids released by conspecifics. Chemical Senses, 21: 692-693.). Indeed, several experiments indicated that a number of bile acids induced behavioral responses in fish (Jones & Hara, 1985Jones, K. A. & T. J. Hara. 1985. Behavioural responses of fishes to chemical cues: results from a new bioassay. Journal of Fish Biology, 27: 495-504.; Hara, 1994Hara, T. J. 1994. The diversity of chemical stimulation in fish olfaction and gustation. Reviews in Fish Biology and Fisheries, 4: 1-35.) and therefore support our hypothesis that bile acids can act as pheromones.
In teleosts, the main bile acids are (Hara, 1994Hara, T. J. 1994. The diversity of chemical stimulation in fish olfaction and gustation. Reviews in Fish Biology and Fisheries, 4: 1-35.) sulphated bile alcohol, mainly 5-cyprinol and 5-chimaerol, and (Zhang et al., 2001Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.) C24 bile acids, mainly cholic acid (CA), chenodeoxycholic acid (CD), deoxycholic acid (DC) and haemulcholic acid (Haslewwod, 1967; Goto et al., 1996Goto, T., T. Ui, T. Une, T. Kuramoto, K. Kirira & T. Hoshita. 1996. Bile salt composition and distribution of the D-cystenolic acid conjugated bile salts in fish. Fisheries Science, 62: 606-609.). The C24 bile acids are taurine conjugated and/or sulphated. Also, cysteinolic acid-conjugated bile acids were found in the bile of some marine species (Une et al., 1991Une, M., T. Goto, K. Kirira, T. Kuramoto, K. Hagiwara, T. Nakagima & T. Hoshita. 1991. Isolation and identification of bile salts conjugated with cysteinolic from bile of the red seabream, Pagrosomus major. Journal of Lipid Research, 32: 1619-1623.; Goto et al., 1996Goto, T., T. Ui, T. Une, T. Kuramoto, K. Kirira & T. Hoshita. 1996. Bile salt composition and distribution of the D-cystenolic acid conjugated bile salts in fish. Fisheries Science, 62: 606-609.). The bile acid composition of rainbow trout was investigated and cholic acid was found to be the main component and constituted over 85% of total. (Denton & Yousef, 1974Denton, J. E. & M. K. Yousef. 1974. Bile acid composition of rainbow trout, Salmo gairdneri. Lipids, 9: 945-951.). Chenodeoxycholic acid accounted for 14% and 3α, 12α-7-keto and 7α,12α-3-keto-5β cholanoates for 1% or less of total. In lake char, taurocholic acid (TCA) and Taurochenodeoxycholic acid (TCD) are the two principal bile acids (Zhang et al., 2001Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.) and these results are consistent with previous reports in other salmonid species (Sasaki, 1966Sasaki, T. 1966. Stereo-bile acids and bile alcohols: LXXXII. Comparative studies on the bile salts of fishes by thin layer chromatography. The Journal of Biochemistry, 60: 56-62.; Haslewood, 1967Haslewood, G. A. D. 1967. Bile salts. Methuen, London .; Denton & Yousef, 1974Denton, J. E. & M. K. Yousef. 1974. Bile acid composition of rainbow trout, Salmo gairdneri. Lipids, 9: 945-951.). In spite that all these substances are well known, no specific bile acids have been identified as pheromones in catfish species. These fish are known to have taste buds all around their body surface used for perceiving environmental chemical cues (Barlow & Northcutt, 1997Barlow, L. A. & R. G. Northcutt. 1997. Taste buds develop autonomously from endoderm without induction by cephalic neural crest or paraxial mesoderm. Development, 124: 949-957.) and have also specific adaptations, the barbells, used for the same purpose (Kotrschal et al., 1996Kotrschal, K., R. C. Peters & K. B. Doving. 1996. Chemosensory and tactile nerve responses from the anterior dorsal fin of a rockling, Gaidropsarus vulgaris. Primary Sensory Neuron, 1: 297-309.). Regarding intraespecific chemical communication, catfish are known to release alarm pheromone when their skin is damaged causing conspecific antipredator responses (Giaquinto & Volpato, 2001Giaquinto, P. C. & G. L. Volpato. 2001. Hunger suppresses the onset and the freezing component of the antipredator response to conspecific skin extract in pintado catfish. Behaviour, 138: 1205-1214.). The pintado, Pseudoplatystoma corruscans, is an interesting South American catfish to study chemical communication because such species show alarm chemicals from club cells and are even able to recognize conspecific size by chemical information (Giaquinto & Volpato, 2005Giaquinto, P. C. & G. L. Volpato. 2005. Chemical cues related to conspecific size in pintado catfish, Pseudoplatystoma corruscans. Acta Ethologica, 8: 65-69.). Also, chemical signals are likely to be an important source of information in feeding behavior (Giaquinto & Hoffmann, 2010Giaquinto, P. C. & A. Hoffmann. 2010. Role of olfaction and vision cues in feeding behavior and alarm reaction in the catfish pintado, Pseudoplatystoma corruscans. Journal of Ethololgy, 28: 21-27.) and in female mate choice (Giaquinto, 2010Giaquinto, P. C. 2010. Female pintado catfish choose well-fed males. Behaviour, 147: 319-332.). These confirm the importance of olfaction for this taxonomic group of fish. Also, as a nocturnal and predatory fish, chemically-modulated reactions are expected to play a major role in intra and inter-specific relationships. Based on these findings, we planned to investigate a possible biological role of bile acids in pintado, Pseudoplatystoma corruscans, by testing which of them would be detected by olfaction.
Despite growing interest in the role of bile acids in fish chemoreception (Zhang et al., 2001Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.), little effort has been made to examine a possible pheromonal role of bile acids in fish. To investigate pheromonal roles of bile acids pintado, our fist study tested which bile acids are detected by olfaction using electro-olfactogram (EOG) recordings. From 30 bile acids tested, five showed evident EOG responses; among them, taurocholic acid (TCA) was investigated in a behavioral study as a possible pheromone because it showed the strongest response and previous reports showing that this bile acid is released in feces in high proportion (Zhang et al., 2001Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.) and authentic bile acids are also important inducing behavioral responses in fish (Jones & Hara, 1985Jones, K. A. & T. J. Hara. 1985. Behavioural responses of fishes to chemical cues: results from a new bioassay. Journal of Fish Biology, 27: 495-504.).
Material and Methods
Animals and housing. Pintado fish, Pseudoplatystoma corruscans , were obtained from a commercial fish farm and kept in indoor 500-L tanks for 30 days prior to the experimental procedures. Water temperature ranged between 28-30º C and a 12L:12D photoperiod was maintained. The tanks were constantly aerated and ammonium and nitrite levels were maintained below 0.5 and 0.05 mg/L, respectively. The tanks were provided with PVC tubes as shelters for the fish. The fish were daily fed with two live worms per fish.
The length and mean weight of these fish was 9.6 ± 0.92 cm and 31.2 ± 0.58 g
respectively. Fish were not separated by sex since they are immature at this size (Godinho et al., 2007Godinho, A. L., B. Kynard & H. P. Godinho. 2007. Migration and
spawning of female surubim (Pseudoplatystoma corruscans, Pimelodidae) in the São
Francisco river, Brazil. Environmental Biology of Fishes.
http://www.sfrancisco.bio.br.
http://www.sfrancisco.bio.br...
).
Experimental design. We tested several types of bile acids to analyze their potential as chemical signal. Thus, we firstly tested the response of chemosensory apparatus by using EOG record. Secondly, we chose the strongest bile acid to test its role in a two-choice maze, running water containing bile acid or a plain water as control.
Stimulus administration and EOG recording. Each fish was tranquilized with MS222 (1:8000), anaesthetized intraperitoneally with amobarbital (30 mg/kg body weight), and immobilized with an intramuscular injection of Flaxedil (gallamine triethiodide, 3-4 mg/kg body weight). The anaesthetized fish was wrapped with an absorbent tissue and secured in a holding apparatus. The right side rosette was exposed by removing the dorsal aspect of the skin and the cartilage of the olfactory sac. The naris and the gills were perfused with plain water. To deliver plain water or stimulants to the naris, the method of Sveinsson (2000)Siefkes, M. J. & W. Li. 2004. Electrophysiological evidence for detection and discrimination of pheromonal bile acids by the olfactory epithelium of female sea lamprey (Petromyzon marinus). Journal of Comparative Physiology A, 190: 193-199. was used. Briefly, a pneumatic activator switches plain water to/from the stimulant solution delivered by two identical polyethylene tubes. The pneumatic valve was controlled by a solenoid and an associated electronic timing device (Hara et al., 1973Hara, T. J., Y. M. C. Law & E. Van der Veen. 1973. A stimulatory apparatus for studying the olfactory activity in fishes. Journal of the Fisheries Research Board of Canada, 30: 282-285.). The solution flowed through a glass capillary positioned over the rosette (10 mL/min). This system provides an approximate square stimulation at required concentration each time with no apparent interruption or disturbance of the flow to the naris. The stimulus duration was 10s.
EOG responses were recorded differentially by a pair of Ag-AgCl electrodes (type MEH1S; World Precision Instruments, FL, USA) filled with 3M KCl bridged by 8% gelatin-saline filled capillaries (tip diameter 100-150 µm) (Evans & Hara, 1985Evans, R. E. & T. J. Hara. 1985. The characteristics of the eletro-olfactogram (EOG): its loss and recovery following olfactory nerve section in rainbow trout (Salmo gairdneri). Brain Research, 330: 65-75.). One electrode was positioned near the central ridge, posterior portion of the rosette, and slightly above the olfactory epithelium. The other electrode (reference) was placed on the skin surface adjacent to the perfused olfactory cavity. Electrical activities were amplified with a DC-preamplifier (Grass 7P1, Grass Instruments, MA, USA).
Chemicals. In total, 30 bile acids were tested, which comprised all known commercially available bile acids produced by fish and common vertebrates. All tested bile acids were purchased from either Sigma Chemical (St. Louis, M.O., USA) or Steraloids Inc. (Wilton, NH, USA). The five most potent bile acids were select for Concentration-Response tests. Names and abbreviations of most potent bile acids are listed in Table 1 (5 out 30 tested) (Table 1). Trivial names for bile acids are used throughout the manuscript.
The five bile salts tested in this study were selected to include two produced by the channel catfish [TCDC and TCA (Kellogg, 1975Kellogg, T. F. 1975. The biliary bile acids of the channel catfish, Ictalurus punctatus, and the blue catfish, Ictalurus furcatus. Comparative Biochemistry and Physiology B, 50: 109-111.)]. Previous investigators (Døving et al., 1980; Goh & Tamura, 1980Goh, Y. & T. Tamura. 1980. Olfactory and gustatory responses to amino acids in two marine teleosts: Red sea bream and mullet. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 66: 217-224.; Jones & Hara, 1985Jones, K. A. & T. J. Hara. 1985. Behavioural responses of fishes to chemical cues: results from a new bioassay. Journal of Fish Biology, 27: 495-504.; Hellstrøm & Døving, 1986Hellstrøm, T. & K. B. Døving. 1986. Chemoreception of taurocholate in anosmic and sham-operated cod Gadus morhua. Behavioural Brain Research, 21: 155-162.; Friedrich & Korsching, 1998Friedrich, R. W. & S. I. Korsching. 1998. Chemtopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. Journal of Neuroscience, 18: 9977-9988.; Nikonov & Caprio, 2001Nikonov, A. A. & J. Caprio. 2001. Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of channel catfish. Journal of Neurophysiology, 8: 1869-1876.; Zhang et al., 2001Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.; Rolen et al., 2003Rolen, S. H., P. W Sorensen, D. Mattson & J. Caprio. 2003. Polyamines as olfactory stimuli in the goldfish, Carassius auratus. Journal of Experimental Biology, 206: 1683-1696.; Rolen & Caprio, 2007Rolen, S. H. & J. Caprio. 2007. Processing of bile salt odor information by single olfactory bulb neurons in the channel catfish. Journal of Neurophysiology, 97: 4058-4068.) commonly utilized one or more of these bile salts in their studies.
Stock solutions of the stimulants (10-3 M concentrations) were prepared with distilled water and stored in a refrigerator. Test solutions were diluted with plain water before testing. To eliminate the effect of distilled water, aliquots of stock solutions were diluted at least 100 times of plain water to form test formulas.
Cross-adaptation. Cross-adaptation, developed by Caprio & Byrd (1984), was used to compare the EOG response to a test stimulus before and during adaptation to an adapting compound using a protocol by Li & Sorensen (1997). In a given trial, baseline olfactory EOG responses of pintado to blank water control (control A), an L-arginine standard and test stimuli (bile acids) were recorded. The test stimuli were used at concentrations that elicited approximately equipotent olfactory responses at about 100% of the L-arginine standard. During adaptation, the olfactory epithelium was continually exposed to the adapting stimulus for 5 min after which a 5-s application of the same adapting stimulus was tested, first at the concentration used in the adaptation (control B) and then at twice the concentration used for the adaptation (self-adapted control). Then the other test stimuli were tested in the adapting solution (adapted response). Each test was interspersed with 5-s applications of the L-arginine standard and control B to confirm the responsiveness of the tested fish. Switching the adapting stimulus back to blank water completed the trial. The epithelium of the fish was allowed to recover for 30 min and then was tested again using another adapting stimulus. Cross-adaptation data were expressed as percent initial response (PIR) using the following formula adapted from Caprio & Byrd (1984) and Li & Sorensen (1997):
where a larger PIR indicates less cross-reactivity between olfactory receptor mechanisms or separate receptor sites and a low PIR indicates more cross reactivity or shared receptor sites and/or a common signal transduction pathway.
Statistical analysis of EOG data. Statistical analysis system (SAS) software was used to conduct all analyses. In concentration-response experiments, responses were visually compared. The lowest concentration at which a stimulus elicited a response larger than the blank water control (Student´s t-test) was considered to be its response threshold. In cross-adaptation experiments, a paired t-test was used to determine if responses to a test stimulus during adaptation were significantly different from the responses to the same test stimulus before adaptation. All PIR data were subsequently analyzed to directly detect cross-reactivity by subjecting all PIR data to a two-way analysis of variance (ANOVA). If the main effect was found to be significant, the PIR data was divided into five groups according to adapting stimulus. For each group, responses of adapted epithelia to test stimuli were analyzed by a one-way ANOVA to determine the affect of the adapting stimulus. Again, if the main effect was significant, the significance of the adapting effect for each test stimulus was determined by comparing all PIRs in the group with the PIR of the self-adapted control using Dunnett´s test, which tests for differences between several treatments and a single control. The following categories were used to classify cross-adaptation responses: (1) not adapted, meaning responses during adaptation were not significantly different from the initial response (paired t-test, P<0.05); (2) partially adapted, meaning responses during adaptation were significantly less than the initial responses (paired t-test, P<0.05), but the PIR were significantly greater than the control (Dunnetts, P<0.05); and (3) adapted to control levels, meaning PIR were not significantly different than the control (Dunnetts, P<0.05).
Avoidance/preference testes. Behavioral tests were conducted in an avoidance/preference behavior apparatus. Water was pumped into a rectangular tank (120 x 18 x 15 cm) of clear acrylic plastic from both ends at the same rate, regulated by PVC valves. The water drained from bottom plate and side walls at the center of the tank. Water bodies from both sections were well separated as evidenced by dye tests. A stock solution of the compound to be tested was kept at the same temperature as the water and injected into either side of the tank by a dosing pump. The other section received the same amount of distilled water delivered in a same manner. The stimulatory section was alternated after each test.
TCA was chosen to be tested in this behavioral test. This stimulants solution (Sigma Chemical Co.) was diluted in distilled water at 10-8 M concentration. This concentration was chosen based on EOG records, with the higher concentration-response relationship before the olfactory adaptation.
Each fish was introduced to the two-choice maze trough three days prior testing for acclimation. Tests were conducted during 11:00-15:00 h, three observations daily for each fish, for four consecutive days. Fish locomotion was recorded for 20 min (5 min before and 15 min after the stimuli administration). During this period, the direction and frequency of each movement (ahead/back, up/down, and turns in relation to each stimulus) were recorded and the total time an individual spent in each section was quantified. Fourteen fish were individually tested. For each preference test, the response of the test population was analyzed using a Wilcoxon signed-rank matched-pairs test (to compare between sets of trial), paired t test (preference stimuli/ latency to each stimuli), and Dunn's multiple comparisons test (number of movements in treatment and distilled water comparison). Significant data were set at 0.05.
Results
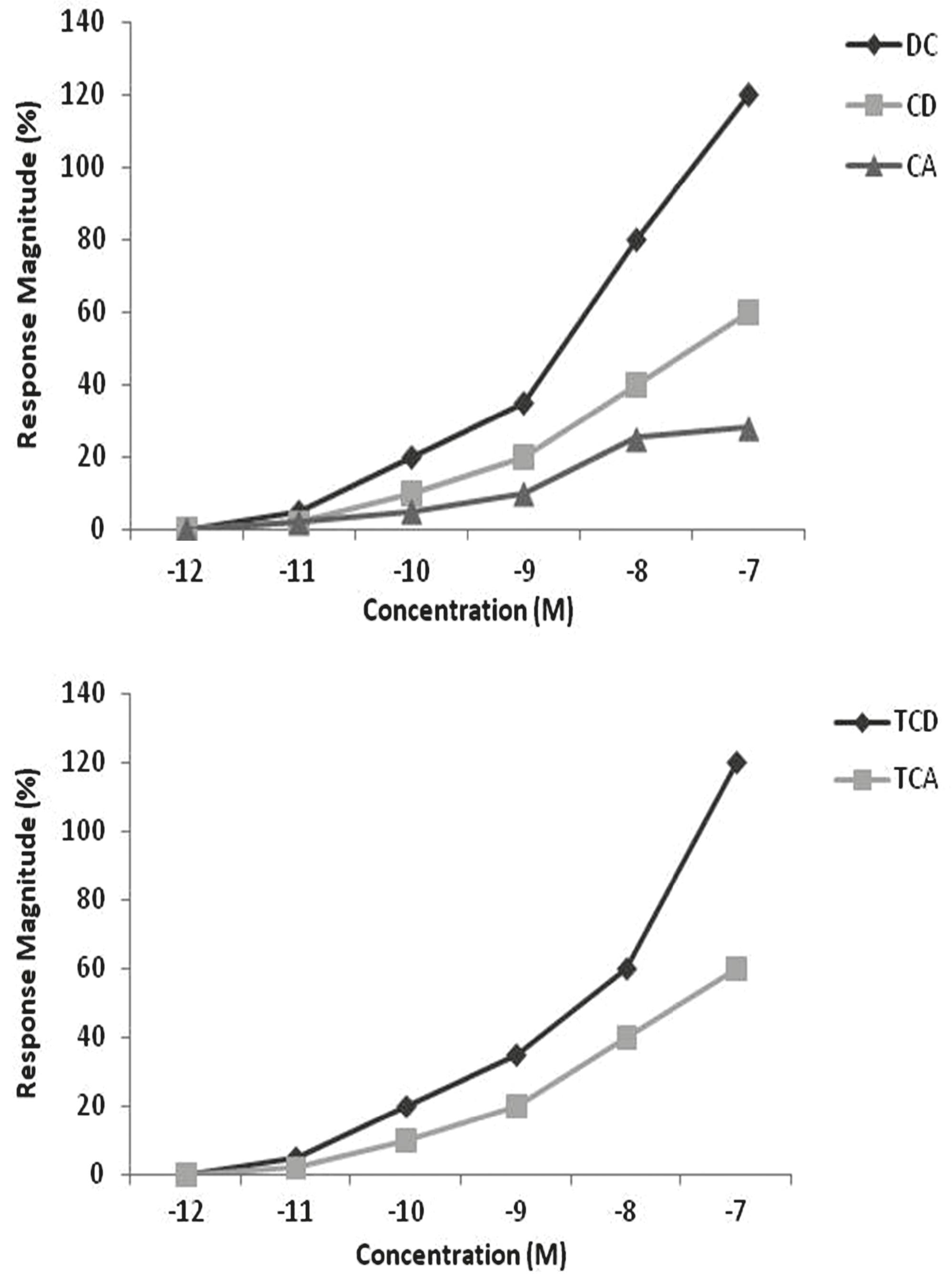
EOG responses. Concentration-response (C-R) relationships of the five bile acids were plotted together as percentages of the L-arginine standard. Five most potent bile acids were DC, CD, CA, TCD, and TCA, with detection thresholds of 0.02 nM, considered extremely low concentrations regarding chemical cues (Døving et al., 1980; Hara et al., 1984; Hara & Zhang, 1996, 1998). Bile acids were grouped according to the molecular structures (Fig. 1). Responses to all concentrations of bile acids ranged from -9% to 640%, and the blank water control elicited a mean response of 7.9±6.5% (mean±SE). The free bile acids deoxycholic acid (DC), chenodeoxycholic (CD) and cholic acid (CA) had similar, nearly parallel C-R curves with detection thresholds 10-9 M 10-11 M and 10-9 M, respectively (Fig. 1). Both the bile acids conjugated with taurine, taurochenodeoxycholic acid (TCD) and taurocholic acid (TCA), had essentially identical C-R curves, with relatively lower detection thresholds of 10-11 M and 10-10 M, respectively (Fig. 1).
Electrolfactogram responses of pintado Pseudoplatystoma corruscans to five representative bile acids. Response magnitudes are normalized as percentages of response to 10-5 M L-serine (mean ± SEM). CA = Cholic acid, TCA = taurocholic acid, TCD = taurochenodeoxicholic acid, CD = chenodeoxycholic acid, DC = deoxycholic acid.
Cross-adaptation. When used as adapting stimuli (self-adaptation), all six bile acids eliminated EOG responsiveness to themselves. The results suggested that the interaction of bile acids with receptors is in a reversible and truly competitive manner and may be mediated by specific receptors (Fig. 2). Adaptation to free bile acid DC inhibited responses to CA and itself, but adaptation to CD did not inhibit TCA responses completely, suggesting that separate receptors may exist for DC and CD and that DC and CA share the same receptors. None of the bile acids significantly inhibited TCD responses (other than itself), suggesting separate receptors for TCD. ARG responses were unaffected by all bile acids.
Remained response magnitudes at 10-7 M during adaptation to 10-7 M taurocholic acid (TCA) and 10-7 M taurochenodeoxycholic (TCD). Responses are expressed as percentages of the response to the standard 10-5 M Arginine (L-ARG). CA = Cholic acid, TCA = taurocholic acid, TCD = taurochenodeoxicholic acid, CD = chenodeoxycholic acid, DC = deoxycholic acid.
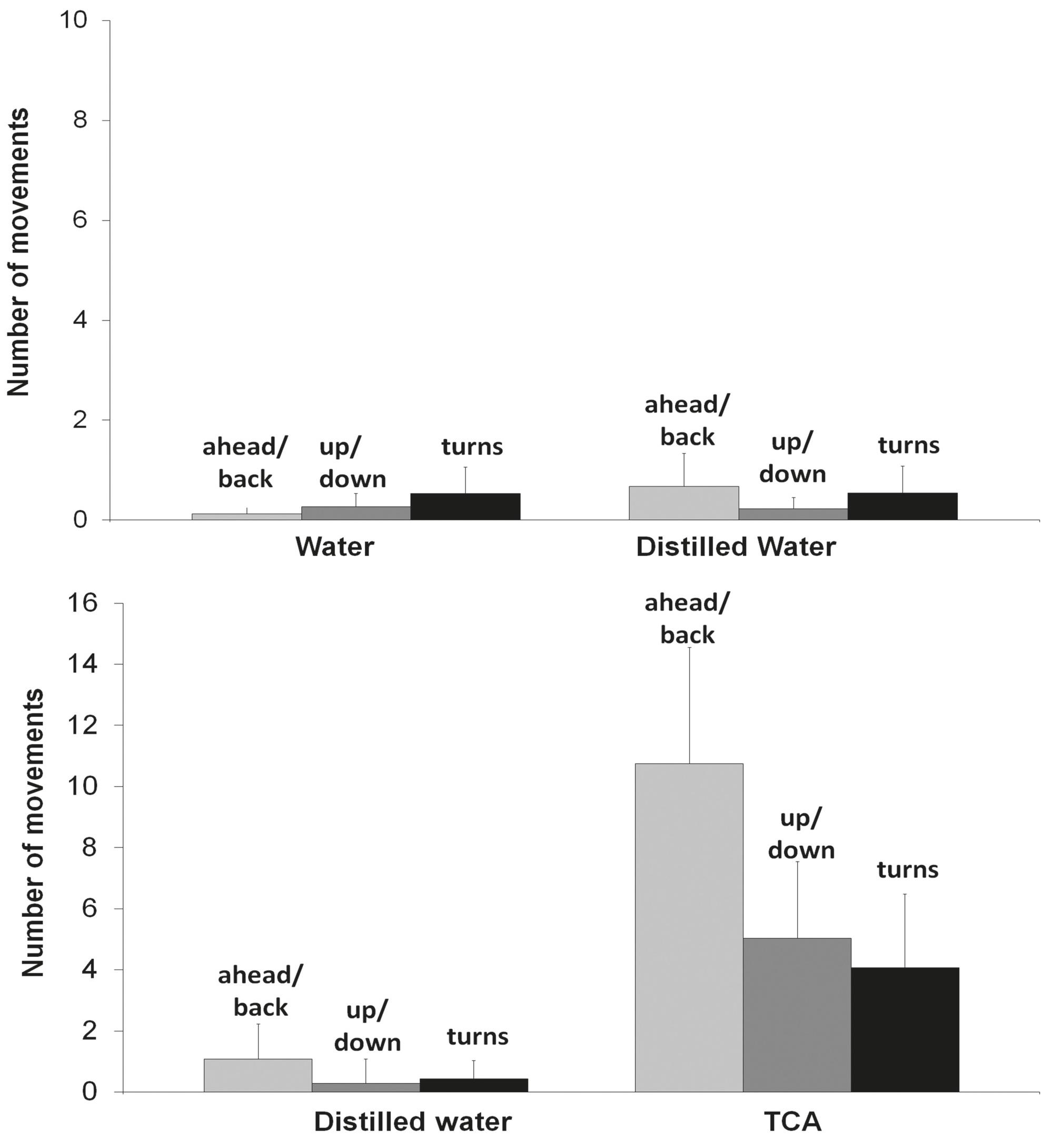
Behavioral responses. When the aquarium sections received water and distilled water (control), fish spent equal amount of time in both sections (Fig. 3 - control), showing no difference to a neutral reaction assumption. However, when given a choice between distilled water and TCA, pintado showed clear preference to the section containing TCA solution (Fig. 3 - TCA test). Frequency of movements was also not affected in the controls (Fig. 4 - control), but increased in the TCA
Time spent in each compartment when Pseudoplatystoma corruscans were stimulated by taurocholic acid (TCA) and controls. TCA response was significantly different from distilled water (paired t test: 3.94, P = 0.0005) as indicated by an asterisk.
Number of movements of Pseudoplatystoma corruscans stimulated by taurocholic acid (TCA) and controls. Response to TCA was significantly higher than distilled water comparison for each behavior. Dunn's multiple comparisons test, P
Discussion
In this study, EOG recording and behavioral studies together demonstrated that catfish pintado, P. corruscans, responds to the tested bile acids. Most importantly, our behavioral studies showed that TCA bile acid were strongly attractive to this species.
Among bile acids tested, the most potent ones were CA, TCA, TCD, CD, and DC. This observation is consistent with EOG data in lake char (Zhang et al., 1996Zhang, C., S. B. Brown & T. J. Hara. 1996. Olfactory sensitivity and behavioral reactions of lake char to bile acids released by conspecifics. Chemical Senses, 21: 692-693.), zebrafish (Danio rerio) (Michel & Lubomudrov, 1995Michel, W. C. & L. M. Lubomudrov. 1995. Specificity and sensitivity of the olfactory organ of the zebrafish, Danio rerio. Journal of Comparative Physiology A, 177: 191-199.), sea lamprey (Li & Sorensen, 1994Li, W. & P. W. Sorensen. 1994. High specificity of the sea lamprey olfactory system to four classes of bile acids. Chemical Senses, 19: 506.) and olfactory bulb recording in grayling and Arctic char (Doving et al., 1980Vermeirssen, E. L. M. & A. P. Scott. 2001. Male priming pheromone is present in bile as well as urine of female rainbow trout. Journal of Fish Biology, 58: 1039-1045.). Conjugated bile acids tested here showed a profile of concentration-response relationship of phasic responses different from the free bile acids (Fig. 1). Also, our cross-adaptation results indicated a competitive interaction of bile acids with their receptors which is fully reversible at the range concentrations tested (Fig. 2). The results suggest that olfactory responses to bile acids may be mediated by specific receptors. This might imply distinct olphatory receptors for these bile acid groups.
TCA showed the strongest tonic-phase response ratios (Fig. 1). In salmonids, this bile acid is copiously released in feces and elicits behavioral responses in conspecifics. Such profile is congruent with the strong attraction of pintado to TCA as revealed in the behavioral study (Figs. 3-4). Pintado is a nocturnal fish and was investigated in the present study during the light period of the day. This explains the very low behavioral response in the control conditions (Figs. 3-4, control) and reinforces that pintado, as salmonids, was strongly attracted to TCA (Figs. 3-4, TCA test).
Extreme olfactory sensitive to bile acids, coupled with their wide distribution and chemical variations, have been implicated for their role in fish behavior (Doving et al., 1980Doving, K. B, R. Selset & G. Thommesen. 1980. Olfactory sensitivity to bile acids in salmonid fishes. Acta Physiologica Scandinavica, 108: 123-131.; Zhang & Hara, 1991Zhang, C. & T. J. Hara. 1991. Olfactory and gustatory responses to bile salts in salmonids. Pp. 16-22. In: Zhang, C. & T. J. Hara. 1991. Chemical signals in vertebrates. Philadelphia, Pennsylvania.; Hara, 1994Hara, T. J. 1994. The diversity of chemical stimulation in fish olfaction and gustation. Reviews in Fish Biology and Fisheries, 4: 1-35.). Bile acid analyses in many salmonid species indicated a high proportion of conjugated bile acids (TCA and TCD) in bile, feces and holding water (Zhang et al., 2001Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.). This observation supports the overall evolutionary pattern proposed by Haslewood (1967)Haslewood, G. A. D. 1967. Bile salts. Methuen, London . and current concepts that non-mammals secrete exclusively taurine conjugated or sulphated bile acids, thus indicating a role of bile acids in chemical communication.
Pintado response to TCA may thus be of biological importance. Faeces and urine represent two sources for this bioactive byproducts in water (Zhang et al., 2001Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.). Although the ecological significance of bile acids in faeces urine and water remains to be established, Foster (1985)Foster, N. R. 1985. Lake trout reproductive behavior: influences of chemosensory cues from young-of-the-year by-products. Transactions of the American Fisheries Society, 114: 794-803. showed that females are attracted to reefs treated with faeces of juvenile male lake char and successful reproduction was observed at these sites. This suggests that TCA emanating from faeces of pintado may play a role in the reproductive behavior of spawning adults. Moreover, faeces chemicals attract fish predators and then some fish localize their defecation away from their foraging areas (Brown et al., 1995Brown, G. E., D. P. Chivers & R. J. F. Smith. 1995. Localized defecation by pike: a response to labeling by cyprinid alarm pheromone? Behavioral Ecology and Sociobiology, 36: 105-110.). Giaquinto & Volpato (2005)Giaquinto, P. C. & G. L. Volpato. 2005. Chemical cues related to conspecific size in pintado catfish, Pseudoplatystoma corruscans. Acta Ethologica, 8: 65-69. showed that pintado discriminates large and size-matched conspecifics by chemical cues. As a larger pintado is a potential predator for smaller conspecifics, this chemical recognition might be an anti-predator mechanism.
Acknowledgments
This study was funded by Fundação para o Amparo da Pesquisa do Estado de São Paulo, FAPESP, doctoral fellowship to P.C.G (process number: 98/03036-4). All experimental procedures employed in this study complied with the Brazilian College for Animal Experimentation (COBEA) ) (http://www.cobea.org.br).
References
- Barlow, L. A. & R. G. Northcutt. 1997. Taste buds develop autonomously from endoderm without induction by cephalic neural crest or paraxial mesoderm. Development, 124: 949-957.
- Brown, G. E., D. P. Chivers & R. J. F. Smith. 1995. Localized defecation by pike: a response to labeling by cyprinid alarm pheromone? Behavioral Ecology and Sociobiology, 36: 105-110.
- Denton, J. E. & M. K. Yousef. 1974. Bile acid composition of rainbow trout, Salmo gairdneri. Lipids, 9: 945-951.
- Doving, K. B, R. Selset & G. Thommesen. 1980. Olfactory sensitivity to bile acids in salmonid fishes. Acta Physiologica Scandinavica, 108: 123-131.
- Evans, R. E. & T. J. Hara. 1985. The characteristics of the eletro-olfactogram (EOG): its loss and recovery following olfactory nerve section in rainbow trout (Salmo gairdneri). Brain Research, 330: 65-75.
- Foster, N. R. 1985. Lake trout reproductive behavior: influences of chemosensory cues from young-of-the-year by-products. Transactions of the American Fisheries Society, 114: 794-803.
- Friedrich, R. W. & S. I. Korsching. 1998. Chemtopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. Journal of Neuroscience, 18: 9977-9988.
- Giaquinto, P. C. 2010. Female pintado catfish choose well-fed males. Behaviour, 147: 319-332.
- Giaquinto, P. C. & T. J. Hara. 2008. Discrimination of bile acids by the rainbow trout olfactory system: evidence as potential pheromone. Biological Research, 41: 33-42.
- Giaquinto, P. C. & A. Hoffmann. 2010. Role of olfaction and vision cues in feeding behavior and alarm reaction in the catfish pintado, Pseudoplatystoma corruscans. Journal of Ethololgy, 28: 21-27.
- Giaquinto, P. C. & G. L. Volpato. 2001. Hunger suppresses the onset and the freezing component of the antipredator response to conspecific skin extract in pintado catfish. Behaviour, 138: 1205-1214.
- Giaquinto, P. C. & G. L. Volpato. 2005. Chemical cues related to conspecific size in pintado catfish, Pseudoplatystoma corruscans. Acta Ethologica, 8: 65-69.
- Godinho, A. L., B. Kynard & H. P. Godinho. 2007. Migration and spawning of female surubim (Pseudoplatystoma corruscans, Pimelodidae) in the São Francisco river, Brazil. Environmental Biology of Fishes. http://www.sfrancisco.bio.br.
» http://www.sfrancisco.bio.br - Goh, Y. & T. Tamura. 1980. Olfactory and gustatory responses to amino acids in two marine teleosts: Red sea bream and mullet. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 66: 217-224.
- Goto, T., T. Ui, T. Une, T. Kuramoto, K. Kirira & T. Hoshita. 1996. Bile salt composition and distribution of the D-cystenolic acid conjugated bile salts in fish. Fisheries Science, 62: 606-609.
- Grosell, M., M. J. O'Donnell & C. M. Wood. 2000. Hepatic versus gallbladder bile composition: in vivo transport physiology of the gallbladder in rainbow trout. American Journal of Physiology, 278: 1674-1684.
- Hara, T. J. 1994. The diversity of chemical stimulation in fish olfaction and gustation. Reviews in Fish Biology and Fisheries, 4: 1-35.
- Hara, T. J., Y. M. C. Law & E. Van der Veen. 1973. A stimulatory apparatus for studying the olfactory activity in fishes. Journal of the Fisheries Research Board of Canada, 30: 282-285.
- Hara, T. J., S. Macdonald, R .E. Evan, T. Marui & S. Arai. 1984. Morpholine, bile acids and skin mucus as possible chemical cues in salmonid. Mechanisms of Migration in Fishes. NATO Conference Series, 14: 363-378.
- Hara, T. J. & C. Zhang. 1995. Olfactory responses to putative pheromones and their neural pathways in salmonids. Proceedings of the workshop "Fish Pheromones: Origins and Mechanisms of Action", University of Algarve, 22-40 May, Faro, Portugal, p. 82-97.
- Haslewood, G. A. D. 1967. Bile salts. Methuen, London .
- Hellstrøm, T. & K. B. Døving. 1986. Chemoreception of taurocholate in anosmic and sham-operated cod Gadus morhua. Behavioural Brain Research, 21: 155-162.
- Huertas, M., P. C. Hubbard, A. V. M. Canario & J. Cerda. 2007. Olfactory sensitivity to conspecific bile fluid and skin mucus in the European eel Anguilla anguilla (L.). Journal of Fish Biology, 70: 1907-1920.
- Jones, K. A. & T. J. Hara. 1985. Behavioural responses of fishes to chemical cues: results from a new bioassay. Journal of Fish Biology, 27: 495-504.
- Karlaganis, G., S. E. Bradley, J. L. Boyer, A. K. Batta, G. Salen, B. Egestad & J. Sjövell. 1989. A bile alcohol sulfate as a major component in the bile of the small skate (Raja erinacea). Journal of Lipid Research, 30: 317-322.
- Kellogg, T. F. 1975. The biliary bile acids of the channel catfish, Ictalurus punctatus, and the blue catfish, Ictalurus furcatus. Comparative Biochemistry and Physiology B, 50: 109-111.
- Kotrschal, K., R. C. Peters & K. B. Doving. 1996. Chemosensory and tactile nerve responses from the anterior dorsal fin of a rockling, Gaidropsarus vulgaris. Primary Sensory Neuron, 1: 297-309.
- Li, W. & P. W. Sorensen. 1994. High specificity of the sea lamprey olfactory system to four classes of bile acids. Chemical Senses, 19: 506.
- Michel, W. C. & L. M. Lubomudrov. 1995. Specificity and sensitivity of the olfactory organ of the zebrafish, Danio rerio. Journal of Comparative Physiology A, 177: 191-199.
- Nikonov, A. A. & J. Caprio. 2001. Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of channel catfish. Journal of Neurophysiology, 8: 1869-1876.
- Quinn, T. P. & T. J. Hara. 1986. Sibling recognition and olfactory sensitive in juvenile coho salmon (Oncorhynchus kisutch). Canadian Journal of Zoology, 64: 921-925.
- Rolen, S. H. & J. Caprio. 2007. Processing of bile salt odor information by single olfactory bulb neurons in the channel catfish. Journal of Neurophysiology, 97: 4058-4068.
- Rolen, S. H., P. W Sorensen, D. Mattson & J. Caprio. 2003. Polyamines as olfactory stimuli in the goldfish, Carassius auratus. Journal of Experimental Biology, 206: 1683-1696.
- Sasaki, T. 1966. Stereo-bile acids and bile alcohols: LXXXII. Comparative studies on the bile salts of fishes by thin layer chromatography. The Journal of Biochemistry, 60: 56-62.
- Siefkes, M. J. & W. Li. 2004. Electrophysiological evidence for detection and discrimination of pheromonal bile acids by the olfactory epithelium of female sea lamprey (Petromyzon marinus). Journal of Comparative Physiology A, 190: 193-199.
- Sorensen, P. W., T. J. Hara & N. E. Stacey. 1987. Extreme olfactory sensitivity of mature and gonadally-regressed goldfish to a potent steroidal pheromone, 17, 20-dihydroxy-4-pregnen-3-one. Journal of Comparative Physiology, 160: 305-313.
- Stabell, O. B. 1992. Olfactory control of homing behavior in salmonids. Pp. 249-270. In: T. J. Hara (Ed.). Fish chemoreception. London, Chapman and Hall.
- Sveinsson, T. & T. J. Hara. 2000. Olfactory sensitivity and specificity of Artic char, Salvelinus alpinus, to a putative pheromone, prostaglandin F2α. Physiology and Behavior, 69: 301-307.
- Une, M., T. Goto, K. Kirira, T. Kuramoto, K. Hagiwara, T. Nakagima & T. Hoshita. 1991. Isolation and identification of bile salts conjugated with cysteinolic from bile of the red seabream, Pagrosomus major. Journal of Lipid Research, 32: 1619-1623.
- Vermeirssen, E. L. M. & A. P. Scott. 2001. Male priming pheromone is present in bile as well as urine of female rainbow trout. Journal of Fish Biology, 58: 1039-1045.
- Zhang, C., S. B. Brown & T. J. Hara. 1996. Olfactory sensitivity and behavioral reactions of lake char to bile acids released by conspecifics. Chemical Senses, 21: 692-693.
- Zhang, C. & T. J. Hara. 1991. Olfactory and gustatory responses to bile salts in salmonids. Pp. 16-22. In: Zhang, C. & T. J. Hara. 1991. Chemical signals in vertebrates. Philadelphia, Pennsylvania.
- Zhang, C., S. B. Scott & T. J. Hara. 2001. Biochemical and physiological evidence that bile acids are produced and released by lake char (Salvelinus namaycush) function as chemical signals. Journal of Comparative Physiology B, 171: 161-171.
Publication Dates
-
Publication in this collection
Jan-Mar 2015
History
-
Received
25 Feb 2014 -
Reviewed
25 Nov 2014 -
Accepted
31 Mar 2015